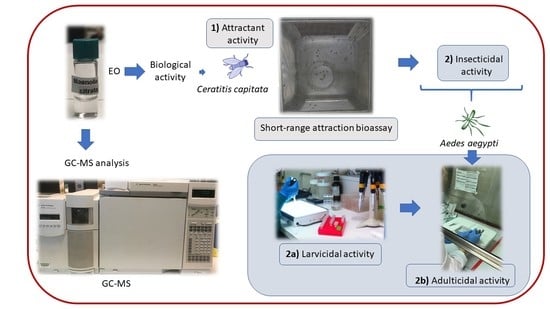
Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae) †
Abstract
1. Introduction
2. Results and Discussion
| RI a | RI b | Compounds | % | Identification Method a,c,d |
|---|---|---|---|---|
| 851 | 850 | (Z)-3-hexen-1-ol | 0.6 | MS, RI, std |
| 920 | 924 | α-thujene | 0.4 | MS, RI |
| 926 | 932 | α-pinene | 0.1 | MS, RI, std |
| 968 | 969 | sabinene | 12.4 | MS, RI, std |
| 971 | 974 | β-pinene | 0.5 | MS, RI, std |
| 981 | 981 | 6-methyl-5-hepten-2-one | 1.2 | MS, RI, std |
| 985 | 988 | myrcene | 0.4 | MS, RI, std |
| 1020 | 1020 | p-cymene | 0.8 | MS, RI, std |
| 1024 | 1024 | limonene | 0.4 | MS, RI, std |
| 1026 | 1026 | 1,8-cineole | 0.3 | MS, RI, std |
| 1032 | 1032 | (Z)-β-ocimene | 1.2 | MS, RI, std |
| 1043 | 1044 | (E)-β-ocimene | 0.1 | MS, RI, std |
| 1053 | 1054 | γ-terpinene | 0.1 | MS, RI, std |
| 1064 | 1065 | cis-sabinene hydrate | 0.9 | MS, RI |
| 1082 | 1067 | cis-linalool oxide (furanoid) | 0.3 | MS, RI, std |
| 1101 | 1095 | linalool | 19.1 | MS, RI, std |
| 1107 | 1118 | cis-p-menth-2-en-1-ol | 0.1 | MS, RI, std |
| 1120 | 1136 | trans-p-menth-2-en-1-ol | 0.1 | MS, RI, std |
| 1137 | 1144 | neo-isopulegol | 0.1 | MS, RI |
| 1142 | 1145 | isopulegol | 0.8 | MS, RI |
| 1150 | 1148 | citronellal | 14.1 | MS, RI |
| 1157 | 1167 | neoiso-isopulegol | 0.1 | MS, RI |
| 1165 | 1173 | rosefuran epoxide | 0.1 | MS, RI |
| 1174 | 1174 | terpinen-4-ol | 1.6 | MS, RI, std |
| 1190 | 1186 | α-terpineol | 0.2 | MS, RI, std |
| 1220 | 1227 | nerol | 0.4 | MS, RI |
| 1226 | 1223 | citronellol | 6.5 | MS, RI, std |
| 1235 | 1235 | neral | 13.5 | MS, RI |
| 1245 | 1249 | piperitone | 0.1 | MS, RI, std |
| 1252 | 1257 | methyl citronellate | 0.9 | MS, RI |
| 1265 | 1264 | geranial | 15.7 | MS, RI |
| 1268 | 1271 | citronellyl formate | 0.4 | MS, RI |
| 1323 | 1312 | citronellic acid | 3.4 | MS, RI |
| 1405 | 1417 | β-caryophyllene | 0.1 | MS, RI, std |
| 1439 | 1452 | α-humulene | tr | MS, RI, std |
| 1443 | 1458 | alloaromadendrene | 0.2 | MS, RI, std |
| 1472 | 1489 | β-selinene | 0.4 | MS, RI |
| 1480 | 1498 | α-selinene | 0.1 | MS, RI |
| 1562 | 1582 | caryophyllene oxide | 0.3 | MS, RI, std |
| 1584 | 1602 | ledol | tr | MS, RI |
| 1589 | 1608 | humulene epoxide II | tr | MS, RI |
| Total | 98.0 |
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Isolation
3.3. GC-MS Analysis
Identification of Components
3.4. Biologicaal Activities
3.4.1. Mosquito Assays
3.4.2. Larvicidal Bioassay
3.4.3. Adulticidal Bioassay
3.4.4. Fruit Fly Assay
Short-Range Attraction Bioassay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Jepson, P.C.; Murray, K.; Bach, O.; Bonilla, M.A.; Neumeister, L. Selection of pesticides to reduce human and environmental health risks: A global guideline and minimum pesticides list. Lancet Planet. Health 2020, 4, e56–e63. [Google Scholar] [CrossRef]
- Van Den Berg, H.; Gu, B.; Grenier, B.; Kohlschmid, E.; Al-Eryani, S.; Bezerra, H.S.D.S.; Nagpal, B.N.; Chanda, E.; Gasimov, E.; Velayudhan, R.; et al. Pesticide lifecycle management in agriculture and public health: Where are the gaps? Sci. Total. Environ. 2020, 742, 140598. [Google Scholar] [CrossRef]
