Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours
Abstract
Simple Summary
Abstract
1. Introduction
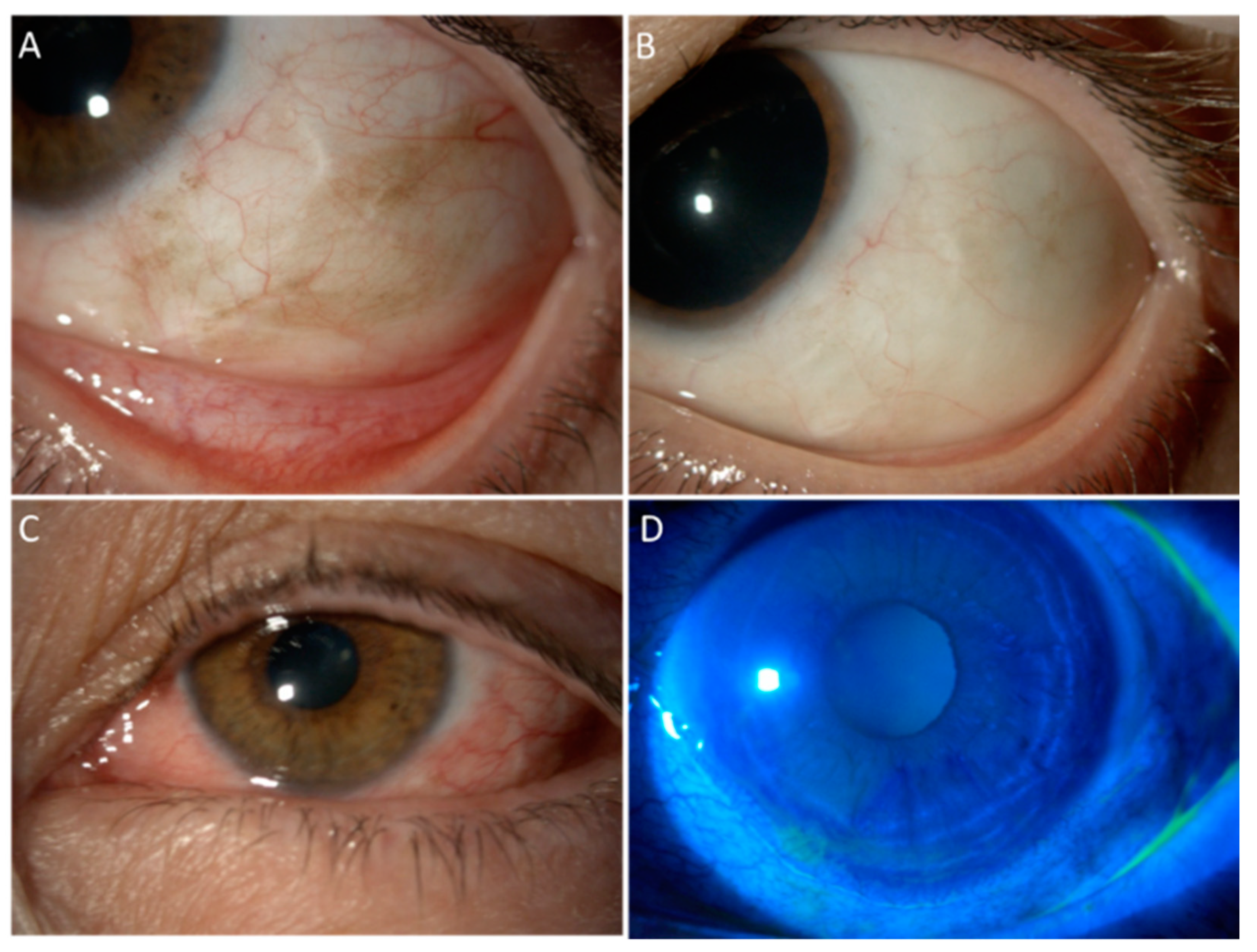
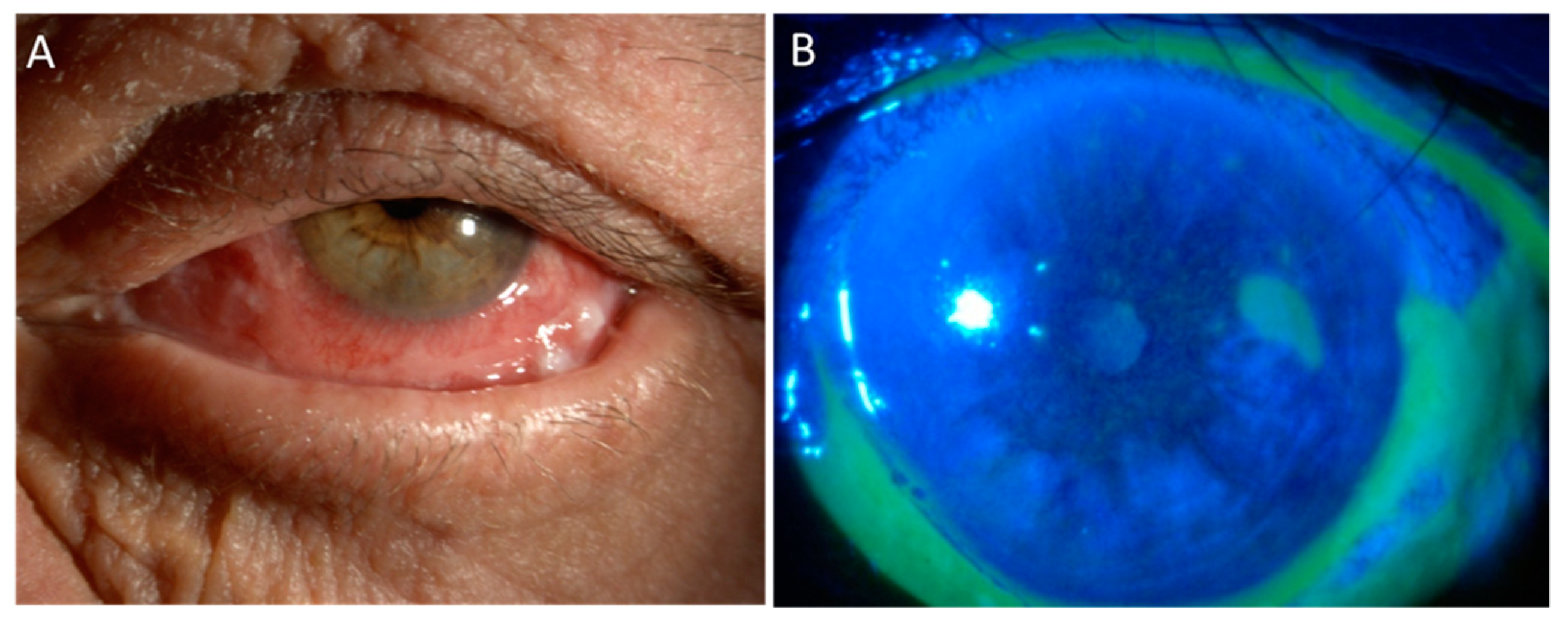
2. Mitomycin C
3. 5-Fluorouracil
Ocular Surface Squamous Neoplasia
4. Local Immunotherapy
5. Cryotherapy
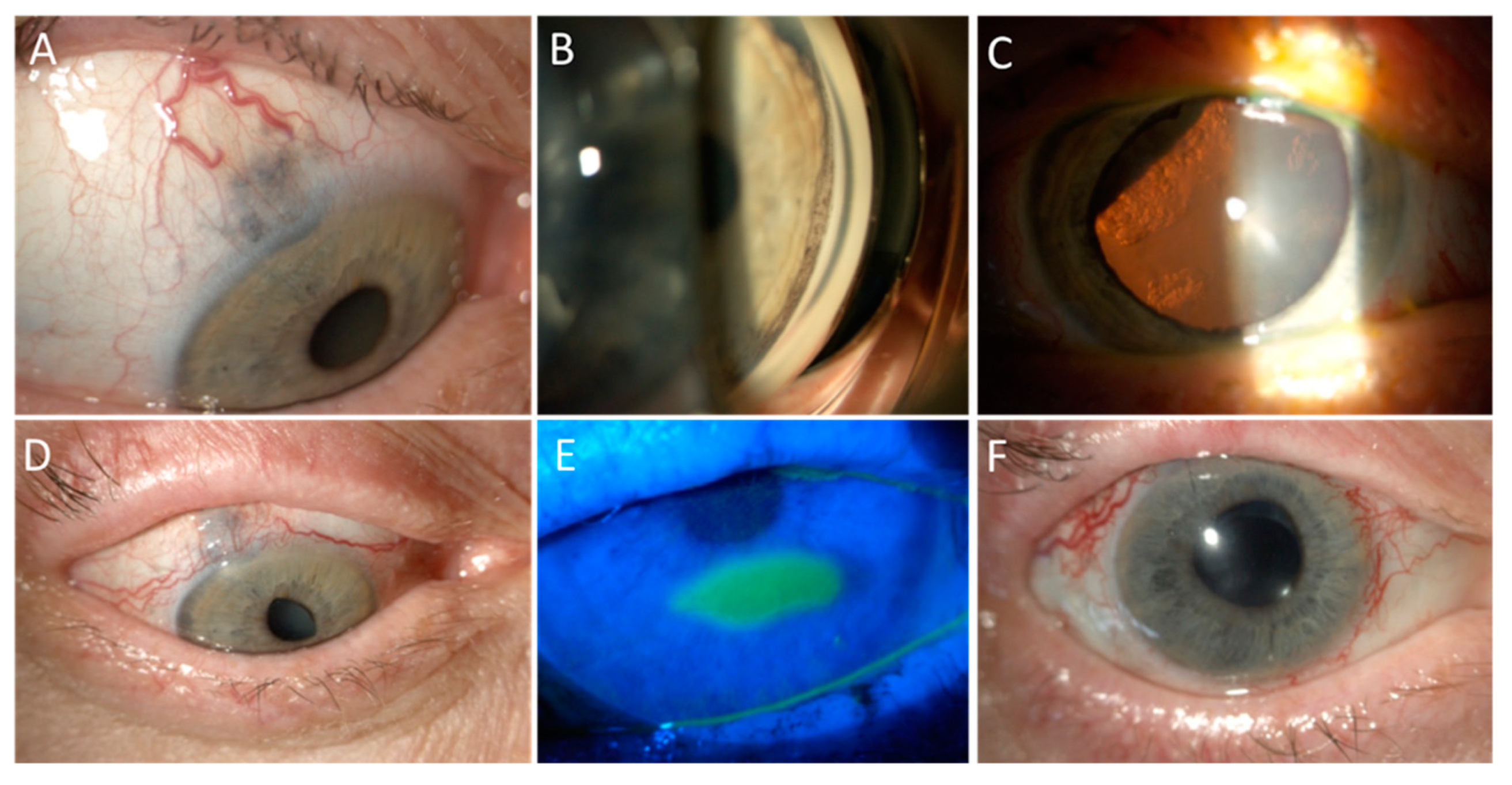
6. Radiotherapy for Uveal Melanoma
6.1. Proton Beam Radiotherapy
6.2. Brachytherapy
6.3. Stereotactic Radiotherapy
7. Radiotherapy for Ocular Surface Tumours
7.1. Ocular Surface Squamous Neoplasia
7.2. Conjunctival Melanoma
7.3. Conjunctival Lymphoma
8. Management of Ocular Surface Side Effects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Singh, A.D.; Seregard, S. (Eds.) Ocular Tumours; ESASO Course Series; S. Karger AG: Basel, Switzerland, 2016; Volume 7, ISBN 978-3-318-05618-1. [Google Scholar]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E. Incidence of Uveal Melanoma in Europe. Ophthalmology 2007, 114, 2309–2315.e2. [Google Scholar] [CrossRef]
- Hammer, H.; Oláh, J.; Tóth-Molnár, E. Dysplastic nevi are a risk factor for uveal melanoma. Eur. J. Ophthalmol. 1996, 6, 472–474. [Google Scholar] [CrossRef]
- Eskelin, S.; Kivelä, T. Mode of presentation and time to treatment of uveal melanoma in Finland. Br. J. Ophthalmol. 2002, 86, 333–338. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registration Statistics, England Statistical Bulletins-Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/previousReleases (accessed on 8 August 2020).
