1. Introduction
Drought stress is a major limiting factor for crop production systems worldwide, especially under arid and semi-arid areas [
1]. Arid and semi-arid regions in Asia have faced acute water shortages for the last few decades, becoming a challenging task for agriculture and water management experts [
2]. Now, drought is considered the most important factor affecting crop yield. More devastatingly, the predicted climate change may enhance the frequency of drought, which may increase crop production losses [
3].
Our food source mainly depends on cereals. Their production is significantly affected by drought stress [
4]. In changing climate scenarios, wheat production decreases because of acute water deficiency in wheat-producing areas [
5]. Drought affects the wheat yield throughout the growing season to some extent, but more yield losses occur during reproductive and grain-filling phases [
6]. The yield losses of wheat during the reproductive phase due to drought might be due to its deleterious effects on morphological and physiological traits. Drought stress adversely affects root depth [
7], may accelerate leaf senescence [
8], oxidative damage to photosynthetic machinery [
9], and decrease carbon dioxide fixation [
10]. It may also affect the assimilate translocation rate [
10], resulting in a reduced grain set [
11]. Reduced chlorophyll causes chlorosis, leads to a reduction in photosynthesis [
12]. Drought adversely affects carbon fixation due to leaf stomata’s closure, reducing the production of photo-assimilates [
13]. Decreased stomatal conductance is the main cause of the reduction in photosynthesis during drought conditions [
14]. However, at later stages, drought leads to tissue dehydration which may cause metabolic injury [
8]. It also reduces leaf relative water content (RWC) and stomatal conductance, which ultimately leads to reducing growth and biomass production [
15,
16].
In addition to physiological and morphological traits, drought also affects traits that confer drought resistance in the specific environment may be very distinct. Drought causes grain loss because of pollen sterility and abscisic acid accumulation in spikes of drought susceptible wheat genotypes [
17]. Last but not least, drought stress could significantly affect wheat kernels milling [
18], flour quality [
19], dough kneading [
20,
21], and the entire breadmaking process [
6]. Being a major cereal crop in Pakistan, the wheat yield must be improved to fulfill the growing population’s increasing food demands [
22]. For this reason, the selection and use of drought-stress resistant cultivars able to guarantee suitable yields and flour quality, is essential [
23]. The response of plants to drought stress depends on different factors, such as growth stages, length and severity of stress, and a cultivar’s genetic makeup [
24].
Drought stress can occur at any growth stage depending upon the local environmental conditions [
3]. Thal region in Punjab Pakistan is an important region regarding wheat cultivation, and this region is also facing drought problems. This region experienced severe drought during 2000–2002 during the rabi season and affected 28% of the area [
25]. Thal region comes under arid as well as irrigated area which receives about 234 mm annual rainfall [
26]. To tackle drought in this region, adopting drought-tolerant genotypes is one of the most sustainable ways to reduce the impacts of different intensities of dry spells on wheat production [
23]. A lot of work has been done to maximize wheat production by adopting different drought mitigating practices. However, no information is available regarding suitable varieties grown in drought-prone arid areas of Punjab province to obtain better genetic potential. To facilitate farmers of arid regions (Thal zone), the present study was conducted to evaluate the effect of drought stress on the tested wheat genotypes to find high-yielding wheat genotypes under limited water conditions in the Thal region of Punjab, Pakistan.
2. Materials and Methods
2.1. Experimental Site and Soil Characteristics
This study was conducted in a green-house at the College of Agriculture, Bahauddin Zakariya University, Layyah, Pakistan. For the experiment, a mixture of soil and farmyard manure (FYM) (3:1 (w/w)) was used. The soil was sandy loam having pH 8.1, electrical conductivity (EC) 2.28 dSm−1, organic matter content 0.49%, available P 7 ppm, and available K 92 ppm.
2.2. Experimental Design and Treatments
Seeds of 14 wheat genotypes, consisted of new and old locally cultivated varieties, was obtained from Ayub Agricultural Research Institute (AARI) Faisalabad, and Arid Zone Research Institute (AZRI) Bhakkar, Punjab, Pakistan. The tested wheat genotypes were: Ujala-2016, Gold-2016, Johar-2016, Galaxy-2013, Ehsan-2016, Fath-e-Jang, Saher-2006, Gandum-1, Panjab-2011, AAS-2011, AS-2002, Faisalabad-2008, Lasani-2008, and Shafaq-2006. Moreover, three drought treatments of Galaxy-2013, i.e., (1) well-watered (control), (2) mild drought stress (60% water holding capacity (WHC)), and (3) severe drought stress (40% WHC), were tested. Pots were arranged in a factorial, completely randomized design (CRD) with three replications. The complete set of genotypes is presented in
Table 1.
