Abstract
Phytotoxic substances released from plants are considered eco-friendly alternatives for controlling weeds in agricultural production. In this study, the leaves of Afzelia xylocarpa (Kurz) Craib. were investigated for biological activity, and their active substances were determined. Extracts of A. xylocarpa leaf exhibited concentration-dependent phytotoxic activity against the seedling length of Lepidium sativum L., Medicago sativa L., Phleum pratense L., and Echinochloa crus-galli (L.) P. Beauv. Bioassay-guided fractionation of the A. xylocarpa leaf extracts led to isolating and identifying two compounds: vanillic acid and trans-ferulic acid. Both compounds were applied to four model plants using different concentrations. The results showed both compounds significantly inhibited the model plants’ seedling length in a species-dependent manner (p < 0.05). The phytotoxic effects of trans-ferulic acid (IC50 = 0.42 to 2.43 mM) on the model plants were much greater than that of vanillic acid (IC50 = 0.73 to 3.17 mM) and P. pratense was the most sensitive to both compounds. In addition, the application of an equimolar (0.3 mM) mixture of vanillic acid and trans-ferulic acid showed the synergistic effects of the phytotoxic activity against the root length of P. pratense and L. sativum. These results suggest that the leaves of A. xylocarpa and its phytotoxic compounds could be used as a natural source of herbicides.
1. Introduction
Weeds are considered an important problem in agriculture worldwide. They compete with crops throughout the growing period and reduce crop yield and quality [1]. On average, approximately 34% of the total yield loss of crops is due to weeds interference worldwide [2,3]. Farmers mostly rely on synthetic herbicides to control weeds in crop fields because of their easy accessibility and a more rapid return [4]. In recent years, the deleterious effects of synthetic herbicides on the environment have been of concern. The application of synthetic herbicides has led to long-term danger to the environment because of their permanence in nature [5,6]. In particular, the widespread practice of using synthetic herbicides has resulted in the evolution of herbicide-tolerant weed species [7]. Consequently, scientific communities have been paying greater attention to eco-friendly alternatives and sustainable biological solutions for weed management [8,9,10]. As a result, allelopathic plants with phytotoxic substances are now considered environmentally friendly alternatives for controlling weeds [11,12].
Allelopathic plants release various phytotoxic substances into the environment through different mechanisms [13,14]. These phytotoxic substances (e.g., phenolics, terpenoids, and alkaloids and their derivatives) can affect target plants’ physiological functions, such as inhibiting cell membrane permeability and cell division as well as interrupting respiration photosynthesis, nutrient uptake, and enzymatic activities [15,16,17]. Most of these substances are entirely or partially water-soluble, making them more eco-friendly and easier to apply as natural herbicides [18,19]. Consequently, there has been much research on isolating and identifying phytotoxic substances from different plant species to develop natural herbicides [20,21,22]. Some of the allelochemicals isolated from plants have been used in developing allelochemical-based herbicides. For instance, cinmethylin herbicide (Cinch®) has been developed based on the chemical structure of 1,4-cineole derived from Eucalyptus sp. This herbicide is effectively used as a pre-emergence herbicide to control annual grass weeds that inhibit the enzyme tyrosine aminotransferase activity [23,24].
However, different structural characteristics of the compounds show different modes of action and target sites on the plants [25]. Therefore, it is important to search for and to identify new species with phytotoxic activity to determine specific properties and unique target sites in target plants.
Recently, researchers have been interested in searching for a potential source of phytotoxic substances in the Fabaceae family [26,27]. Fabaceae is a large family of diverse plants known for a high diversity of secondary metabolites with phytotoxic potential [28,29]. Research on their phytotoxic potential suggests future applications as natural herbicides [30,31].
Afzelia xylocarpa (Kurz) Craib. is a dicotyledonous plant belonging to the subfamily Caesalpinioideae in the Fabaceae family. This species is both ecologically and economically important deciduous tree in agroforestry systems in the tropics and is mainly distributed in Thailand, Vietnam, Cambodia, Laos, and Burma [32]. It is used as a traditional medicine against inflammatory ocular diseases, sore throat, and food poisoning [33]. Previous studies have shown different biological activities of this plant, such as antioxidant, antidiabetic, antimicrobial, and anti-inflammatory [34,35,36]. A. xylocarpa is one of the endangered species in the world. This plant suffers from overexploitation as a timber source, and its habitat has also been decreasing [32]. One possible reason for the reduction of the habitat of the species may be its allelopathic potential and/or autotoxicity. Allelopathic effects are more explicated in the forest communities because of the huge biomass of the canopy or released phytotoxic substances through fallen leaves/fruits [37,38]. Various compounds have been reported in A. xylocarpa seeds, such as chlorogenic acid, ferulic acid, gallic acid, taxifolin, caffeic acid, rosmarinic acid, daidzein, isovanillic acid, cinnamic acid, vanillin, naringenin, p-coumaric acid, cholesterine, campesterol, campestanol, and stigmasterol [39]. Twelve compounds were isolated from A. xylocarpa leaves, including kaempferol-7-O-β-d-glucopyranoside, friedelin, β-sitosterol, butyl benzoate, stigmas-ta-4, 25-dien-3-one, epifriedelanol, stigmasterol, palmitic acid, linoleic acid, α-linolenic acid, and (3S,5R,6R,7E)-3,5,6-trihydroxy-7-megastigmen-9-one [33]. It was reported that some phenolic acids, such as p-coumaric acid, act as phototoxic substances, which are released from different plant parts or decomposed plant residues. These compounds accumulate in the soil and have detrimental effects on the seed germination and growth of the species nearby and autotoxic effects on the species itself [40,41,42]. Therefore, the understanding of allelopathy is important to preserve this plant species. In addition, the allelopathic substances may potentially be used in biological weed management.
Although some bioactive compounds have been isolated from A. xylocarpa, it was not clear which compounds truly act as active substances in this plant. Thus, this study was conducted to determine the phytotoxic activity of A. xylocarpa leaves and to isolate active substances by bioassay-guided fractionations. The activities of the identified compounds and a mixture of these compounds were tested on the seedling growth under laboratory conditions. It was envisaged that this study would highlight the effect of active substances isolated from A. xylocarpa leaves on the model plants. This information could be useful for assessing the interrelations between active substances and action sites in the target plants. This research may lead to developing a natural herbicide in the future. More bioactive substances produced by this forest species can help better understand their impact on the local environment and natural ecosystem.