- Shields, C.L.; Fasiudden, A.; Mashayekhi, A.; Shields, J.A. Conjunctival Nevi: Clinical Features and Natural Course in 410 Consecutive Patients. Arch. Ophthalmol. 2004, 122, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Demirci, H.; Karatza, E.; Shields, J.A. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumours. Ophthalmology 2004, 111, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, N.; Lake, S.L.; Coupland, S.E.; Damato, B.E. Conjunctival melanoma and melanocytic intra-epithelial neoplasia. Eye 2013, 27, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Eberhart, C.G.; Kivela, T.T. WHO Classification of Tumours of the Eye, 4th ed.; IARC: Lyon, France, 2018; pp. 30–39. [Google Scholar]
- Paridaens, A.D.A.; McCartney, A.C.E.; Hungerford, J.L. Multifocal amelanotic conjunctival melanoma and acquired melanosis sine pigmento. Br. J. Ophthalmol. 1992, 76, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Gündüz, K.; Cater, J.; Mercado, G.V.; Gross, N.; Lally, B. Conjunctival melanoma: Risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch. Ophthalmol. 2000, 118, 1497–1507. [Google Scholar] [CrossRef]
- Lee, G.A.; Hirst, L.W. Ocular surface squamous neoplasia. Surv. Ophthalmol. 1995, 39, 429–450. [Google Scholar] [CrossRef]
- Erie, J.C.; Campbell, R.J.; Liesegang, T.J. Conjunctival and Corneal Intraepithelial and Invasive Neoplasia. Ophthalmology 1986, 93, 176–183. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A. Tumours of the conjunctiva and cornea. Indian J. Ophthalmol. 2019, 67, 1930–1948. [Google Scholar] [CrossRef]
- Mathis, T.; Cassoux, N.; Tardy, M.; Piperno, S.; Gastaud, L.; Dendale, R.; Maschi, C.; Nguyen, A.-M.; Meyer, L.; Bonnin, N.; et al. Management of uveal melanomas, guidelines for oncologists. Bull. Cancer 2018, 105, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Bernabei, F.; Moscardelli, F.; Vagge, A.; Scotto, R.; Bovone, C.; Scorcia, V.; Giannaccare, G. Assessment of corneal fluorescein staining in different dry eye subtypes using digital image analysis. Transl. Vis. Sci. Technol. 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Greenwell, T.; Muecke, J.S. Long-term outcomes after adjunctive topical 5-flurouracil or mitomycin C for the treatment of surgically excised, localized ocular surface squamous neoplasia. Clin. Exp. Ophthalmol. 2014, 42, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Marquez, E.; Katsanos, A.; Kozobolis, V.P.; Konstas, A.G.P.; Teus, M.A. A Critical Overview of the Biological Effects of Mitomycin C Application on the Cornea Following Refractive Surgery. Adv. Ther. 2019, 36, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Ballalai, P.L.; Erwenne, C.M.; Martins, M.C.; Lowen, M.S.; Barros, J.N. Long-term results of topical mitomycin c 0.02% for primary and recurrent conjunctival-corneal intraepithelial neoplasia. Ophthal. Plast. Reconstr. Surg. 2009, 25, 296–299. [Google Scholar] [CrossRef]
- Birkholz, E.S.; Goins, K.M.; Sutphin, J.E.; Kitzmann, A.S.; Wagoner, M.D. Treatment of ocular surface squamous cell intraepithelial neoplasia with and without mitomycin C. Cornea 2011, 30, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.A.; Maceroni, M.; Sammarco, M.G.; Pagliara, M.M. Mitomycin C or interferon as adjuvant therapy to surgery for ocular surface squamous neoplasia: Comparative study. Eur. J. Ophthalmol. 2018, 28, 204–209. [Google Scholar] [CrossRef]
- Daniell, M.; Maini, R.; Tole, D. Use of mitomycin C in the treatment of corneal conjunctival intraepithelial neoplasia. Clin. Exp. Ophthalmol. 2002, 30, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Muecke, J. Treatment of ocular surface squamous neoplasia with mitomycin C. Br. J. Ophthalmol. 2010, 94, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; Muecke, J. Complications of mitomycin C therapy in 100 eyes with ocular surface neoplasia. Br. J. Ophthalmol. 2006, 90, 819–822. [Google Scholar] [CrossRef]
- Rudkin, A.K.; Dempster, L.; Muecke, J.S. Management of diffuse ocular surface squamous neoplasia: Efficacy and complications of topical chemotherapy. Clin. Exp. Ophthalmol. 2015, 43, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, S.; Soni, A.; SinghSethi, H.; Sudan, R.; Sony, P.; Pangtey, M.S. Combined surgery, cryotherapy, and Mitomycin-C for recurrent ocular surface squamous neoplasia. Cornea 2002, 21, 189–191. [Google Scholar] [CrossRef]
- Stone, D.U.; Butt, A.L.; Chodosh, J. Ocular surface squamous neoplasia: A standard of care survey. Cornea 2005, 24, 297–300. [Google Scholar] [CrossRef]
- Hirst, L.W. Randomized Controlled Trial of Topical Mitomycin C for Ocular Surface Squamous Neoplasia. Early Resolution. Ophthalmology 2007, 114, 976–982. [Google Scholar] [CrossRef]
- Yamamoto, N.; Ohmura, T.; Suzuki, H.; Shirasawa, H. Successful treatment with 5-fluorouracil of conjunctival intraepithelial neoplasia refractive to mitomycin-C. Ophthalmology 2002, 109, 249–252. [Google Scholar] [CrossRef]
- Boehm, M.D.; Huang, A.J.W. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alfa 2b. Ophthalmology 2004, 111, 1755–1761. [Google Scholar] [CrossRef]
- Billing, K.; Karagiannis, A.; Selva, D. Punctal-canalicular stenosis associated with mitomycin-C for corneal epithelial dysplasia. Am. J. Ophthalmol. 2003, 136, 746–747. [Google Scholar] [CrossRef]
- Dudney, B.W.; Malecha, M.A. Limbal stem cell deficiency following topical mitomycin c treatment of Conjunctival-Corneal intraepithelial neoplasia. Am. J. Ophthalmol. 2004, 137, 950–951. [Google Scholar] [CrossRef]
- Lichtinger, A.; Pe’er, J.; Frucht-Pery, J.; Solomon, A. Limbal Stem Cell Deficiency after Topical Mitomycin C Therapy for Primary Acquired Melanosis with Atypia. Ophthalmology 2010, 117, 431–437. [Google Scholar] [CrossRef]
- Pe’er, J.; Frucht-Pery, J. The treatment of Primary Acquired Melanosis (PAM) with atypia by topical Mitomycin, C. Am. J. Ophthalmol. 2005, 139, 229–234. [Google Scholar] [CrossRef]
