NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cohort
2.2. Nucleic Acid Extraction
2.3. NTRK Fusion Genes in Pediatric Samples
2.4. Analyses of Point Mutations, RET/PTC and PAX8/PPARγ Rearrangements
2.5. NTRK Fusion Genes Detection
2.6. Statistical Analysis
3. Results
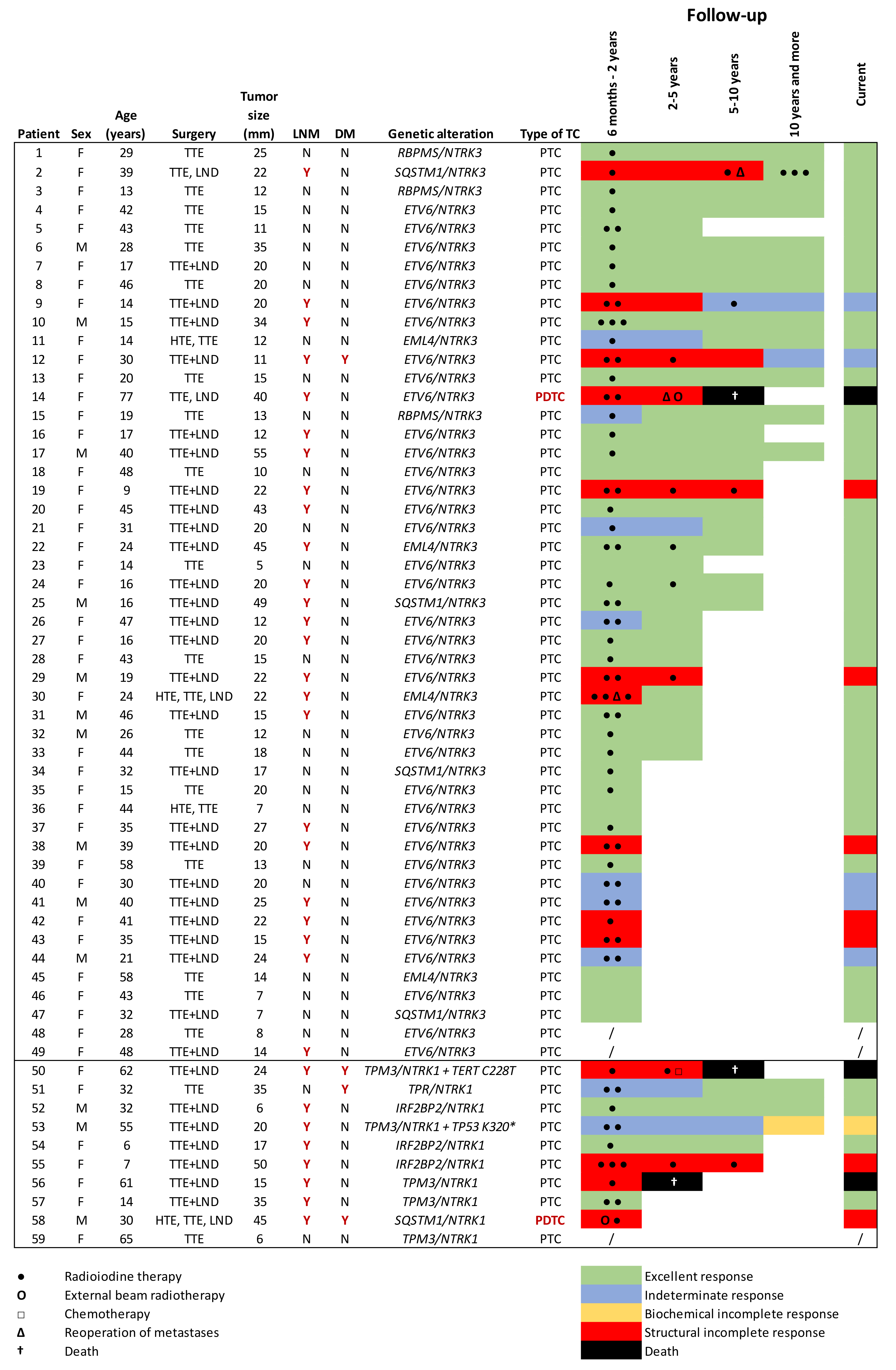
3.1. Clinical and Pathological Features
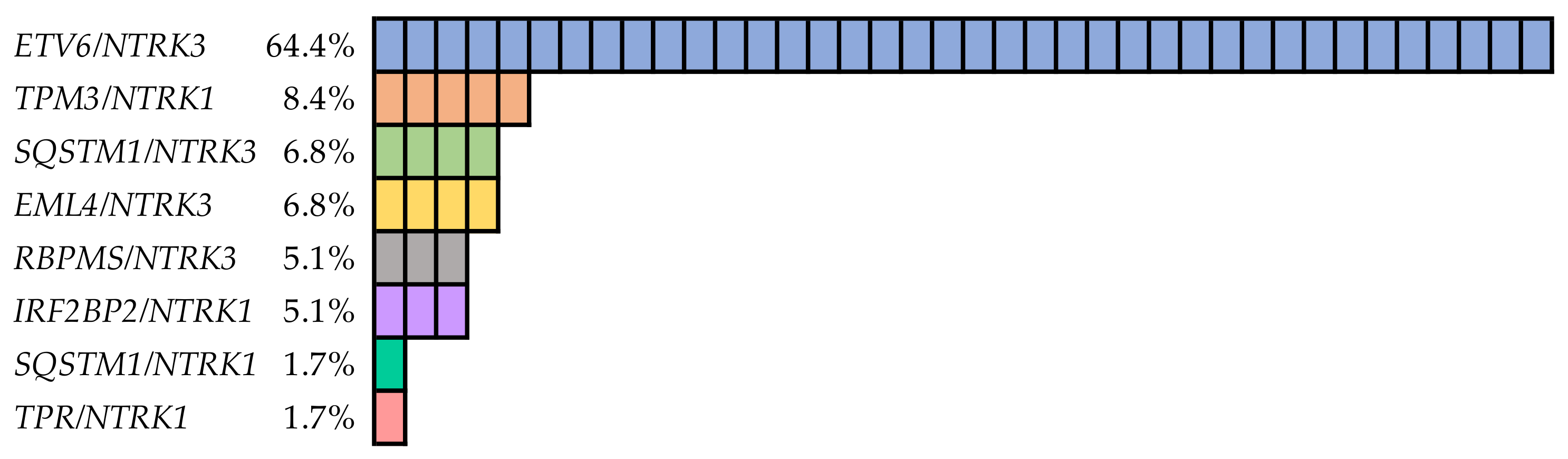
3.2. NTRK Fusion Genes
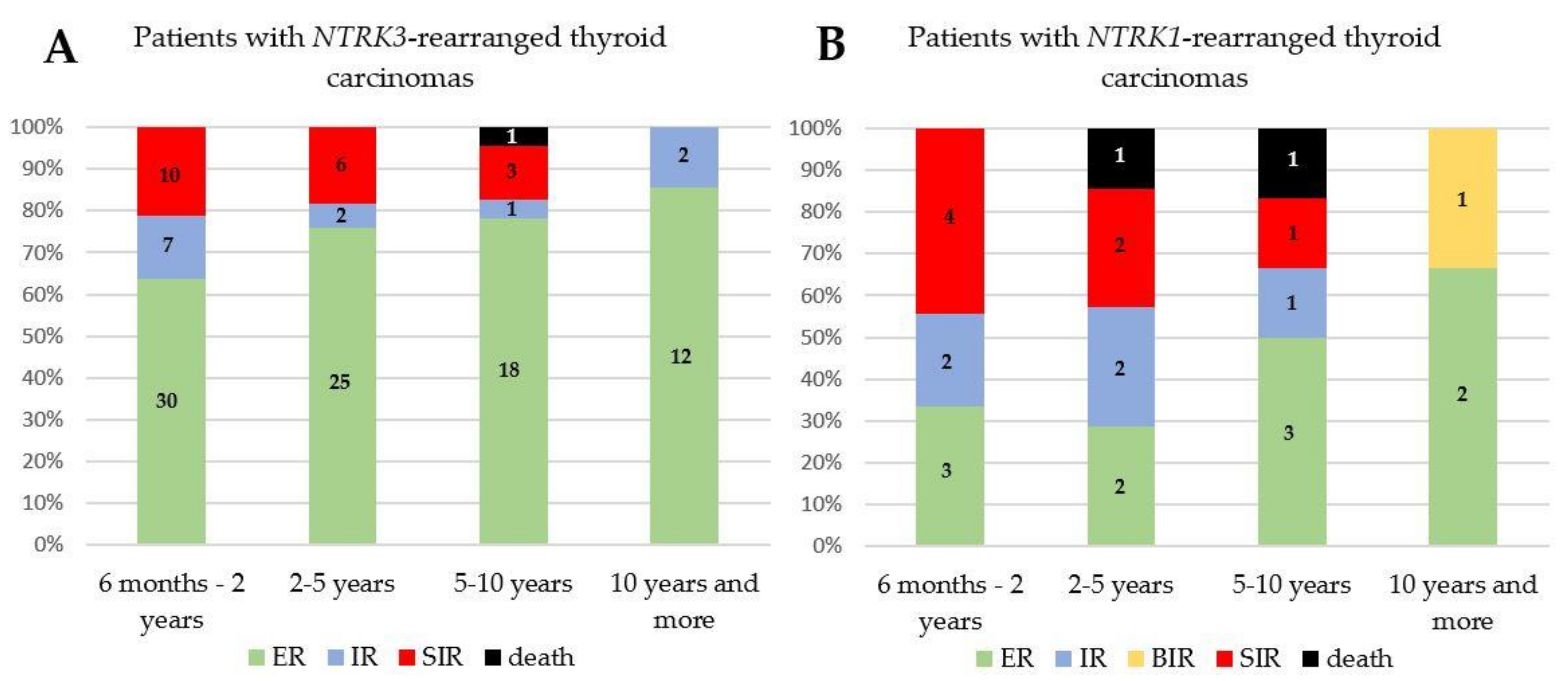
3.3. Treatment and Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ito, Y.; Miyauchi, A.; Kihara, M.; Fukushima, M.; Higashiyama, T.; Miya, A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J. Surg. 2018, 42, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Nachalon, Y.; Stern-Shavit, S.; Bachar, G.; Shvero, J.; Limon, D.; Popovtzer, A. Aggressive Palliation and Survival in Anaplastic Thyroid Carcinoma. JAMA Otolaryngol. Neck Surg. 2015, 141, 1128–1132. [Google Scholar] [CrossRef]
- Enumah, S.; Fingeret, A.; Parangi, S.; Dias-Santagata, D.; Sadow, P.M.; Lubitz, C.C. BRAFV600E Mutation is Associated with an Increased Risk of Papillary Thyroid Cancer Recurrence. World J. Surg. 2020, 44, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, H. The Impact of BRAF Mutation on the Recurrence of Papillary Thyroid Carcinoma: A Meta-Analysis. Cancers 2020, 12, 2056. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2019, 67, 44–57. [Google Scholar] [CrossRef]
- Xing, M. Clinical utility of RAS mutations in thyroid cancer: A blurred picture now emerging clearer. BMC Med. 2016, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, A.; Le, A.T.; Doebele, R.C. TRKing Down an Old Oncogene in a New Era of Targeted Therapy. Cancer Discov. 2014, 5, 25–34. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Van Der Tuin, K.; Garcia, M.V.; Corver, W.E.; Khalifa, M.N.; Neto, D.R.; Corssmit, E.P.M.; Hes, F.J.; Links, T.P.; Smit, J.W.A.; Plantinga, T.S.; et al. Targetable gene fusions identified in radioactive iodine refractory advanced thyroid carcinoma. Eur. J. Endocrinol. 2019, 180, 235–241. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, J.-Y.; Huang, C.-J.; Chen, H.-S.; Yang, A.-H.; Hang, J.-F. Detection of NTRK1/3 Rearrangements in Papillary Thyroid Carcinoma Using Immunohistochemistry, Fluorescent In Situ Hybridization, and Next-Generation Sequencing. Endocr. Pathol. 2020, 31, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cai, W.; Feng, D.; Teng, H.; Mao, F.; Jiang, Y.; Huajing, T.; Li, X.; Zhang, Y.; Liu, B.; et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J. Pathol. 2018, 244, 215–226. [Google Scholar] [CrossRef]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. RET, NTRK, ALK, BRAF, and MET Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid 2020, 30, 1771–1780. [Google Scholar] [CrossRef]
- Picarsic, J.L.; Buryk, M.A.; Ozolek, J.A.; Ranganathan, S.; Monaco, S.E.; Simons, J.P.; Witchel, S.F.; Gurtunca, N.; Joyce, J.; Zhong, S.; et al. Molecular Characterization of Sporadic Pediatric Thyroid Carcinoma with the DNA/RNA ThyroSeq v2 Next-Generation Sequencing Assay. Pediatr. Dev. Pathol. 2016, 19, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.L.; Vyas, M.; Horne, M.J.; Virk, R.K.; Morotti, R.; Liu, Z.; Tallini, G.; Nikiforova, M.N.; Christison-Lagay, E.R.; Udelsman, R.; et al. NTRKfusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 2016, 122, 1097–1107. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Pekova, B.; Dvorakova, S.; Sykorova, V.; Vacinova, G.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Vlcek, P.; Sykorova, P.; Kodetova, D.; et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr. Connect. 2019, 8, 796–805. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Paschke, R.; Cantara, S.; Crescenzi, A.; Jarzab, B.; Musholt, T.J.; Simoes, M.S. European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur. Thyroid J. 2017, 6, 115–129. [Google Scholar] [CrossRef]
- Seethala, R.R.; Chiosea, S.I.; Liu, C.Z.; Nikiforova, M.; Nikiforov, Y.E. Clinical and Morphologic Features of ETV6-NTRK3 Translocated Papillary Thyroid Carcinoma in an Adult Population Without Radiation Exposure. Am. J. Surg. Pathol. 2017, 41, 446–457. [Google Scholar] [CrossRef]