2. Materials and Methods
2.1. Plant Material and Model Plants
Leaves of Afzelia xylocarpa (Kurz) Craib. were collected from Phitsanulok Province, Thailand, in August 2017. The collected materials were thoroughly washed with tap water, immediately dried under shade to avoid direct sunlight, and finely ground into powder form for further extraction. Four plant species were used as model plants for bioassay experiments. Two dicotyledonous species (Lepidium sativum L. and Medicago sativa L.) were selected because of their known growth characteristics and sensitivity to allelopathic extracts. Two monocotyledonous species (Phleum pratense L. and Echinochloa crus-galli (L.) P. Beauv) were selected for their worldwide distribution in crop fields [43,44]. In the isolation process, L. sativum was selected as the model plant due to its high sensitivity to phytotoxic substances at low concentrations [45].
2.2. Preparation of the A. xylocarpa Leaf Extracts
The A. xylocarpa powder (100 g dry weight) was extracted in 70% aqueous methanol in a 1:5 (w/v) ratio at room temperature and then kept under dark conditions to avoid light degradation. After 48 h extraction, the solution was then vacuum filtered through filter paper (No.2, 12.5 cm; Toyo Ltd., Tokyo, Japan). The residue was re-extracted with methanol (500 mL) for another 24 h and filtered. The combined filtrates were concentrated in a vacuum using a rotary evaporator (40 °C) to produce a crude extract (~52 g).
2.3. Bioassay Procedure
To assess the biological activity of the A. xylocarpa leaf extracts, concentrations of 1, 3, 10, 30, 100, and 300 mg dry weight (DW) equivalent extract/mL were used. An aliquot of the extracts at assay concentrations (0.6 mL) was transferred into Petri dishes (2.8 cm in diameter) containing a sheet of filter paper (No. 2; 2.8 cm; Toyo Ltd.). After the solvent evaporated, filter paper sheets were moistened with 0.6 mL of 0.05% Tween-20 in distilled water (polyoxyethylene sorbitan monolaurate; Nacalai, Kyoto, Japan). Ten seeds each of L. sativum and M. sativa, and ten sprouted seeds each of P. pratense and E. crus-galli were placed into Petri dishes (sprouted in distilled water under darkness at 25 °C for 48 h). A solution of 0.05% Tween-20 served as control. All plates were incubated in a growth chamber under darkness (25 °C), and six replicates were performed for each treatment. After 48 h incubation, the lengths of the shoots and roots of the model plants were measured. The results were expressed as a percentage ratio using the following formula [46]: seedling length (%) = (treated length/length of control) ×100.
2.4. Isolation and Identification of the Active Substances
The A. xylocarpa leaves (1400 g) were extracted using the same method described earlier. The aqueous methanol leaf extracts were concentrated in vacuo (40 °C) to produce an aqueous solution for further isolation and identification. The isolation methods were modified from those performed by Boonmee et al. [47]. The separated fractions obtained from each isolation step were examined for their activity using an L. sativum bioassay as the same assay method described above. After adjusting the pH to 7 using 1 M phosphate buffer, the aqueous solution was partitioned with ethyl acetate at an equal volume and separated into aqueous and ethyl acetate fractions. The ethyl acetate fraction was subjected to silica gel column chromatography (60 g, silica gel 60, 70–230 mesh; Nacalai, Kyoto, Japan), and eluted with stepwise gradient mixtures of n-hexane: ethyl acetate (9:1 to 2:8 (v/v), 150 mL), and methanol for final elution (300 mL). The most active fraction (F5) was then chromatographed on a Sephadex LH-20 column (100 g; GE Healthcare, Uppsala, Sweden). The mobile phase was used as stepwise water: methanol mixtures (8:2 to 2:8 (v/v), 150 mL), and methanol (300 mL). The most active fraction (F2) eluted with 40% aqueous methanol. Therefore, fraction 2 was loaded onto a reverse-phase C18 cartridge (1.2 × 6.5 cm; YMC-Dispo Pack AT ODS-25; YMC Ltd., Kyoto, Japan). The cartridge was eluted step-by-step with water: methanol mixtures (9:1 to 2:8 (v/v), 15 mL), and methanol (30 mL). The most active fraction was eluted with 20% aqueous methanol (F1) and finally purified using reverse-phase HPLC (column size 10 mm i.d. × 500 mm S-5 μm; ODS AQ-325, YMC Ltd.), eluted at a flow rate of 1.5 mL/min with 20% aqueous methanol, and detected at a wavelength of 220 nm. Finally, two isolated substances were found, and their chemical structures were assessed using spectral analysis.
2.5. Biological Activity of the Isolated Active Substances
Two isolated substances were prepared in methanol to produce concentrations of 0.1, 0.3, 1, 3, and 10 mM. The prepared concentrations of each isolated substance (0.6 mL) were applied on L. sativum, M. sativa, P. pratense, and E. crus-galli to perform bioassays as the same protocol mentioned above. The growth parameters (shoot and root length) of the model plants were measured, and all results were calculated using the formula described above.
2.6. The Synergistic Effects of a Mixture of the Two Isolated Substances
The synergistic effects of the two isolated substances were determined. A stock solution of each isolated substance was prepared with methanol (0.3 mL) to get a concentration of 0.3 mM. The isolated substances 1 and 2 were mixed at a ratio of 1:1. The mixture of the two substances was applied on two model plants: L. sativum and P. pratense. The experimental design, incubation conditions, and data collection were the same as in the bioassay described above.
2.7. Statistical Analysis
The data from each experiment were analyzed using Tukey’s test with SPSS version 16.0 (IBM, Chicago, IL, USA). Student’s t-test was used to analyze two-group comparisons. The concentration required for 50% inhibition of each test plant was determined using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) by nonlinear regression of the inhibition results. The relationship between the seedling length of the test plants and the concentrations was determined using a two-tailed Pearson’s correlation.