- Kurli, M.; Finger, P.T. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: 12 years’ experience. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Choi, J.S.; Joo, C.K. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am. J. Ophthalmol. 2009, 147, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Pavesio, C.E.; Decory, H.H. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br. J. Ophthalmol. 2008, 92, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.; Katz, J.; Majmudar, P.; Rostov, A. Loteprednol Etabonate for the Treatment of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2020, 36, 497–511. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Thakur, S.K.; Dutta, J.; Prakash, R.; Shaw, C.; Gangopadhyay, D.N.; Dutta, H.; Bhaduri, G. Effect of mitomycin C-aided trabeculectomy on conjunctival goblet cell density. Nepal J. Ophthalmol. 2012, 4, 68–72. [Google Scholar] [CrossRef]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review [published online ahead of print, 2020 Jul 23]. Br. J. Ophthalmol 2021. [Google Scholar] [CrossRef]
- Abraham, L.M.; Selva, D.; Casson, R.; Leibovitch, I. The clinical applications of fluorouracil in ophthalmic practice. Drugs 2007, 67, 237–255. [Google Scholar] [CrossRef]
- Midena, E.; Angeli, C.D.; Valenti, M.; De Belvis, V.; Boccato, P. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. Br. J. Ophthalmol. 2000, 84, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Parrozzani, R.; Frizziero, L.; Trainiti, S.; Testi, I.; Miglionico, G.; Pilotto, E.; Blandamura, S.; Fassina, A.; Midena, E. Topical 1% 5-fluoruracil as a sole treatment of corneoconjunctival ocular surface squamous neoplasia: Long-term study. Br. J. Ophthalmol. 2017, 101, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Gichuhi, S.; Macharia, E.; Kabiru, J.; Zindamoyen, A.M.; Rono, H.; Ollando, E.; Wachira, J.; Munene, R.; Maina, J.; Onyuma, T.; et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: A randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e378–e385. [Google Scholar] [CrossRef]
- Joag, M.G.; Sise, A.; Murillo, J.C.; Sayed-Ahmed, I.O.; Wong, J.R.; Mercado, C.; Galor, A.; Karp, C.L. Topical 5-Fluorouracil 1% as Primary Treatment for Ocular Surface Squamous Neoplasia. Ophthalmology 2016, 123, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Mercado, C.; Galor, A.; Karp, C.L. Comparison of Topical 5-Fluorouracil and Interferon Alfa-2b as Primary Treatment Modalities for Ocular Surface Squamous Neoplasia. Am. J. Ophthalmol. 2019, 199, 216–222. [Google Scholar] [CrossRef] [PubMed]
- de Keizer, R.J.; de Wolff-Rouendaal, D.; van Delft, J.L. Topical application of 5-fluorouracil in premalignant lesions of cornea, conjunctiva and eyelid. Doc. Ophthalmol. 1986, 64, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Karp, C.L.; Chhabra, S.; Barnes, S.; Alfonso, E.C. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: A dose comparison study. Br. J. Ophthalmol. 2010, 94, 551–554. [Google Scholar] [CrossRef]
- Kusumesh, R.; Ambastha, A.; Sinha, B.; Kumar, R. Topical interferon α-2b as a single therapy for primary ocular surface squamous Neoplasia. Asia-Pac. J. Ophthalmol. 2015, 4, 279–282. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Kim, H.J.; Al-Dahmash, S.; Shah, S.U.; Lally, S.E.; Shields, J.A. Interferon for ocular surface squamous neoplasia in 81 cases: Outcomes based on the american joint committee on cancer classification. Cornea 2013, 32, 248–256. [Google Scholar] [CrossRef]
- Shah, S.U.; Kaliki, S.; Kim, H.J.; Lally, S.E.; Shields, J.A.; Shields, C.L. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: Outcomes based on american joint committee on cancer classification. Arch. Ophthalmol. 2012, 130, 159–164. [Google Scholar] [CrossRef]
- Aldave, A.J.; Nguyen, A. Ocular surface toxicity associated with topical interferon α-2b [5]. Br. J. Ophthalmol. 2007, 91, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.A.; Tiberti, A.C.; Valente, P.; Laguardia, M.; Sammarco, M.G.; Balestrazzi, A.; Larocca, L.M.; Balestrazzi, E. Intralesional interferon-α for conjunctival mucosa-associated lymphoid tissue lymphoma: Long-term results. Ophthalmology 2012, 119, 494–500. [Google Scholar] [CrossRef]
- Jakobiec, F.A.; Iwamoto, T. Cryotherapy for untraepithelial conjunctival melanocytic proliferations. Arch Ophthalmol. 1983, 101, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Jakobiec, F.A.; Brownstein., S.; Wilkinson, R.D.; Khalil, M.; Cooper, W.C.; Shibata, H.R. Combined surgery and cryotherapy for diffuse malignant melanoma of the conjunctiva. Arch. Ophthalmol. 1980, 98, 1390–1396. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; De Potter, P. Surgical management of conjunctival tumours. Arch Ophthamol. 1997, 115, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.D.; Frauenfelder, F.T. Principles of cryosurgery. Ophthalmic Surg. 1979, 10, 21–30. [Google Scholar] [PubMed]
- De Potter, P.; Shields, C.L.; Shields, J.A.; Menduke, H. Clinical predictive factors for development of recurrence and metastasis in conjunctival melanoma: A review of 68 cases. Br. J. Ophthalmol. 1993, 77, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Perez, N.; Singh, A.D.; Cater, J. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: Outcomes and limitations. Ophthalmology 2002, 109, 225–234. [Google Scholar] [CrossRef]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Böhringer, T.; Chamot, L.; Goitein, G. Eye retention after proton beam radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 867–880. [Google Scholar] [CrossRef]
- Takiar, V.; Gombos, D.S.; Mourtada, F.; Rechner, L.A.; Lawyer, A.A.; Morrison, W.H.; Garden, A.S.; Beadle, B.M. Disease control and toxicity outcomes using ruthenium eye plaque brachytherapy in the treatment of uveal melanoma. Pract. Radiat. Oncol. 2014, 4, 189–194. [Google Scholar] [CrossRef]
- Eleuteri, A.; Taktak, A.F.G.; Coupland, S.E.; Heimann, H.; Kalirai, H.; Damato, B. Prognostication of metastatic death in uveal melanoma patients: A Markov multi-state model. Comput. Biol. Med. 2018, 102, 151–156. [Google Scholar] [CrossRef]
- Chang, M.Y.; McCannel, T.A. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br. J. Ophthalmol. 2013, 97, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Dendale, R.; Lumbroso-Le Rouic, L.; Noel, G.; Feuvret, L.; Levy, C.; Delacroix, S.; Meyer, A.; Nauraye, C.; Mazal, A.; Mammar, H.; et al. Proton beam radiotherapy for uveal melanoma: Results of Curie Institut-Orsay Proton Therapy Center (ICPO). Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 780–787. [Google Scholar] [CrossRef]