2.3. Plants Growing
The earthen pots (12 cm in diameter and 60 cm in height) were filled with 10 kg of sieved soil and FYM at a ratio of 3:1, respectively. Ten seeds per pot were sown uniformly at a depth of 2 cm. After germination of seeds, five seedlings per pot were maintained till maturity. Fertilizer application was done following the recommended dose, i.e., nitrogen (N) at 0.25 g as urea, phosphorus (P) at 0.4 g as di-ammonium phosphate, and potassium (K) at 0.35 g pot−1 as potassium sulphate was applied as basal dose, while the remaining half dose of N as urea at 0.25 g pot−1 was applied at jointing stage. Drought was imposed from the jointing stage and maintained till crop maturity.
2.4. Maintenance of Water Holding Capacity
For measuring soil WHC/field capacity (
FC), the gravimetric method was followed as described by [
27]. Before sowing the seed, three soil samples were collected from each pot and measured for their fresh weight. After that, the samples were oven-dried at 105 °C till constant weight. After drying, three representative samples were taken and saturated with distilled water to make a saturated paste.
FC was determined by the following formula, as proposed by [
27]:
Through this formula, 100%, 60%, and 40% WHC were calculated, which was maintained throughout the experiment by weighing the pots. The difference in weight was corrected by applying the required amount of water according to the calculation.
2.5. Measurement of Relative Water Content (RWC) and SPAD-Chlorophyll Value
To measure RWC, fully expanded younger leaves from each treatment were collected. The leaf surface was gently dried using tissue paper, then wrapped in polythene bags and brought to the laboratory. Leaf samples were weighed to obtain leaf fresh weight (
FW). The samples were then soaked in plastic tubes containing distilled water and left overnight in the dark. The next morning, these leaves were carefully bloated with tissue paper and weighed to determine the turgid weight (
TW). Leaves were then dried at 70 °C till constant weight using a hot air oven. Dried leaves were then weighed to record dry weight (
DW). RWC was calculated by following the formula as described by [
28].
The SPAD-Chlorophyll value was estimated by using a portable SPAD-502 Chlorophyll Meter (Minolta Co., Ltd., Osaka, Japan). For this purpose, randomly selected 10 plants from each treatment were used to measure the chlorophyll content. The reading was taken from the flag leaf at the anthesis stage from three different points (bottom, middle, and tip of the leaf) and then averaged to get the final SPAD-Chlorophyll value.
2.6. Gas Exchange Characteristics
Gas exchange characteristics, including stomatal conductance (g), net photosynthesis (Pn), and transpiration rate (E), were recorded from fully expanded flag leaf for each treatment at the anthesis stage by using an infra-red gas analyzer (IRGA) Leaf Chamber Analyzer (Type LCA-4, USA). The measurements were made from 10:00 a.m. to 11:59 a.m. on a sunny day under a CO
2 concentration of 400 μmol mol
−1 [
29].
2.7. Yield and Related Traits
Five representative plants were randomly selected from each pot, and fertile tillers were counted manually, then averaged to find tillers per plant. At maturity, the plant height from five plants was measured from stem base to the ear’s tip using a meter scale and then averaged. Similarly, five spikes were randomly selected from each pot, and their length was measured with the help of a ruler and worked out their average. The same spikes were used to calculate the number of spikelets in each spike, and their mean was worked out. These five plants were harvested manually from each pot, sun-dried for six days. After drying, the weight of whole plants was measured on electrical balance and averaged to get biological yield per plant. After measuring biological yield, five spikes from each pot were threshed manually, and grains were cleaned, weighed, and averaged to obtain the grain yield per plant. Similarly, from the same threshed grains, 100 grains were counted manually from each pot and weighed to measure 100-grain weight.
2.8. Statistical Analysis
Data collected on all parameters were analyzed using Statistix software (Version 8.1. USA). Tukey’s HSD test at 5% probability level for 2-way ANOVA was applied to compare the treatment means [
30]. Further data were processed in Microsoft Excel-2010 to get values of standard error (±S.E.) as well as bar graphs for a graphical representation of data.