3. Results and Discussion
3.1. Biological Activity of the Aqueous Methanol Extracts of A. xylocarpa Leaves
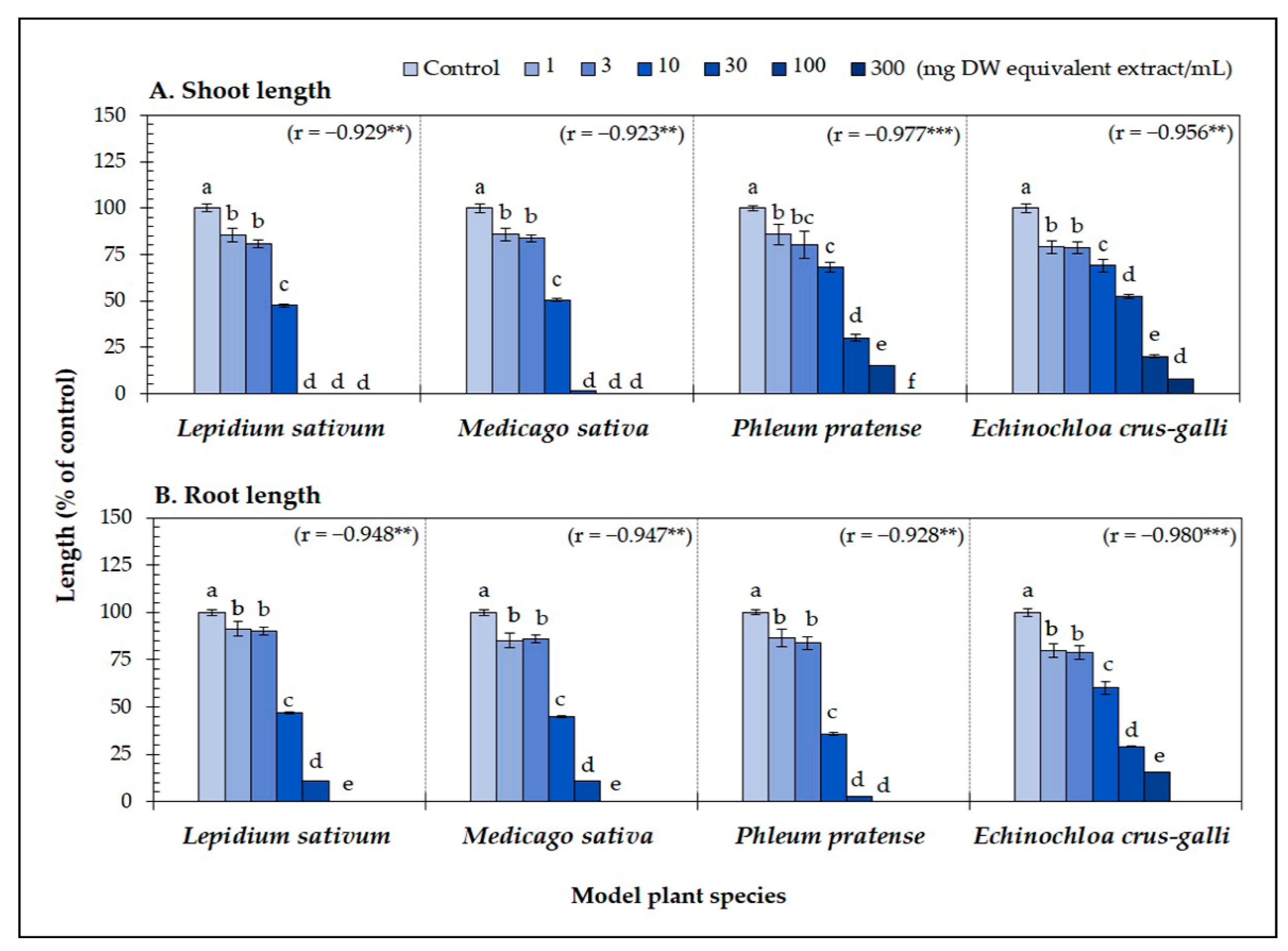
The A. xylocarpa leaf extracts significantly reduced the seedling length of the four model plants (p < 0.05) (Figure 1). At the concentration of 30 mg DW equivalent extract/mL, the shoots of L. sativum were completely inhibited, whereas the shoots of M. sativa, P. pratense, and E. crus-galli were inhibited to 1.68, 30.08, and 52.44% of control length, respectively (Figure 1A). In contrast to the shoot length, the leaf extracts inhibited the root length of L. sativum, M. sativa, P. pratense, and E. crus-galli to 10.85, 10.60, 2.65, and 29.04% of control length, respectively (Figure 1B). The phytotoxic activity of the leaf extracts against the model plants significantly correlated with the extract concentrations (r = −0.92 to −0.98, p < 0.01) (Figure 1A,B). The IC50 values of the leaf extracts for the seedling length of the model plants ranged from 6.42 to 24.21 mg DW equivalent extract/mL (Table 1). In addition, the leaf extracts inhibited the root length of L. sativum, P. pratense, and E. crus-galli more than the shoot length (P. pratense was most sensitive to the extracts; inhibition of its roots was 2.3-times greater than for its shoots).
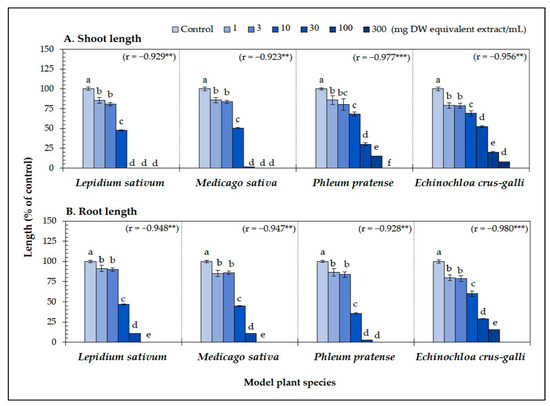
Figure 1.
Effect of the Afzelia xylocarpa leaf extracts on the (A) shoot and (B) root length of the model plants exposed to six concentrations. Means ± SE with six replicates (10 seeds/replicates). The different letters on the columns of each model plant indicate significant differences (Tukey’s HSD, p < 0.05). Statistical significance is represented by asterisks (two-tailed Pearson’s correlation, ** p < 0.01 and *** p < 0.001). correlation coefficient (r).

Table 1.
The concentration of the aqueous methanol leaf extracts and vanillic acid and trans-ferulic acid from Afzelia xylocarpa required for 50% inhibition (IC50 value) of the shoot and root length of the model plants.