- Egger, E.; Schalenbourg, A.; Zografos, L.; Bercher, L.; Boehringer, T.; Chamot, L.; Goitein, G. Maximizing local tumour control and survival after proton beam radiotherapy of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 138–147. [Google Scholar] [CrossRef]
- Kent, D.; Noonan, C.P.; Damato, B.E. Management of Irish patients with intraocular melanoma referred to Liverpool, England. Acta Ophthalmol. Scand. 1998, 76, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Bogner, J.; Verwey, J.; Georg, D.; Dieckmann, K.; Escudé, L.; Caro, M.; Pötter, R.; Goitein, G.; Lomax, A.J.; et al. Proton beam radiotherapy versus fractionated stereotactic radiotherapy for uveal melanomas: A comparative study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 373–384. [Google Scholar] [CrossRef]
- Munzenrider, J.E.; Verhey, L.J.; Gragoudas, E.S.; Seddon, J.M.; Urie, M.; Gentry, R.; Birnbaum, S.; Ruotolo, D.M.; Crowell, C.; McManus, P.; et al. Conservative treatment of uveal melanoma: Local recurrence after proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 493–498. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Prim. 2020, 6, 24. [Google Scholar] [CrossRef]
- Mishra, K.K.; Daftari, I.K. Proton therapy for the management of uveal melanoma and other ocular tumours. Chin. Clin. Oncol. 2016, 5, 50. [Google Scholar] [CrossRef]
- Damato, B.; Kacperek, A.; Errington, D.; Heimann, H. Proton beam radiotherapy of uveal melanoma. Saudi J. Ophthalmol. 2013, 27, 151–157. [Google Scholar] [CrossRef]
- Munzenrider, J.E. Proton therapy for uveal melanomas and other eye lesions. Strahlenther. Onkol. 1999, 175, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Paton, K.; Ma, R.; Pickles, T. Outcomes of proton beam radiotherapy for large non-peripapillary choroidal and ciliary body melanoma at TRIUMF and the BC cancer agency. Ocul. Oncol. Pathol. 2016, 2, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Foss, A.J.E.; Whelehan, I.; Hungerford, J.L.; Anderson, D.F.; Errington, D.; Kacperek, A.; Restori, M.; Kongerud, J.; Sheen, M. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br. J. Ophthalmol. 1997, 81, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Mehta, M.P. Clinical Outcomes of Proton Radiotherapy for Uveal Melanoma. Clin. Oncol. 2016, 28, e17–e27. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, S.; Asencio-Durán, M.; Lavín-Dapena, C.; Manzano-Muñoz, B.; D’Anna-Mardero, O.; Cordero-Ros, R.; Pellegrini, M.; Bernabei, F.; Mercanti, A.; Scorcia, V.; et al. Ultrasound cyclo plasty for the management of glaucoma secondary to ocular irradiation for choroidal melanoma. Int. J. Ophthalmol. 2020, 13, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Seddon, J.; Goitein, M.; Verhey, L.; Munzenrider, J.; Urie, M.; Suit, H.D.; Blitzer, P.; Koehler, A. Current results of proton beam irradiation of uveal melanomas. Ophthalmology 1985, 92, 284–291. [Google Scholar] [CrossRef]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Macfaul, P.A.; Bedford, M.A. Ocular complications after therapeutic irradiation. Br. J. Ophthalmol. 1970, 54, 237–247. [Google Scholar] [CrossRef]
- Finger, P.T.; Chin, K.J.; Duvall, G. Palladium-103 Ophthalmic Plaque Radiation Therapy for Choroidal Melanoma: 400 Treated Patients. Ophthalmology 2009, 116, 790–796. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Lane, A.M.; Regan, S.; Li, W.; Judge, H.E.; Munzenrider, J.E.; Seddon, J.M.; Egan, K.M. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch. Ophthalmol. 2000, 118, 773–778. [Google Scholar] [CrossRef]
- Singh, A.D.; Dupps, W.J., Jr.; Biscotti, C.V.; Suh, J.H.; Lathrop, K.L.; Nairn, J.P.; Shih, H. Limbal Stem Cell Preservation during Proton Beam Irradiation for Diffuse Iris Melanoma. Cornea 2017, 36, 119–122. [Google Scholar] [CrossRef]
- Groenewald, C.; Konstantinidis, L.; Damato, B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye 2013, 27, 163–171. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Roberts, D.; Errington, R.D.; Kacperek, A.; Heimann, H.; Damato, B. Transpalpebral proton beam radiotherapy of choroidal melanoma. Br. J. Ophthalmol. 2015, 99, 232–235. [Google Scholar] [CrossRef]
- Leblanc, A.; Lumbroso-Le Rouic, L.; Desjardins, L.; Dendale, R.; Cassoux, N. Diffuse Iris Melanoma: Conservative Treatment with Proton Beam Therapy after Limbal Stem Cell Preservation or Enucleation? Ocul. Oncol. Pathol. 2019, 5, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Tseng, V.L.; Coleman, A.L.; Zhang, Z.-F.; McCannel, T.A. Complications from Plaque versus Proton Beam Therapy for Choroidal Melanoma: A Qualitative Systematic Review. J. Cancer Ther. 2016, 07, 169–185. [Google Scholar] [CrossRef][Green Version]
- Desjardins, L.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Cassoux, N.; Dendale, R.; Mazal, A.; Delacroix, S.; Sastre, X.; Plancher, C.; Asselain, B. Treatment of uveal melanoma by accelerated proton beam. Dev. Ophthalmol. 2012, 49, 41–57. [Google Scholar] [PubMed]
- Petrovic, A.; Bergin, C.; Schalenbourg, A.; Goitein, G.; Zografos, L. Proton therapy for uveal melanoma in 43 juvenile patients: Long-term results. Ophthalmology 2014, 121, 898–904. [Google Scholar] [CrossRef]
- Courdi, A.; Caujolle, J.P.; Grange, J.D.; Diallo-Rosier, L.; Sahel, J.; Bacin, F.; Zur, C.; Gastaud, P.; Iborra-Brassart, N.; Hérault, J.; et al. Results of proton therapy of uveal melanomas treated in Nice. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 5–11. [Google Scholar] [CrossRef]
- Desjardins, L.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Dendale, R.; Delacroix, S.; Nauraye, C.; Estève, M.; Plancher, C.; Asselain, B. Combined proton beam radiotherapy and transpupillary thermotherapy for large uveal melanomas: A randomized study of 151 patients. Ophthalmic Res. 2006, 38, 255–260. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Lane, A.M. Uveal melanoma: Proton beam irradiation. Ophthalmol. Clin. N. Am. 2005, 18, 111–118. [Google Scholar] [CrossRef]
- Kincaid, M.C.; Folberg, R.; Torczynski, E.; Zakov, Z.B.; Shore, J.W.; Liu, S.J.; Planchard, T.A.; Weingeist, T.A. Complications after proton beam therapy for uveal malignant melanoma. A clinical and histopathologic study of five cases. Ophthalmology 1988, 95, 982–991. [Google Scholar] [CrossRef]