4. Discussion
The current study revealed that the RWC under well-watered conditions showed the highest values, which remarkably reduced under mild and severe drought conditions (
Table 2). Out of 14 tested genotypes, Galaxy-2013 maintained the highest RWC under severe drought conditions, followed by AAS-2011, which showed their tolerance to severe drought stress and might be due to genetic differences, making them superior to other studied genotypes. The association between higher RWC and drought tolerance for various wheat varieties has already been reported [
31,
32], which elaborates that drought-tolerant varieties maintained more water contents under limited water availability [
33]. Water retention under drought stress conditions could be a desired trait. However, when the plants keep closing their stomata, it may decrease the transpiration rate, affecting the uptake and transport of nutrients [
34]. It was observed that plants exhibited the maximum SPAD-chlorophyll values under well-watered conditions. In contrast, the values were reduced when the crop experienced drought stress, and the minimum values were recorded under severe drought conditions (
Table 2). A reduction in chlorophyll content under drought conditions was observed in different crop plants, including wheat, by another research [
35]. However, the genotype Galaxy-2013 produced the highest SPAD-chlorophyll value under severe drought conditions (
Table 2), which illustrates that Galaxy-2013 is a drought-tolerant variety staying green longer period which is the main character of drought-tolerant varieties. In contrast, reduced chlorophyll contents were observed in drought-sensitive wheat cultivars (
Table 2). It has already been documented that some wheat varieties have undergone a reduction in chlorophyll contents under drought stress [