These results suggest that the leaves of A. xylocarpa may contain phytotoxic substances with phytotoxic activity. In addition, the results indicate that seedling length varied among the model plants, and the level of inhibition depended on the extract concentration (Figure 1). Similarly, several previous studies have also reported that the phytotoxic effect is concentration-dependent [48,49,50,51,52,53]. Moreover, many previous studies noted that test plant species’ response varied depending on the types of phytotoxic substances [54]. Hence, it is important to understand which compounds play a major role in plant allelopathy to be used for weed control.
3.2. Isolation and Identification of the Active Substances in the A. xylocarpa Leaf Extracts
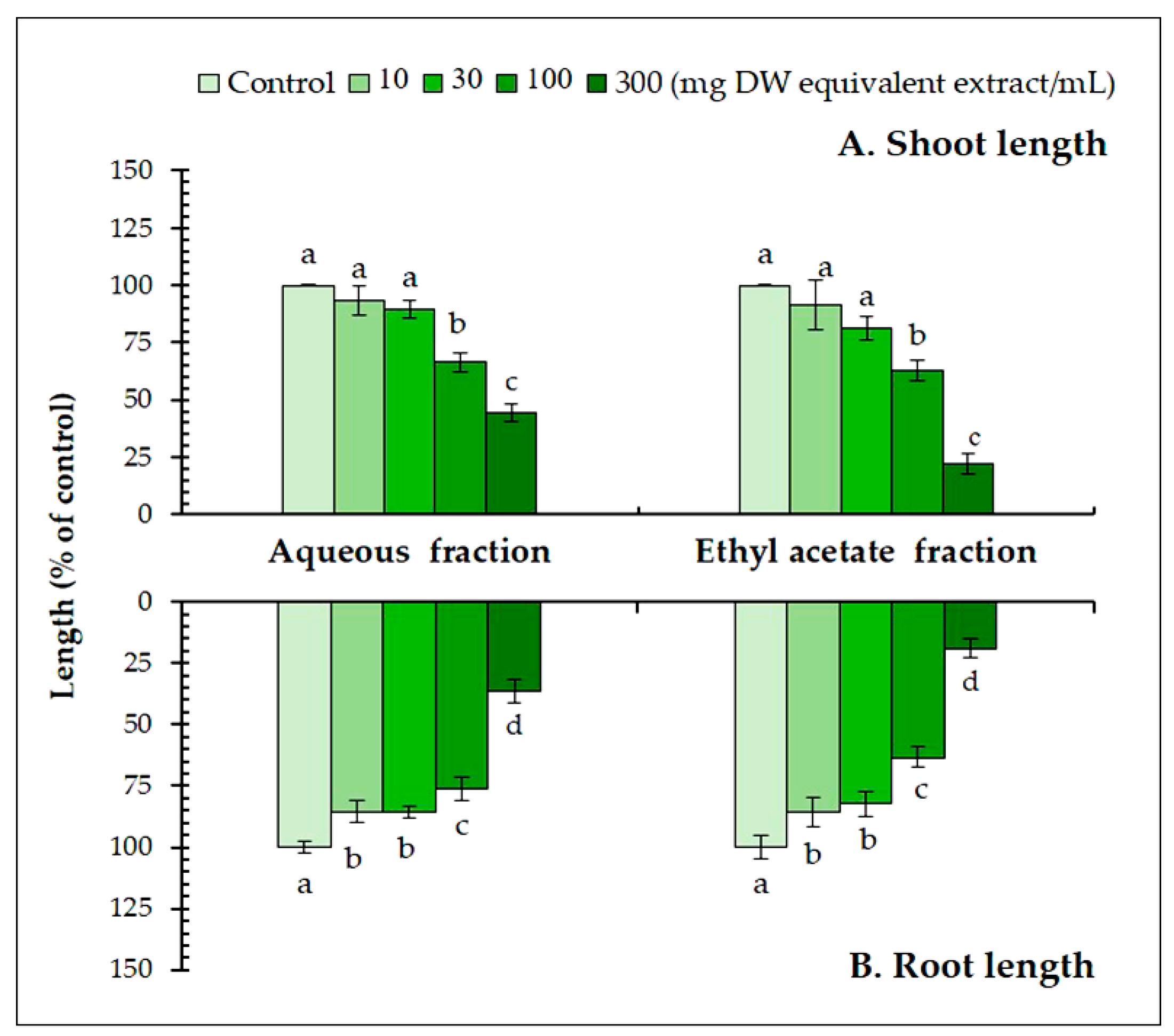
Partitioning showed that the ethyl acetate fraction had higher inhibitory activity than the aqueous fraction (Figure 2). At 300 mg DW equivalent extract/mL, the ethyl acetate fraction inhibited the seedling length of L. sativum by 1.9–2.1 times greater than that of the aqueous fraction. Therefore, the growth inhibitory substances in the ethyl acetate fraction were separated through a series of bioassay-guided fractionations. The most active fraction was then purified using HPLC, and two active substances were found. The molecular formula of substance 1 was determined as C8H8O4 using HRESIMS at m/z 167.03 [M−H] − (calcd. for C8H7O4, 167.03). The 1H NMR spectrum (400 MHz, CD3OD) of the substance showed δH 3.89 (3H, s), 7.56 (1H, d, J = 1.7), 7.55 (1H, dd, J = 8.7, 1.7), and 6.84 (1H, d, J = 8.7). The substance was identified as 4-hydroxy-3-methoxy benzoic acid (vanillic acid). Substance 2 was determined as C10H10O4 using HRESIMS at m/z 193.05 [M−H] − (calcd. for C10H9O4, 193.05). The 1H NMR spectrum (400 MHz, CD3OD) of the substance showed δH 3.90 (3H, s), 7.60 (1H, d, J = 15.9), 6.31 (1H, d, J =15.9), 7.18 (1H, d, J = 1.9), 7.06 (1H, dd, J = 8.1, 1.9), and 6.81 (1H, d, J = 8.1). The substance was identified as trans-4-hydroxy-3-methoxy cinnamic acid (trans-ferulic acid).
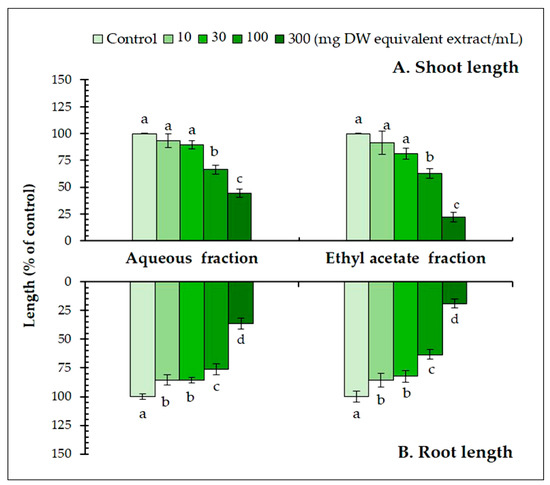
Figure 2.