- Dinesh, D.S.; Kumari, S.; Kumar, V.; Das, P. The potentiality of botanicals and their products as an alternative to chemical insecticides to sandflies (Diptera: Psychodidae): A review. J. Vector Borne Dis. 2014, 51, 1–7. [Google Scholar] [PubMed]
- Senthil-Nathan, S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents Against Mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Shah, F.; Razaq, M.; Ali, Q.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Field evaluation of synthetic and neem-derived alternative insecticides in developing action thresholds against cauliflower pests. Sci. Rep. 2019, 9, 7684. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Ahl, H.A.S.-A. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Vu, L.V.; Vu, C.Q. Diversity pattern of butterfly communities (Lepidoptera, Papilionoidae) in different habitat types in a tropical rain forest of southern Vietnam. Biodivers. Conserv. 2003, 12, 1099–1111. [Google Scholar] [CrossRef][Green Version]
- Van, Y.T.; Cochard, R. Tree species diversity and utilities in a contracting lowland hillside rainforest fragment in Central Vietnam. For. Ecosyst. 2017, 4, 9. [Google Scholar] [CrossRef]
- Hung, D.V.; Potokin, A.F. Diversity of Plant Species Composition and Forest Vegetation Cover of Dong Nai Culture and Nature Reserve, Vietnam. IOP Conf. Ser. Earth Environ. Sci. 2019, 316, 012009. [Google Scholar] [CrossRef]
- Mohammed, A.; Chadee, D.D. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011, 119, 38–43. [Google Scholar] [CrossRef]
- Al-Massarani, S.; El-Shaibany, A.; Tabanca, N.; Ali, A.; Estep, A.S.; Becnel, J.J.; Goger, F.; Demirci, B.; El-Gamal, A.; Baser, K.H.C. Assessment of selected Saudi and Yemeni plants for mosquitocidal activities against the yellow fever mosquito Aedes aegypti. Saudi Pharm. J. 2019, 27, 930–938. [Google Scholar] [CrossRef]
- Niogret, J.; Montgomery, W.S.; Kendra, P.E.; Heath, R.R.; Epsky, N.D. Attraction and electroantennogram responses of male Mediterranean fruit fly to volatile chemicals from Persea, Litchi and Ficus Wood. J. Chem. Ecol. 2011, 37, 483–491. [Google Scholar] [CrossRef]
- Papadopoulos, N.T. Fruit Fly Invasion: Historical, Biological, Economical Aspects and Management. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, 1st ed.; Shelly, T., Epsky, N.D., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: New York, NY, USA, 2014; pp. 219–252. [Google Scholar]
- Vargas, R.I.; Leblanc, L.; Pinero, J.C.; Hoffman, K.M. Male Annihilation, Past, Present, and Future. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, 1st ed.; Shelly, T., Epsky, N.D., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: New York, NY, USA, 2014; pp. 493–511. [Google Scholar]
- Kim, S.; Park, C.-W.; Kim, Y.-D.; Suh, Y.; Suh, Y.-D.K. Phylogenetic relationships in family Magnoliaceae inferred from ndhF sequences. Am. J. Bot. 2001, 88, 717–728. [Google Scholar] [CrossRef]
- Cires, E.; De Smet, Y.; Cuesta, C.; Goetghebeur, P.; Sharrock, S.; Gibbs, D.; Oldfield, S.; Kramer, A.; Samain, M.-S. Gap analyses to support ex situ conservation of genetic diversity in Magnolia, a flagship group. Biodivers. Conserv. 2013, 22, 567–590. [Google Scholar] [CrossRef]