- Macdonald, E.C.A.; Cauchi, P.; Kemp, E.G. Proton beam therapy for the treatment of uveal melanoma in Scotland. Br. J. Ophthalmol. 2011, 95, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, T.D.; Lane, A.M.; Morrison, M.; Gragoudas, E.S.; Kim, I.K. Long-term outcomes after proton beam irradiation in patients with large choroidal melanomas. JAMA Ophthalmol. 2017, 135, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Cordini, D.; Rehak, M.; Hager, A.; Riechardt, A.I.; Böker, A.; Heufelder, J.; Weber, A.; Gollrad, J.; Besserer, A.; et al. Local Recurrence after Primary Proton Beam Therapy in Uveal Melanoma: Risk Factors, Retreatment Approaches, and Outcome. Am. J. Ophthalmol. 2015, 160, 628–636. [Google Scholar] [CrossRef]
- Zografos, L.; Gailloud, C.; Perret, C.; Uffer, S.; Raimondi, S.; Chamot, L.; Carrel, S.; Greiner, R. Rapport sur le traitement conservateur des melanomes de l’uvee a la Clinique ophtalmologique universitaire de Lausanne. Klin. Monbl. Augenheilkd. 1988, 192, 572–578. [Google Scholar] [CrossRef]
- Hawkins, B.S. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings: COMS report no. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar]
- Jampol, L.M.; Moy, C.S.; Murray, T.G.; Reynolds, S.M.; Albert, D.M.; Schachat, A.P.; Diddie, K.R.; Engstrom, R.E.; Finger, P.T.; Hovland, K.R.; et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002, 109, 2197–2206. [Google Scholar] [CrossRef]
- Kaiserman, N.; Kaiserman, I.; Hendler, K.; Frenkel, S.; Pe’er, J. Ruthenium-106 plaque brachytherapy for thick posterior uveal melanomas. Br. J. Ophthalmol. 2009, 93, 1167–1171. [Google Scholar] [CrossRef]
- Van Ginderdeuren, R.; Van Limbergen, E.; Spileers, W. 18 Years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br. J. Ophthalmol. 2005, 89, 1306–1310. [Google Scholar] [CrossRef]
- Abrams, M.J.; Gagne, N.L.; Melhus, C.S.; Mignano, J.E. Brachytherapy vs. external beam radiotherapy for choroidal melanoma: Survival and patterns-of-care analyses. Brachytherapy 2016, 15, 216–223. [Google Scholar] [CrossRef]
- Chaudhry, I.A.; Liu, M.; Shamsi, F.A.; Arat, Y.O.; Shetlar, D.J.; Boniuk, M. Corneoscleral necrosis after episcleral au-198 brachytherapy of uveal melanoma. Retina 2009, 29, 73–79. [Google Scholar] [CrossRef]
- Quivey, J.M.; Char, D.H.; Phillips, T.L.; Weaver, K.A.; Castro, J.R.; Kroll, S.M. High intensity 125-Iodine (1251) plaque treatment of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 613–618. [Google Scholar] [CrossRef]
- Lumbroso-Le Rouic, L.; Charif Chefchaouni, M.; Levy, C.; Plancher, C.; Dendale, R.; Asselain, B.; Solignac, S.; Mazal, A.; Desjardins, L. 125l plaque brachytherapy for anterior uveal melanomas. Eye 2004, 18, 911–916. [Google Scholar] [CrossRef]
- Sia, S.; Harper, C.; McAllister, I.; Perry, A. Iodine-125 episcleral plaque therapy in uveal melanoma. Clin. Exp. Ophthalmol. 2000, 28, 409–413. [Google Scholar] [CrossRef]
- Bechrakis, N.E.; Bornfeld, N.; Zöller, I.; Foerster, M.H. Iodine 125 plaque brachytherapy versus transscleral tumour resection in the treatment of large uveal melanomas. Ophthalmology 2002, 109, 1855–1861. [Google Scholar] [CrossRef]
- King, B.A.; Awh, C.; Gao, B.T.; Wang, J.; Kocak, M.; Morales-Tirado, V.M.; Ballo, M.T.; Wilson, M.W. Iodine-125 episcleral plaque brachytherapy for AJCC T4 posterior uveal melanoma: Clinical outcomes in 158 patients. Ocul. Oncol. Pathol. 2019, 5, 340–349. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Cater, J.; Gündüz, K.; Miyamoto, C.; Micaily, B.; Brady, L.W. Plaque radiotherapy for uveal melanoma: Long-term visual outcome in 1106 consecutive patients. Arch. Ophthalmol. 2000, 118, 1219–1228. [Google Scholar] [CrossRef]
- Takiar, V.; Voong, K.R.; Gombos, D.S.; Mourtada, F.; Rechner, L.A.; Lawyer, A.A.; Morrison, W.H.; Garden, A.S.; Beadle, B.M. A choice of radionuclide: Comparative outcomes and toxicity of ruthenium-106 and iodine-125 in the definitive treatment of uveal melanoma. Pract. Radiat. Oncol. 2015, 5, e169–e176. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Chen, A.; Cook, T.; Faber, D.; Winward, K.; Sause, W. Outcomes and Control Rates for I-125 Plaque Brachytherapy for Uveal Melanoma: A Community-Based Institutional Experience. ISRN Ophthalmol. 2014, 2014, 950975. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.; Finger, P.T. Palladium-103 plaque radiation therapy for American Joint Committee on Cancer T3- and T4-staged choroidal melanomas. JAMA Ophthalmol. 2014, 132, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Belaïd, A.; Nasr, C.; Jmour, O.; Cherif, A.; Kochbati, L.; Bouguila, H.; Besbes, M.; Benna, F. Brachytherapy of Uveal Melanomas with Ruthenium-106 Plaques. Asian Pac. J. Cancer Prev. 2016, 17, 5281. [Google Scholar]
- Lommatzsch, P.K.; Werschnik, C.; Schuster, E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 129–137. [Google Scholar] [CrossRef]
- Mossböck, G.; Rauscher, T.; Winkler, P.; Kapp, K.S.; Langmann, G. Impact of dose rate on clinical course in uveal melanoma after brachytherapy with ruthenium-106. Strahlenther. Onkol. 2007, 183, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, M.M.; Tagliaferri, L.; Azario, L.; Lenkowicz, J.; Lanza, A.; Autorino, R.; Caputo, C.G.; Gambacorta, M.A.; Valentini, V.; Blasi, M.A. Ruthenium brachytherapy for uveal melanomas: Factors affecting the development of radiation complications. Brachytherapy 2018, 17, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Markiewicz, A.; Debicka-Kumela, M.; Bogdali, A.; Romanowska-Dixon, B. Outcomes of I-125 brachytherapy for uveal melanomas depending on irradiation dose applied to the tumour apex—A single institution study. J. Contemp. Brachytherapy 2018, 10, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Rospond-Kubiak, I.; Wróblewska-Zierhoffer, M.; Twardosz-Pawlik, H.; Kociecki, J. Ruthenium brachytherapy for uveal melanoma—single institution experience. J. Contemp. Brachytherapy 2017, 9, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Seregard, S.; Af Trampe, E.; Lax, I.; Kock, E.; Lundell, G. Results following episcleral ruthenium plaque radiotherapy for posterior uveal melanoma. The Swedish experience. Acta Ophthalmol. Scand. 1997, 75, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tarmann, L.; Wackernagel, W.; Ivastinovic, D.; Schneider, M.; Winkler, P.; Langmann, G. Tumour parameters predict the risk of side effects after ruthenium-106 plaque brachytherapy of uveal melanomas. PLoS ONE 2017, 12, e0183833. [Google Scholar] [CrossRef]