The effect of the aqueous and ethyl acetate fractions on the (A) shoot and (B) root length of Lepidium sativum exposed to four concentrations. Means ± SE with six replicates (10 seeds/replicates). The different letters on each column indicate significant differences (Tukey’s HSD, p < 0.05).
Vanillic acid and trans-ferulic acid are phenolic compounds, biosynthesized from phenylalanine via the shikimic acid pathway in plants. Vanillic acid is a hydroxybenzoic acid derivative found in several natural sources [55,56,57]. This compound has been reported to have multiple activities, including anti-inflammatory, antimicrobial, anticancer, and antioxidant [58,59]. On the other hand, trans-ferulic acid, a hydroxycinnamic acid derivative, is present in many edible plants [60,61]. Many studies have found that trans-ferulic acid possesses different beneficial biological activities, including antioxidants [62,63]. To our best knowledge, A. xylocarpa leaves are a source of vanillic acid and trans -ferulic acid with phytotoxic effects.
3.3. Biological Activity of Vanillic Acid and Trans-Ferulic Acid
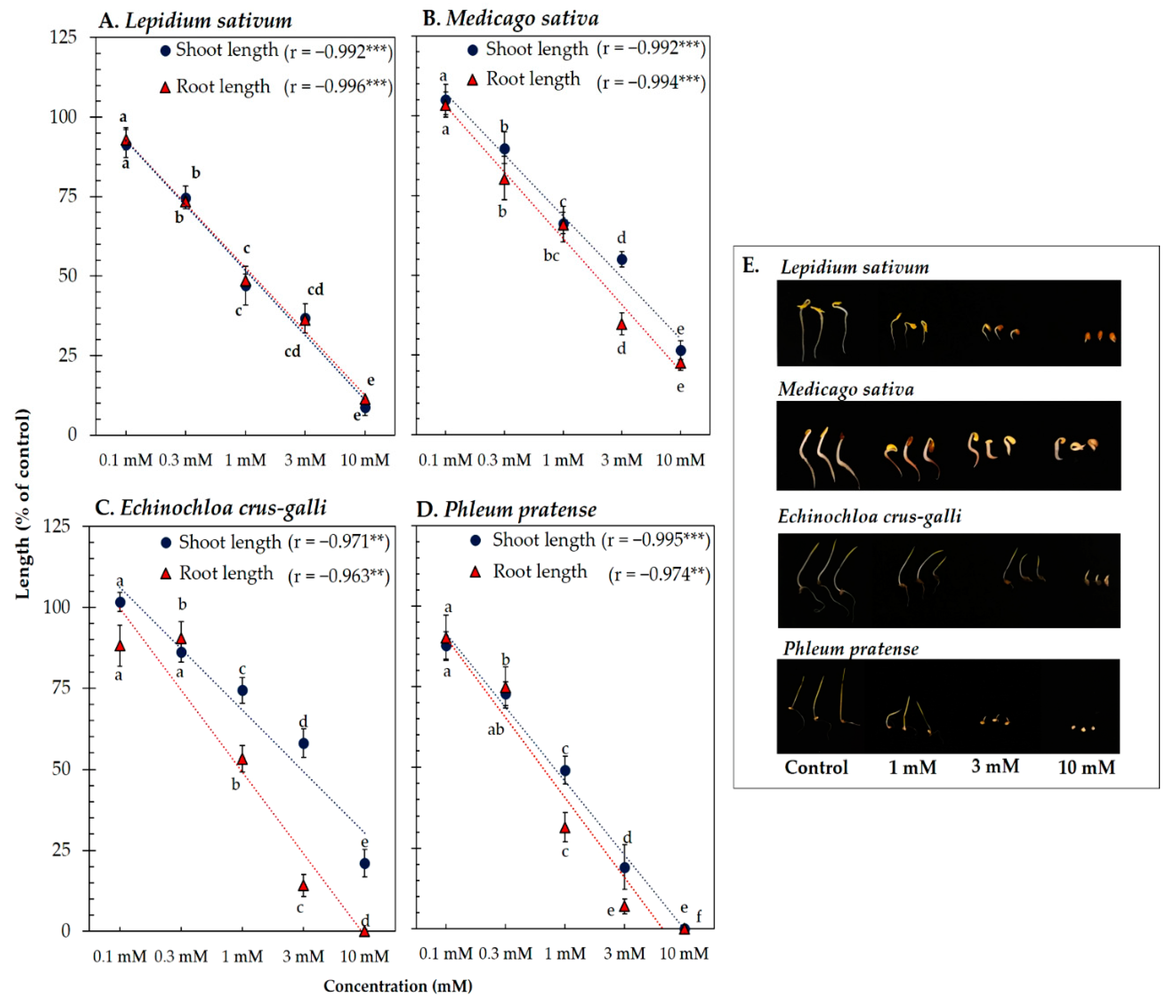
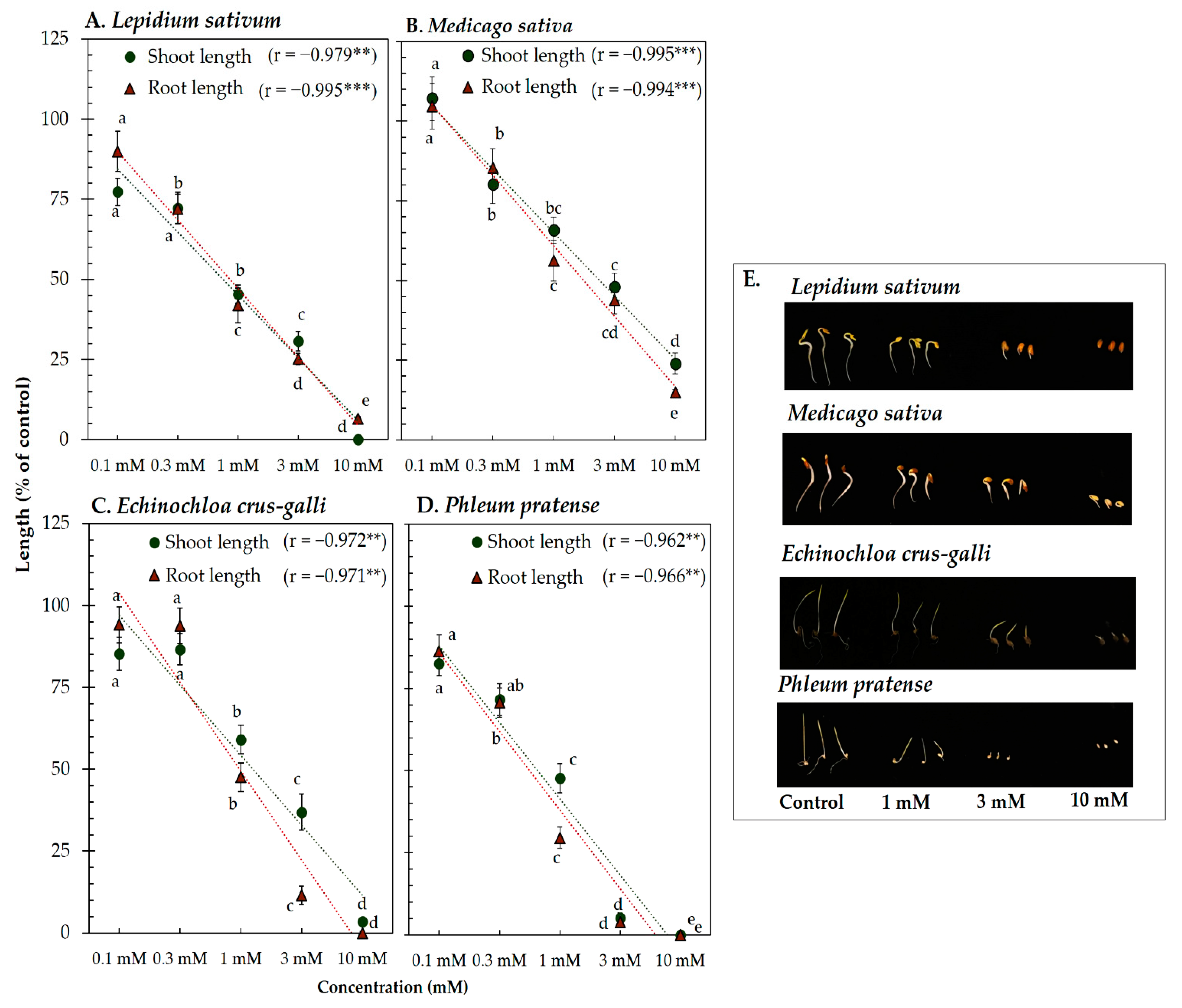
Vanillic acid and trans-ferulic acid showed phytotoxicity against the four model plants’ seedling length (Figure 3 and Figure 4). At the concentration 1 mM of vanillic acid, the shoot length of L. sativum, M. sativa, P. pratense, and E. crus-galli was inhibited to 47.42, 66.32, 74.34, and 66.87% of control