- Ho, K.-Y.; Tsai, C.-C.; Chen, C.-P.; Huang, J.-S.; Lin, C.-C. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother. Res. 2001, 15, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Sun, W.; Gao, L.; Kim, K.S.; Kim, K.T.; Cai, L.; Zhang, Z.; Zheng, Y. Extracts of Magnolia Species-Induced Prevention of Diabetic Complications: A Brief Review. Int. J. Mol. Sci. 2016, 17, 1629. [Google Scholar] [CrossRef] [PubMed]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Ishikawa, Y.; Kasahara, H.; Yamanaka, J.-I.; Kameoka, H. An insect growth inhibitory lignan from flower buds of Magnolia fargesii. Phytochemistry 1994, 35, 611–613. [Google Scholar] [CrossRef]
- Kelm, M.A.; Nair, M.G.; Schutzki, R.A. Mosquitocidal Compounds from Magnolia salicifolia. Int. J. Pharmacogn. 1997, 35, 84–90. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Demirci, B.; Raman, V.; Budel, J.M.; Baser, K.H.C.; Khan, I.A. Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti. Molecules 2020, 25, 1359. [Google Scholar] [CrossRef] [PubMed]
- Flores-Estevez, N.; Vasquez-Morales, S.G.; Cano-Medina, T.; Sanchez-Velasquez, L.R.; Noa Carrazana, J.C.; Diaz-Fleischer, F. Insecticidal activity of raw extracts from Magnolia dealbata Zucc on a tephritidae pest. J. Environ. Sci. Health B. 2013, 48, 585–589. [Google Scholar] [CrossRef]
- Vasquez-Morales, S.G.; Flores-Estevez, N.; Sanchez-Velasquez, L.R.; Pineda-Lopez, M.D.R.; Viveros-Viveros, H.; Diaz-Fleischer, F. Bioprospecting of botanical insecticides: The case of ethanol extracts of Magnolia schiedeana Schltl. applied to a Tephritid, fruit fly Anastrepha ludens Loew. J. Entomol. Zool. 2015, 3, 1–5. [Google Scholar]
- Global Biodiversity Information Facility. 2021. Available online: https://www.gbif.org/species/3153028 (accessed on 14 January 2021).
- The Plant List. 2013. A Working List of All Known Plant Species. Available online: http://www.theplantlist.org/tpl/record/kew-348947 (accessed on 14 January 2021).
- Wheeler, L.; Rivers, M.C.; Khela, S. Magnolia citrata. The IUCN Red List of Threatened Species. Available online: http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T191871A2011039.en (accessed on 31 March 2021).
- Ngoc Anh, L.-D.; Graduate University of Science and Technology, Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam; Vietnam National Museum of Nature, Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam. Personal communication, 30 March 2021.
- Huong, B.V.; Ngan, T.B.; Ngoc Anh, L.D.; Tao, N.T. Preliminary study on chemical constituent of essential oil from the leaves of Michelia citrata (Noot. & Chalenglin) Q.N.Vu & N.H. Xia collected in Quan Ba District, Ha Giang Province. J. Sci. Technol. 2014, 30, 337–340. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. 17 Mass Spectrometer database, NIST/EPA/NIH. In NIST/EPA/NIH Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA; Department of Commerce: Washington, DC, USA, 2017. [Google Scholar]
- Wiley. Wiley Registry of Mass Spectral Data, 11th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Koenig, W.A.; Joulain, D.; Hochmuth, D. GC/MS Library: Terpenoids and Related Constituents of Essential Oils; Library of MassFinder: 2004. Available online: https://massfinder.com (accessed on 22 March 2021).