36]. Reduction in photosynthetic pigments, including chlorophyll in wheat with the increasing water stress, was observed previously [
37,
38]. However, drought-tolerant genotypes retained many photosynthetic pigments under drought stress [
38,
39,
40]. Drought stress can destroy or reduce chlorophyll content and inhibit its synthesis [
41].
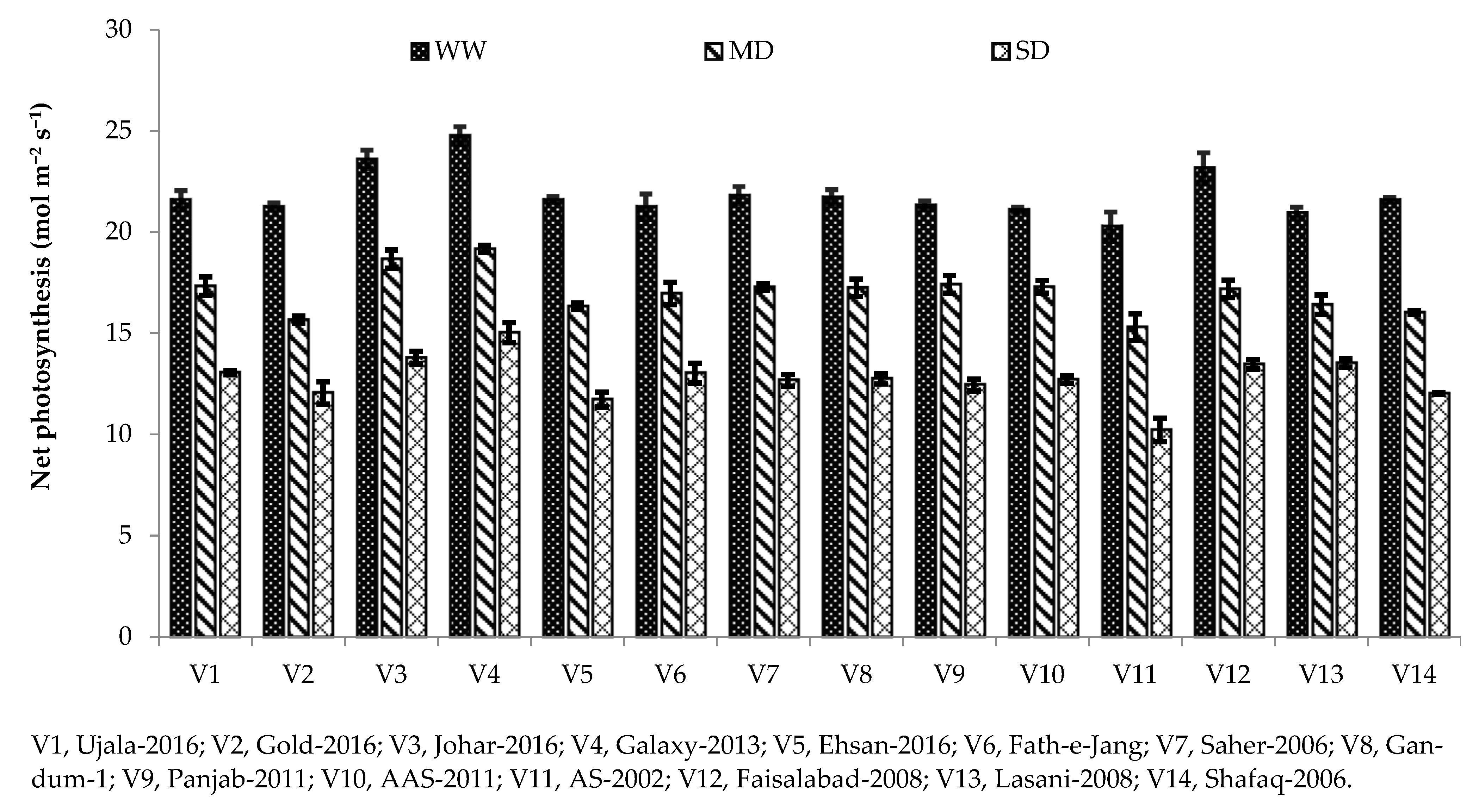
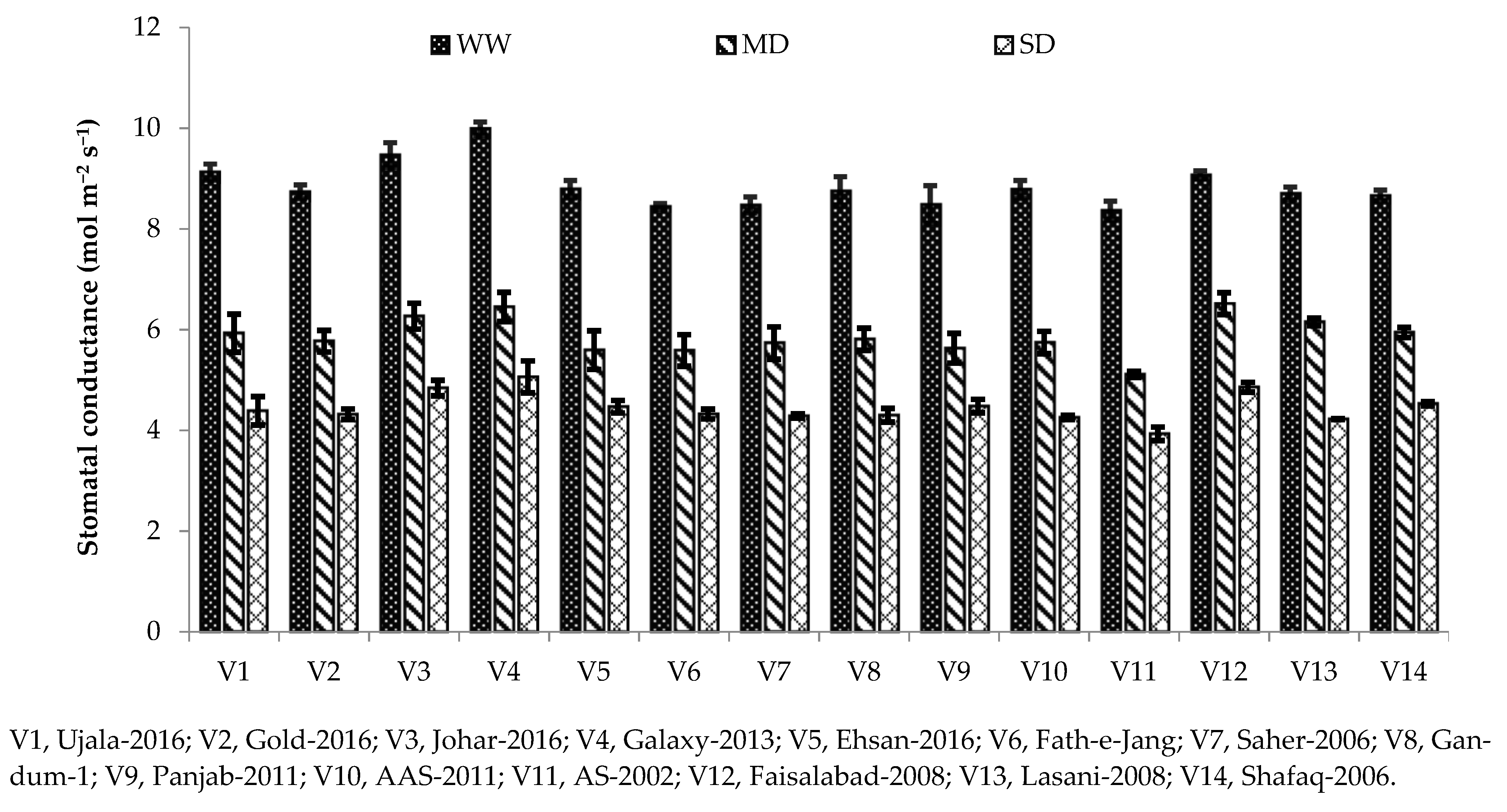
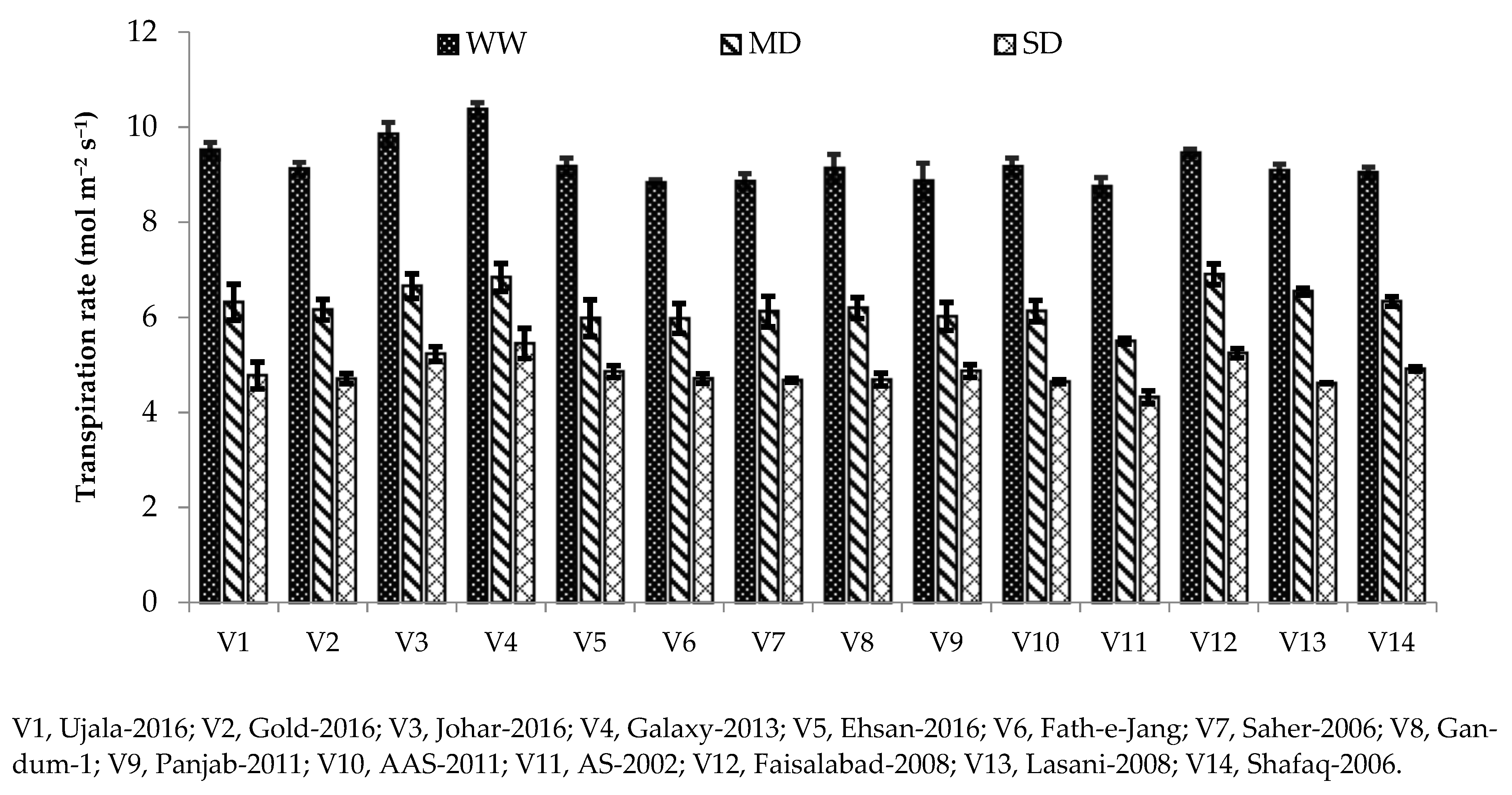
Photosynthesis plays a major role in the growth and yield of crop plants. Variation in photosynthetic pigments is the key indicator of the rate of photosynthesis in plants grown under water deficit [
3]. It is well known that plant exposure to drought stress decreases the photosynthetic crop rate, including cereals [
42]. In our study, different photosynthetic and gas exchange parameters, including Pn, gs, and transpiration rate, were reduced under drought stress conditions compared with the well-watered condition (
Table 3). Chlorophyll is the main contributing factor towards photosynthesis rate; hence, decreases in net photosynthesis might be due to reduced chlorophyll contents under severe drought conditions (
Table 1 and
Table 2). In addition to chlorophyll, another contributing factor for reduced Pn is limited CO
2 diffusion due to early stomatal closure under drought stress [
43]. Stomatal conductance was also reduced in our study under drought stress, which might be due to the closure of stomata to combat drought [
44]. Stomatal closure limits water loss through transpiration and helps plants to conserve water during water stress [
44]. Drought affects photosynthesis pigments differently depending on the variety. Genotypes with higher chlorophyll content have a more photosynthetic rate due to their genetic make-up under drought stress [