length, respectively, whereas that of root length was inhibited to 47.79, 66.01, 63.22, and 54.75% of control length, respectively (Figure 3). On the other hand, 1 mM of trans-ferulic acid inhibited the shoot length of L. sativum, M. sativa, P. pratense, and E. crus-galli to 45.87, 65.81, 57.00, and 53.68% of control length, respectively, whereas root length was inhibited to 41.91, 56.25, 57.00, and 29.28% of control length, respectively (Figure 4). The correlation coefficient between vanillic acid and trans-ferulic acid and the seedling length of the model plants was very high, with values ranging from −0.962 to −0.996 (p < 0.01). The IC50 values of vanillic acid and trans-ferulic acid for the seedling length of the model plants were 0.73 to 3.17 mM and 0.42 to 2.43 mM, respectively (Table 1).
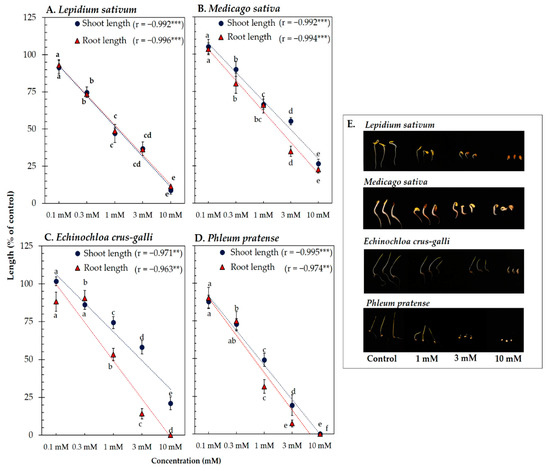
Figure 3.
Effect of vanillic acid isolated from the Afzelia xylocarpa leaf extracts on the model plants (A) Lepidium sativum, (B) Medicago sativa, (C) Phleum pratense, (D) Echinochloa crus-galli, and (E) effect of vanillic acid on the seedling length of the model plants after treatment for 48 h. Means ± SE with three replicates (10 seeds/replicates). The different letters along a curve indicate significant differences (Tukey’s HSD, p < 0.05). Statistical significance is represented by asterisks (two-tailed Pearson’s correlation, ** p < 0.01 and *** p < 0.001). correlation coefficient (r).
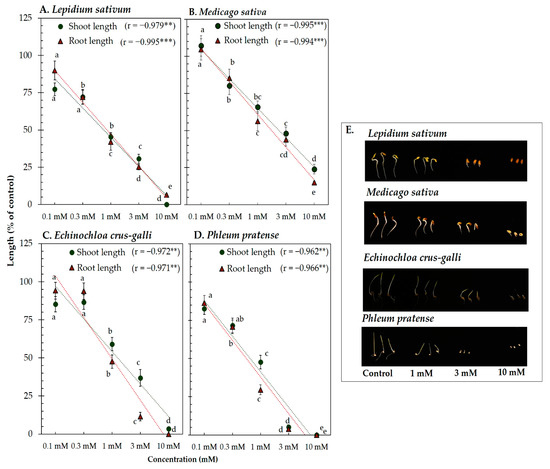
Figure 4.