- Leeman-Neill, R.J.; Bs, L.M.K.; Liu, P.; Brenner, A.V.; Leeman-Neill, R.J.; Bogdanova, T.I.; Evdokimova, V.N.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014, 120, 799–807. [Google Scholar] [CrossRef]
- Abi-Raad, R.; Prasad, M.L.; Adeniran, A.J.; Cai, G. Fine-needle aspiration cytomorphology of papillary thyroid carcinoma withNTRKgene rearrangement from a case series with predominantly indeterminate cytology. Cancer Cytopathol. 2020, 128, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, S.; Dadu, R.; Waguespack, S.G.; Sherman, S.I.; Busaidy, N.L.; Hu, M.I.; Jimenez, C.; Habra, M.A.; Williams, M.; Altameemi, L.; et al. MON-491 TRK-Fusion Thyroid Cancer: A Clinical Overview in a Large Population at a Single Cancer Center. J. Endocr. Soc. 2020, 4. [Google Scholar] [CrossRef]
- Pfeifer, A.; Rusinek, D.; Żebracka-Gala, J.; Czarniecka, A.; Chmielik, E.; Zembala-Nożyńska, E.; Wojtaś, B.; Gielniewski, B.; Szpak-Ulczok, S.; Oczko-Wojciechowska, M.; et al. NovelTG-FGFR1andTRIM33-NTRK1transcript fusions in papillary thyroid carcinoma. Genes Chromosom. Cancer 2019, 58, 558–566. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Dias-Santagata, D.; Farahani, A.A.; Boyraz, B.; Faquin, W.C.; Nosé, V.; Sadow, P.M. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod. Pathol. 2020, 33, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Wirth, L.J.; Farahani, A.A.; Nosé, V.; Faquin, W.C.; Dias-Santagata, D.; Sadow, P.M. Clinicopathologic features of kinase fusion-related thyroid carcinomas: An integrative analysis with molecular characterization. Mod. Pathol. 2020, 33, 2458–2472. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.L.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; TuttleMD, R.M.; Sherman, E.J.; Gill, A.J.; GhosseinMD, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.; Melo, M.; Teijeiro, J.M.C.; Soares, P.; Sobrinho-Simões, M. ENDOCRINE TUMOURS: Genetic predictors of thyroid cancer outcome. Eur. J. Endocrinol. 2016, 174, R117–R126. [Google Scholar] [CrossRef]