45]. The same was observed in the current study, as Galaxy-2013 being a drought-tolerant variety, has higher net photosynthesis and stomatal conductance under severe drought (
Figure 1 and
Figure 2). A high photosynthetic rate and stomatal conductance in drought-tolerant cultivars were also reported in [
33].
Yield and yield-related traits were reduced when the drought was imposed, and its effect was more significant under severe drought conditions compared with non-stressed or controlled treatments (
Table 3,
Table 4,
Table 5 and
Table 6). Amongst all wheat cultivars, the maximum fertile tillers, spikelets per spike, grains per spike, 100-grain weight, grain yield, and biological yield were recorded in Galaxy-2013, followed by Johar-2016 under severe drought conditions (
Table 3,
Table 4,
Table 5 and
Table 6). Enhanced yield and yield-related traits in Galaxy-2013 might be due to improved chlorophyll contents and photosynthetic and gas exchange parameters under severe drought conditions (
Table 2;
Figure 1,
Figure 2 and
Figure 3). Decreased yield in drought-sensitive genotypes might be due to a reduction in chlorophyll as well photosynthetic parameters. It has been studied that under low water stress conditions, plants maintain their internal moisture through stomatal closure, resulting in a reduction of gas exchange parameters [
46]. On the contrary, low transpiration in drought-sensitive genotypes due to stomata closure may reduce uptake of water from the soil solution, reducing nutrient uptake and ultimately reduced grain yield [
34]. Meanwhile, in the case of drought-tolerant genotypes, a higher grain yield was due to sustained photosynthesis [
47]. A reduction in the growth and metabolic activities leads to a reduction in agronomic and yield attributes under water deficit conditions [