Effect of trans-ferulic acid isolated from the Afzelia xylocarpa leaf extracts on the model plants (A) Lepidium sativum, (B) Medicago sativa, (C) Phleum pratense, (D) Echinochloa crus-galli, and (E) effect of trans-ferulic acid on the seedling length of the model plants after treatment for 48 h. Means ± SE with three replicates (10 seeds/replicates). The different letters along a curve indicate significant differences (Tukey’s HSD, p < 0.05). Statistical significance is represented by asterisks (two-tailed Pearson’s correlation, ** p < 0.01 and *** p < 0.001). correlation coefficient (r).
These results indicate that vanillic acid and trans-ferulic acid have phytotoxic activity against the seedling length of the model plants in a concentration-dependent manner. According to the results of the bioassay-directed fractionation, both compounds may be the main compounds responsible for the phytotoxic activity of A. xylocarpa leaf. These results are supported by previous studies, which reported that vanillic acid and trans-ferulic acid isolated from Triticum estivum and Saccharum officinarum act as potential phytotoxic chemicals [64,65]. In addition, the IC50 values showed the different responses of both compounds against the four model plants, indicating inhibition was species-specific (Table 1). Differences in phytotoxic effects of compounds against dicotyledonous and monocotyledonous plants have been found in many previous studies [66,67]. The different sensitivities of the model plant species may be related to the genetic, biochemical, and physiological characteristics of each specific species [68,69].
In the present study, the P. pratense seedlings (monocotyledonous weed) were found to be the most sensitive to the phytotoxic effects of vanillic acid and trans-ferulic acid. Of the model plants used in this study, the seeds of P. pratense were the smallest; hence, seed size may be one factor that contributed to the sensitivity to the phytotoxic substances. This supposition is consistent with the research of Leishman et al. [70], who noted that small-seeded species have greater root length per unit of root mass, which provides a greater surface area for the uptake of phytotoxic substances.
In addition, both compounds inducing the generation of reactive oxygen species (ROS) and oxidative stress may be responsible for the phytotoxic activity against test plant species [61,71,72]. Pezzani et al. [73] and Huang et al. [74] found that the accumulation of reactive oxygen species during seedling growth caused cellular damage, protein, nucleic acid, leading to irreversible damage and cell death. Nevertheless, the relationship between structure–activity and target plant species of both compounds is little understood. Both compounds contain a methoxy group at the C-3 position and a hydroxyl group at the C-4 position on the benzene ring skeleton, which may be important for their phytotoxic activity because of their radical-scavenging activity [62]. Moreover, the IC50 values indicate that the effectiveness of trans-ferulic acid against the seedling length of the model plants was greater than that of vanillic acid. The structural difference between the two compounds is present in the functional group at the C-1 position. Trans-ferulic acid contains the carboxylic acid group with the adjacent unsaturated carbon–carbon double bond in the functional group. It has strong electron-donating substituents that enhance its antioxidant properties compared with vanillic acid with only the carboxylic acid group [75].
3.4. The Synergistic Effects of the Mixture of Vanillic Acid and Trans-Ferulic Acid
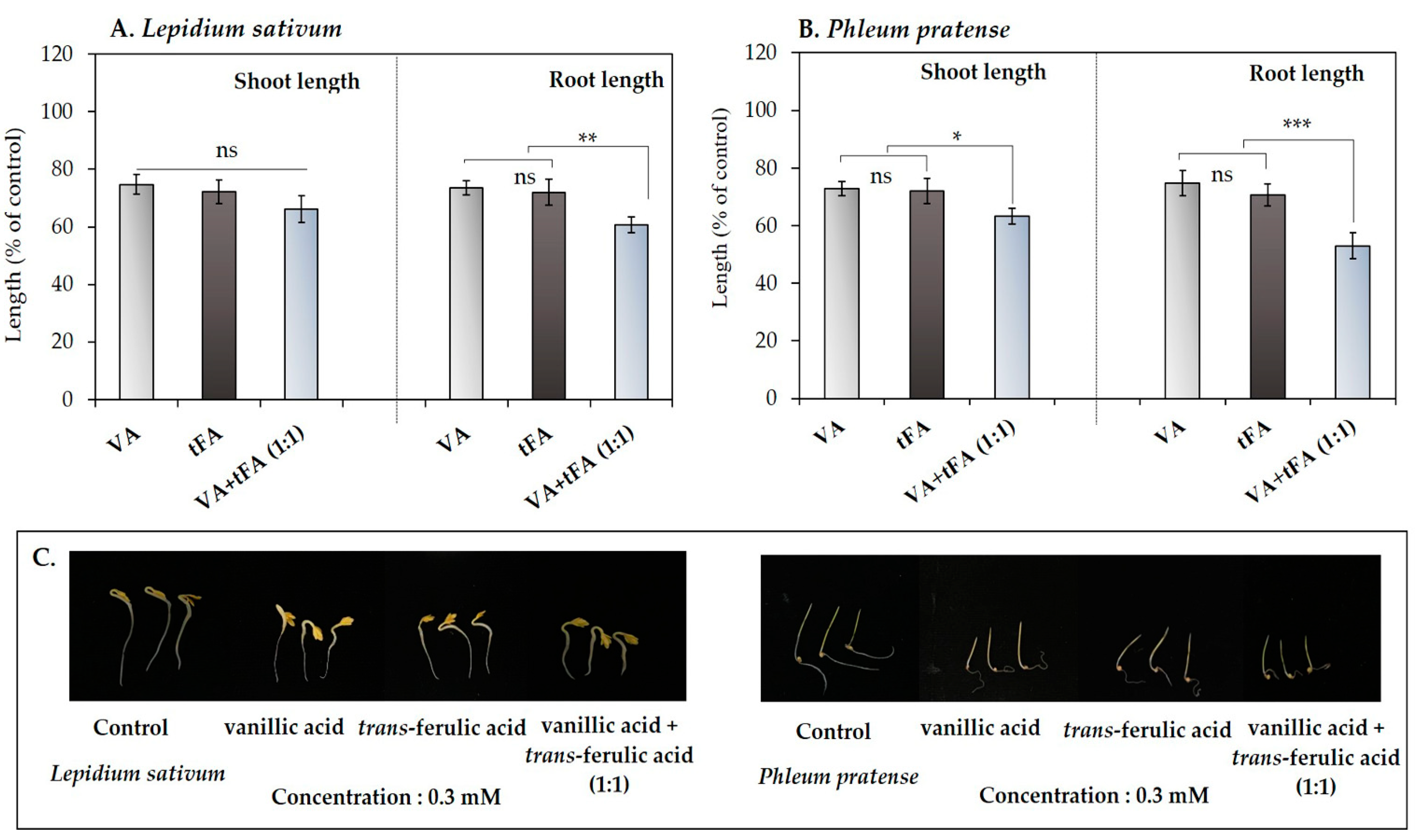
The results of using the compounds individually at a low concentration (0.3 mM) showed a <25% decrease in the seedling length of the model plants (Figure 3 and Figure 4). Therefore, the synergistic effects of the mixture of vanillic acid and trans-ferulic acid were determined at low concentrations. The results showed that the mixture of the two compounds inhibited the shoot and root length of L. sativum to 68.19 and 60.61% of control length, respectively (Figure 5A), whereas the P. pratense seedlings were inhibited to 63.22 and 52.97% of control length, respectively (Figure 5B). The effectiveness of the mixture of the compounds against the L. sativum roots was 1.21- and 1.15-times higher than that of vanillic acid and trans-ferulic acid alone, respectively. On the other hand, the effectiveness of the mixture of these compounds against the P. pratense shoots and roots was 1.15- and 1.41-times higher than that of vanillic acid, respectively, and 1.12- and 1.33-times higher than that of trans-ferulic acid alone, respectively.