- Wiley. Flavors and Fragrances of Natural and Synthetic Compounds 3 (FFNSC 3), Mass Spectral Database; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pandey, S.K.; Tandon, S.; Ahmad, A.; Singh, A.K.; Tripathi, A.K. Structure-activity relationships of monoterpenes and acetyl derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2013, 69, 1235–1238. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, C.; Chung, M.; Liu, Y.; Huang, C.; Chang, S. Larvicidal activities of wood and leaf essential oils and ethanolic extracts from Cunninghamia konishii Hayata against the dengue mosquitoes. Ind. Crop. Prod. 2013, 47, 310–315. [Google Scholar] [CrossRef]
- Reid, W.R.; Thornton, A.; Pridgeon, J.W.; Becnel, J.J.; Tang, F.; Estep, A.; Clark, G.G.; Allan, S.; Liu, N. Transcriptional analysis of four family 4 P450s in a Puerto Rico strain of Aedes aegypti (Diptera: Culicidae) compared with an Orlando strain and their possible functional roles in permethrin resistance. J. Med. Entomol. 2014, 51, 605–615. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Meepagala, M.M.; Becnel, J.J.; Clark, G.G.; Pereira, R.M.; Linthicum, K.J. Structure-activity relationships of 33 piperidines as toxicants against female adults of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 263–269. [Google Scholar] [CrossRef]
- Norris, E.; Johnson, J.; Gross, A.; Bartholomay, L.; Coats, J. Plant essential oils enhance diverse pyrethroids against multiple strains of mosquitoes and inhibit detoxification enzyme processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Niogret, J.; Kendra, P.E.; Epsky, N.D. TLC-based bioassay to isolate kairomones from tea tree essential oil that attract male mediterranean fruit flies, Ceratitis capitata (Wiedemann). Biomolecules 2020, 10, 683. [Google Scholar] [CrossRef]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus var. chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef] [PubMed]
- Blythe, E.K.; Tabanca, N.; Demirci, B.; Kendra, P.E. Chemical composition of essential oil from Tetradenia riparia and its attractant activity for mediterranean fruit fly, Ceratitis capitata. Nat. Prod. Commun. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Stappen, I.; Wanner, J.; Tabanca, N.; Bernier, U.R.; Kendra, P.E. Blue tansy essential oil: Chemical composition, repellent activity against Aedes aegypti and attractant activity for Ceratitis capitata. Nat. Prod. Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Kendra, P.E.; Owens, D.; Montgomery, W.S.; Narvaez, T.I.; Bauchan, G.R.; Schnell, E.Q.; Tabanca, N.; Carrillo, D. α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2017, 12, e0179416. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Owens, D.; Kendra, P.E.; Tabanca, N.; Narvaez, T.I.; Montgomery, W.S.; Schnell, E.Q.; Carrillo, D. Quantitative analysis of contents and volatile emissions from α-copaene and quercivorol lures, and longevity for attraction of Euwallacea nr. fornicatus in Florida. J. Pest Sci. 2019, 92, 237–252. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. A high-throughput screening method to identify potential pesticides for mosquito control. J. Med. Entomol. 2009, 46, 335–341. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Becnel, J.J.; Estep, A.S. Phomalactone as the active constituent against mosquitoes from Nigrospora spherica. Agric. Sci. 2015, 6, 1195–1201. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Louton, J.E.; Becnel, J.J. Resistance status and resistance mechanisms in a strain of Aedes aegypti (Diptera: Culicidae) from Puerto Rico. J. Med Entomol. 2017, 54, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Systat Software. SigmaPlot for Windows, version 14.0; Systat Software, Inc.: San Jose, CA, USA, 2017. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. This work was produced by US government employees and is in the public domain in the US. Sub-mitted for possible open access publication under the terms and conditions of the Creative Com-mons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu-Dam, N.A.; Tabanca, N.; Estep, A.S.; Nguyen, D.H.; Kendra, P.E. Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae). Molecules 2021, 26, 2311. https://doi.org/10.3390/molecules26082311
Luu-Dam NA, Tabanca N, Estep AS, Nguyen DH, Kendra PE. Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae). Molecules. 2021; 26(8):2311. https://doi.org/10.3390/molecules26082311
Chicago/Turabian StyleLuu-Dam, Ngoc Anh, Nurhayat Tabanca, Alden S. Estep, Duy Hung Nguyen, and Paul E. Kendra. 2021. "Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae)" Molecules 26, no. 8: 2311. https://doi.org/10.3390/molecules26082311
APA StyleLuu-Dam, N. A., Tabanca, N., Estep, A. S., Nguyen, D. H., & Kendra, P. E. (2021). Insecticidal and Attractant Activities of Magnolia citrata Leaf Essential Oil against Two Major Pests from Diptera: Aedes aegypti (Culicidae) and Ceratitis capitata (Tephritidae). Molecules, 26(8), 2311. https://doi.org/10.3390/molecules26082311