- Kowal, J.; Romanowska-Dixon, B. Late complications after brachytherapy of I-125 uveal melanoma. Klin. Ocz. 2016, 118, 226–230. [Google Scholar]
- Krohn, J.; Monge, O.R.; Skorpen, T.N.; Mørk, S.J.; Dahl, O. Posterior uveal melanoma treated with I-125 brachytherapy or primary enucleation. Eye 2008, 22, 1398–1403. [Google Scholar] [CrossRef]
- Le, B.H.A.; Kim, J.W.; Deng, H.; Rayess, N.; Jennelle, R.L.; Zhou, S.Y.; Astrahan, M.A.; Berry, J.L. Outcomes of choroidal melanomas treated with eye physics plaques: A 25-year review. Brachytherapy 2018, 17, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Rao, Y.J.; Acharya, S.; Schwarz, J.; Rao, P.K.; Grigsby, P. Patterns of care and outcomes of proton and eye plaque brachytherapy for uveal melanoma: Review of the National Cancer Database. Brachytherapy 2017, 16, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- McCannel, T.A.; McCannel, C.A. Iodine 125 brachytherapy with vitrectomy and silicone oil in the treatment of uveal melanoma: 1-to-1 matched case-control series. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.G.; Markoe, A.M.; Gold, A.S.; Ehlies, F.; Bermudez, E.; Wildner, A.; Latiff, A. Long-term followup comparing two treatment dosing strategies of 125i plaque radiotherapy in the management of small/medium posterior uveal melanoma. J. Ophthalmol. 2013, 2013, 517032. [Google Scholar] [CrossRef] [PubMed]
- Perez, B.A.; Mettu, P.; Vajzovic, L.; Rivera, D.; Alkaissi, A.; Steffey, B.A.; Cai, J.; Stinnett, S.; Dutton, J.J.; Buckley, E.G.; et al. Uveal melanoma treated with iodine-125 episcleral plaque: An analysis of dose on disease control and visual outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Dunavoelgyi, R.; Dieckmann, K.; Gleiss, A.; Sacu, S.; Kircher, K.; Georgopoulos, M.; Georg, D.; Zehetmayer, M.; Poetter, R. Local tumour control, visual acuity, and survival after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 199–205. [Google Scholar] [CrossRef]
- Zehetmayer, M.; Dieckmann, K.; Kren, G.; Kitz, K.; Ruhswurm, I.; Georgopoulos, M.; Pötter, R. Fractionated stereotactic radiotherapy with linear accelerator for uveal melanoma—Preliminary Vienna results. Strahlenther. Onkol. 1999, 175, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.; Georg, D.; Zehetmayer, M.; Rottenfusser, A.; Pötter, R. Stereotactic photon beam irradiation of uveal melanoma: Indications and experience at the university of Vienna since 1997. Strahlenther. Onkol. 2007, 183, 11–13. [Google Scholar] [CrossRef]
- Langmann, G.; Pendl, G.; Papaefthymiou, G.; Guss, H. Gamma knife radiosurgery for uveal melanomas: An 8-year experience. J. Neurosurg. 2000, 93 (Suppl. 3), 184–188. [Google Scholar] [CrossRef]
- Mueller, A.J.; Talies, S.; Schaller, U.C.; Horstmann, G.; Wowra, B.; Kampik, A. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology 2000, 107, 1381–1387. [Google Scholar] [CrossRef]
- Sikuade, M.J.; Salvi, S.; Rundle, P.A.; Errington, D.G.; Kacperek, A.; Rennie, I.G. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye 2015, 29, 1194–1198. [Google Scholar] [CrossRef]
- Sarici, A.M.; Pazarli, H. Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 285–294. [Google Scholar] [CrossRef]
- Furdova, A.; Slezak, P.; Chorvath, M.; Waczulikova, I.; Sramka, M.; Kralik, G. No differences in outcome between radical surgical treatment (enucleation) and stereotactic radiosurgery in patients with posterior uveal melanoma. Neoplasma 2010, 57, 377–381. [Google Scholar]
- Somani, S.; Sahgal, A.; Krema, H.; Heydarian, M.; McGowan, H.; Payne, D.; Xu, W.; Michaels, H.; Laperriere, N.; Simpson, E.R. Stereotactic radiotherapy in the treatment of juxtapapillary choroidal melanoma: 2-Year follow-up. Can. J. Ophthalmol. 2009, 44, 61–65. [Google Scholar] [CrossRef]
- Zehetmayer, M.; Menapace, R.; Kitz, K.; Ertl, A.; Strenn, K.; Ruhswurm, I. Stereotactic irradiation of uveal melanoma with the Leksell gamma unit. Front. Radiat. Ther. Oncol. 1997, 30, 47–55. [Google Scholar]
- Dunavoelgyi, R.; Dieckmann, K.; Gleiss, A.; Sacu, S.; Kircher, K.; Georgopoulos, M.; Georg, D.; Zehetmayer, M.; Poetter, R. Radiogenic side effects after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 121–128. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, S.C.; Park, Y.G.; Chang, J.H. Long-term results of Gamma Knife surgery for uveal melanomas. J. Neurosurg. 2012, 117, 108–114. [Google Scholar] [CrossRef]
- Furdova, A.; Babal, P.; Kobzova, D.; Zahorjanova, P.; Kapitanova, K.; Sramka, M.; Kralik, G.; Furda, R.; Krasnik, V. Uveal melanoma survival rates after single dose stereotactic radiosurgery. Neoplasma 2018, 65, 965–971. [Google Scholar] [CrossRef]
- Marchini, G.; Babighian, S.; Torneinoli, L.; Gerosa, M.A.; Nicolato, A.; Bricolo, A.; Piovan, E.; Zampieri, P.G.; Alessandrini, F.; Benati, A.; et al. Stereotactic radiosurgery of uveal melanomas: Preliminary results with gamma knife treatment. Stereotact. Funct. Neurosurg. 1995, 64, 72–79. [Google Scholar] [CrossRef]
- Marchini, G.; Gerosa, M.; Piovan, E.; Pasoli, A.; Babighian, S.; Rigotti, M.; Rossato, M.; Bonomi, L. Gamma Knife stereotactic radiosurgery for uveal melanoma: Clinical results after 2 years. Proc. Stereotact. Funct. Neurosurg. 1997, 66, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Modorati, G.; Miserocchi, E.; Galli, L.; Picozzi, P.; Rama, P. Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br. J. Ophthalmol. 2009, 93, 40–44. [Google Scholar] [CrossRef]