48]. Such a reduction in grain yield under severe drought compared with well-watered conditions might also be due to less retention of RWC, reflecting fewer metabolic activities (
Table 2) [
49]. The genotypes with reduced leaf water loss are believed to be more drought tolerant and are less affected by water losses through evapotranspiration, thus able to conserve more water content and improve its yield [
50]. Reduced CO
2 assimilation and transpiration rate, stomatal closure, RWC, and increased malondialdehyde content were observed in all cultivars under severe drought. However, drought-tolerant cultivars retained higher RWC and Pn, which ultimately improved grain yield [
33].
The magnitude of correlations was stronger under severe water stress conditions than under mild and normal stress conditions. In a water-stress environment, sensitive physiological processes like turgor pressure, cell enlargement, division, and differentiation affect plant growth [
51]. Under such conditions, the stomatal conductance is also decreased due to the reduction of leaf RWC, which reduces the net photosynthesis. The same was observed in our studies, where RWC was strongly correlated with gas exchange parameters under both extreme conditions (well water,
Table 8; severe water stress,
Table 10), and the same was responded in stronger correlations with the GY and BY. The significant and positive correlations between Pn, gs, and E under all treatments (
Table 8 and
Table 9) explain that the higher gs under normal water conditions than under severe stress condition might improve the access of CO
2 into the chloroplast and resulted in more Pn and E. Regardless of the dynamics of stomatal conductance, all three parameters are strongly correlated with each other, and this can be observed in our findings (
Table 8 and
Table 9). These results are also consistent with the findings in wheat [
52], rice [
53], and peanut [
54].