- Borre, P.V.; Schrock, A.B.; Anderson, P.M.; Morris, J.C.; Heilmann, A.M.; Holmes, O.; Wang, K.; Johnson, A.; Waguespack, S.G.; Ou, S.I.; et al. Pediatric, Adolescent, and Young Adult Thyroid Carcinoma Harbors Frequent and Diverse Targetable Genomic Alterations, Including Kinase Fusions. Oncologist 2017, 22, 255–263. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Lassen, U.N. TRK Inhibition: A New Tumor-Agnostic Treatment Strategy. Target. Oncol. 2018, 13, 545–556. [Google Scholar] [CrossRef] [PubMed]
| NTRK Fusion-Positive Carcinomas (%) n = 59 | |
|---|---|
| Patients | |
| Females/males | 46/13 |
| Age at diagnosis (mean ± SD) | 32.8 ± 16.0 |
| History of radiation exposure before the diagnosis of TC | 1 (1.7) |
| History of prior malignancy before the diagnosis of TC | 1 (1.7) |
| Tumor size | |
| Mean ± SD (mm) | 20.9 ± 11.9 |
| Microcarcinoma (≤10 mm) | 8 (13.6) |
| Histology | |
| Predominantly papillary growth pattern | 8 (13.6) |
| Mixture of papillary and follicular growth pattern | 18 (30.5) |
| Predominantly follicular growth pattern | 28 (47.5) |
| Tall cell variant | 2 (3.4) |
| Clear cell variant | 1 (1.7) |
| PDTC | 2 (3.4) |
| Pathological characteristics | |
| Multifocality | 26 (44.1) |
| Extrathyroidal extension | 24 (40.7) |
| Intravascular invasion 1 | 12 (23.5) |
| Lymph node metastases | 32 (54.2) |
| Distant metastases | 4 (6.8) |
| Chronic lymphocytic thyroiditis 2 | 38 (65.5) |
| Frequent psammoma bodies 3 | 6 (10.3) |
| NTRK3 Fusion-Positive Carcinomas (%) n = 49 | NTRK1 Fusion-Positive Carcinomas (%) n = 10 | p | |
|---|---|---|---|
| Patients | |||
| Females/males | 39/10 | 7/3 | 0.383 |
| Age at diagnosis (mean ± SD) | 32.0 ± 14.4 | 36.4 ± 21.9 | 0.426 |
| History of radiation exposure before the diagnosis of TC | 0 | 1 (10.0) | 0.169 |
| History of prior malignancy before the diagnosis of TC | 0 | 1 (10.0) | 0.169 |
| Tumor size | |||
| Mean ± SD (mm) | 20.0 ± 11.0 | 25.3 ± 14.6 | 0.195 |
| Microcarcinoma (≤10 mm) | 6 (12.2) | 2 (20.0) | 0.409 |
| Histology | |||