- Reynolds, M.M.; Arnett, A.L.; Parney, I.F.; Kumar, R.; Laack, N.N.; Maloney, P.R.; Kozelsky, T.F.; Garces, Y.I.; Foote, R.L.; Pulido, J.S. Gamma knife radiosurgery for the treatment of uveal melanoma and uveal metastases. Int. J. Retin. Vitr. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Muacevic, A.; Nentwich, M.; Wowra, B.; Staerk, S.; Kampik, A.; Schaller, U. Development of a streamlined, non-invasive robotic radiosurgery method for treatment of uveal melanoma. Technol. Cancer Res. Treat. 2008, 7, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, S.; Foerster, R.; Nicolay, N.H.; Arians, N.; Bostel, T.; Debus, J.; Hauswald, H. Linear accelerator-based stereotactic fractionated photon radiotherapy as an eye-conserving treatment for uveal melanoma. Radiat. Oncol. 2018, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Zorlu, F.; Selek, U.; Kiratli, H. Initial results of fractionated CyberKnife radiosurgery for uveal melanoma. J. Neurooncol. 2009, 94, 111–117. [Google Scholar] [CrossRef]
- Arepalli, S.; Kaliki, S.; Shields, C.L.; Emrich, J.; Komarnicky, L.; Shields, J.A. Plaque radiotherapy in the management of scleral-invasive conjunctival squamous cell carcinoma: An analysis of 15 eyes. JAMA Ophthalmol. 2014, 132, 691–696. [Google Scholar] [CrossRef]
- Ramonas, K.M.; Conway, R.M.; Daftari, I.K.; Crawford, J.B.; O’Brien, J.M. Successful treatment of intraocularly invasive conjunctival squamous cell carcinoma with proton beam therapy. Arch. Ophthalmol. 2006, 124, 126–128. [Google Scholar] [CrossRef]
- Laskar, S.G.; Lewis, S.; Agarwal, J.P.; Mishra, S.; Mehta, S.; Patil, P. Combined brachytherapy and external beam radiation: An effective approach for palliation in esophageal cancer. J. Contemp. Brachytherapy 2015, 7, 453–461. [Google Scholar] [CrossRef]
- Walsh-Conway, N.; Conway, R.M. Plaque brachytherapy for the management of ocular surface malignancies with corneoscleral invasion. Clin. Exp. Ophthalmol. 2009, 37, 577–583. [Google Scholar] [CrossRef]
- Kearsley, J.H.; Fitchew, R.S.; Taylor, R.G.S. Adjunctive radiotherapy with strontium-90 in the treatment of conjunctival squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 435–443. [Google Scholar] [CrossRef]
- Karim, R.; Conway, R.M. Conservative resection and adjuvant plaque brachytherapy for early-stage conjunctival melanoma. Clin. Exp. Ophthalmol. 2011, 39, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Coupland, S.E. An audit of conjunctival melanoma treatment in Liverpool. Eye 2009, 23, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Wuestemeyer, H.; Sauerwein, W.; Meller, D.; Chauvel, P.; Schueler, A.; Steuhl, K.P.; Bornfeld, N.; Ana-stassiou, G. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 438–446. [Google Scholar] [CrossRef]
- Tanenbaum, R.E.; Galor, A.; Dubovy, S.R.; Karp, C.L. Classification, diagnosis, and management of conjunctival lymphoma. Eye Vis. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Oh, D.; Kim, W.S.; Kim, S.J.; Ko, Y.H.; Woo, K.I.; Kim, Y.D.; Ahn, Y.C. Low-dose radiation therapy for primary conjunctival marginal zone B-cell lymphoma. Cancer Res. Treat. 2018, 50, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Martinet, S.; Ozsahin, M.; Belkacémi, Y.; Landmann, C.; Poortmans, P.; Oehlere, C.; Scandolaro, L.; Krengli, M.; Maingon, P.; Miralbell, R.; et al. Outcome and prognostic factors in orbital lymphoma: A Rare Cancer Network study on 90 consecutive patients treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 892–898. [Google Scholar] [CrossRef]
- Shu, L.L.; Kao, S.C.S.; Ping, K.H.; Muh, S.C. Results of radiotherapy for orbital and adnexal lymphoma. Orbit 2002, 21, 117–123. [Google Scholar]
- Bhatia, S.; Paulino, A.C.; Buatti, J.M.; Mayr, N.A.; Wen, B.C. Curative radiotherapy for primary orbital lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 818–823. [Google Scholar] [CrossRef]
- Regueiro, C.A.; Valcárcel, F.J.; Romero, J.; de la Torre, A. Treatment of conjunctival lymphomas by beta-ray brachytherapy using a strontium-90-yttrium-90 applicator. Clin. Oncol. 2002, 14, 459–463. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26. [Google Scholar] [CrossRef]

| Study | Number of Patients | MMC Concentration | Allergy | Corneal Epitheliopathy | Epithelial Defect | Epiphora | Lid Inflammation | Ectropion | Ptosis |
|---|---|---|---|---|---|---|---|---|---|
| Bahrami 2013 [19] | 64 | 0.04% | 28% | 0% | 0% | 17% | 0% | 0% | 0% |
| Ballalai 2009 [21] | 23 | 0.02% | 0% | 0% | 17% | 0% | 0% | 0% | 0% |
| Birkholz 2011 [22] | 17 | 0.02% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Blasi 2018 [23] | 16 | 0.02% | 13% | 13% | 0% | 0% | 0% | 0% | 0% |
| Daniell 2002 [24] | 20 | 0.02-0.04% | 0% | 50% | NS | 0% | 10% | 0% | 0% |
| Gupta 2010 [25] | 91 | 0.04% | 23% | 0% | 2% | 15% | 0% | 0% | 1% |
| Khong 2006 [26] | 100 | 0.04% | 34% | 0% | 0% | 17% | 1% | 0% | 1% |
| Rudkin 2014 [27] | 39 | 0.04% | 18% | 23% | 18% | 5% | 0% | 3% | 0% |
| Study | Number of Patients | Corneal Epitheliopathy | Epithelial Defect | Epiphora | Lid Inflammation | Ectropion |
|---|---|---|---|---|---|---|
| Bahrami 2013 [19] | 89 | 6% | 1% | 10% | 62% | 1% |
| Gichuhi 2016 [46] | 47 | 0% | 0% | 49% | 14% | 0% |
| Joag 2016 [47] | 44 | 0% | 0% | 10% | 2% | 0% |
| Midena 2000 [44] | 8 | 100% | 0% | 0% | 0% | 0% |
| Parrozzani 2016 [45] | 41 | 28% | 0% | 0% | 8% | 0% |
| Rudkin 2014 [27] | 12 | 0% | 8% | 0% | 42% | 0% |
| Venkateswaran 2018 [48] | 54 | 7% | 0% | 22% | 9% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannaccare, G.; Bernabei, F.; Angi, M.; Pellegrini, M.; Maestri, A.; Romano, V.; Scorcia, V.; Rothschild, P.-R. Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours. Cancers 2021, 13, 1933. https://doi.org/10.3390/cancers13081933
Giannaccare G, Bernabei F, Angi M, Pellegrini M, Maestri A, Romano V, Scorcia V, Rothschild P-R. Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours. Cancers. 2021; 13(8):1933. https://doi.org/10.3390/cancers13081933
Chicago/Turabian StyleGiannaccare, Giuseppe, Federico Bernabei, Martina Angi, Marco Pellegrini, Antonio Maestri, Vito Romano, Vincenzo Scorcia, and Pierre-Räphael Rothschild. 2021. "Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours" Cancers 13, no. 8: 1933. https://doi.org/10.3390/cancers13081933
APA StyleGiannaccare, G., Bernabei, F., Angi, M., Pellegrini, M., Maestri, A., Romano, V., Scorcia, V., & Rothschild, P.-R. (2021). Iatrogenic Ocular Surface Diseases Occurring during and/or after Different Treatments for Ocular Tumours. Cancers, 13(8), 1933. https://doi.org/10.3390/cancers13081933