| Predominantly papillary growth pattern | 8 (16.3) | 0 | 0.203 |
| Mixture of papillary and follicular growth pattern | 11 (22.4) | 7 (70.0) | 0.006 |
| Predominantly follicular growth pattern | 26 (53.1) | 2 (20.0) | 0.057 |
| Tall cell variant | 2 (4.1) | 0 | 1 |
| Clear cell variant | 1 (2.0) | 0 | 1 |
| PDTC | 1 (2.0) | 1 (10.0) | 0.313 |
| Pathological characteristics | |||
| Multifocality | 18 (36.7) | 8 (80.0) | 0.015 |
| Extrathyroidal extension | 16 (32.7) | 8 (80.0) | 0.008 |
| Intravascular invasion 1 | 7 (16.7) | 5 (55.6) | 0.024 |
| Lymph node metastases | 24 (49.0) | 8 (80.0) | 0.072 |
| Distant metastases | 1 (2.0) | 3 (30.0) | 0.013 |
| Chronic lymphocytic thyroiditis 2 | 34 (70.8) | 4 (40.0) | 0.069 |
| Frequent psammoma bodies 3 | 4 (8.3) | 2 (20.0) | 0.274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekova, B.; Sykorova, V.; Mastnikova, K.; Vaclavikova, E.; Moravcova, J.; Vlcek, P.; Lastuvka, P.; Taudy, M.; Katra, R.; Bavor, P.; et al. NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis. Cancers 2021, 13, 1932. https://doi.org/10.3390/cancers13081932
Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, Lastuvka P, Taudy M, Katra R, Bavor P, et al. NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis. Cancers. 2021; 13(8):1932. https://doi.org/10.3390/cancers13081932
Chicago/Turabian StylePekova, Barbora, Vlasta Sykorova, Karolina Mastnikova, Eliska Vaclavikova, Jitka Moravcova, Petr Vlcek, Petr Lastuvka, Milos Taudy, Rami Katra, Petr Bavor, and et al. 2021. "NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis" Cancers 13, no. 8: 1932. https://doi.org/10.3390/cancers13081932
APA StylePekova, B., Sykorova, V., Mastnikova, K., Vaclavikova, E., Moravcova, J., Vlcek, P., Lastuvka, P., Taudy, M., Katra, R., Bavor, P., Kodetova, D., Chovanec, M., Drozenova, J., Astl, J., Hrabal, P., Vcelak, J., & Bendlova, B. (2021). NTRK Fusion Genes in Thyroid Carcinomas: Clinicopathological Characteristics and Their Impacts on Prognosis. Cancers, 13(8), 1932. https://doi.org/10.3390/cancers13081932