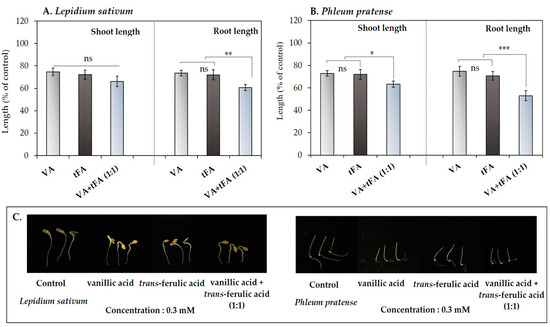
Figure 5.
Effects of the mixture of vanillic acid (VA) and trans-ferulic acid (tFA) on the model plants (A) Lepidium sativum and (B) Phleum pratense. (C) effect of the mixture compound on the model plants after treatment for 48 h. The reported values are expressed as mean ± SE with three replicates (10 seeds/replicates). The asterisks represent a statistically significant comparison between treatments according to Student’s t-test: * p < 0.05, ** p < 0.01, and *** p < 0.001.
Such inhibitory effects indicate that these two compounds work synergistically to inhibit the seedling growth of the model plants. These results support the findings of Blum [76], who reported that some phenolic compounds at low concentrations could have pronounced phytotoxic effects through synergistic action. The level of those synergistic actions depends on various factors, such as the chemical composition of each pair of compounds and the receptor species [77,78]. From our results, the mixture of vanillic acid and trans-ferulic acid inhibited the seedling growth of P. pratense more than L. sativum. Therefore, these results confirmed the synergistic effects of the phenolic compound mixture related to the target plant species. Consequently, the synergistic effects of the mixture of vanillic acid and trans-ferulic acid may lead to developing more effective activity against selected weeds and the application of smaller amounts of compounds in a mixture to achieve efficacy.
4. Conclusions
This study demonstrated the phytotoxic effect of A. xylocarpa leaf extracts on the model plants and identified vanillic acid and trans ferulic acid as the major bioactive compounds. Both compounds exhibited concentration- and species-dependent inhibition. Consequently, the concentration of those compounds and differences in the model plant’s sensitivity are keys to their phytotoxic action. Additionally, a mixture of both compounds at low concentrations had synergistic effects on phytotoxic activity. Our findings suggest that vanillic acid and trans-ferulic acid are responsible for the phytotoxic activity of A. xylocarpa leaves and may be useful for weed management. However, further experiments under field conditions are required to confirm our research findings.
Author Contributions
Conceptualization, R.K. and H.K.-N.; methodology, R.K., K.O., T.T., and H.K.-N.; software R.K.; validation, K.O., T.T., and H.K.-N.; formal analysis, R.K.; investigation, R.K.; data curation, H.K.-N.; writing (original draft preparation), R.K. and H.K.-N.; writing (review and editing), R.K. and H.K.-N; visualization, R.K.; supervision, H.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to Dennis Murphy, The United Graduate School of Agricultural Sciences, Ehime University, Japan, for editing the English of our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qasem, J.R.; Foy, C.L. Weed allelopathy, its ecological impacts and future prospects. J. Crop. Prod. 2001, 4, 43–119. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. J. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdisc. Toxicol. 2009, 2, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, J.P. Impact of pesticides application in agricultural industry: An Indian scenario. Int. J. Agric. Food Sci. Technol. 2013, 4, 817–822. [Google Scholar]
- Awan, T.H.; Cruz, P.C.S.; Chauhan, B.S. Agronomic indices, growth, yield-contributing traits, and yield of dry-seeded rice under varying herbicides. Field Crop. Res. 2015, 177, 15–25. [Google Scholar] [CrossRef]
- Délye, C. Unraveling the genetics bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1720. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Poolkum, S. Utilization of Bidens pilosa var. radiata (Sch. Bip.) Sherff integrated with water irrigation for paddy weed control and rice yield production. Weed Biol. Manag. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Kato-Noguchi, H. Allelopathic potential of Acacia pennata (L.) Willd. leaf extracts against the seedling growth of six test plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1534–1542. [Google Scholar] [CrossRef]
- Singh, N.B.; Pandey, B.N.; Singh, A. Allelopathic effects of Cyperus rotundus extract in vitro and ex vitro on banana. Acta Physiol. Plant 2009, 31, 633–638. [Google Scholar] [CrossRef]
- Abbas, T.; Tanveer, A.; Khaliq, A.; Safdar, M.E. Comparative allelopathic potential of native and invasive weeds in rice ecosystem. Pak. J. Weed. Sci. Res. 2016, 22, 269–283. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 67–68. [Google Scholar]
- Chon, S.U.; Nelson, C.J. Allelopathy in compositae plants. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef]
- Teerarak, M.; Charoenying, P.; Laosinwattana, C. Physiological and cellular mechanisms of natural herbicide resource from Aglaia odorata Lour. on bioassay plants. Acta Physiol. Plant 2012, 34, 1277–1285. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Ned. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suwitchayanon, P.; Boonmee, S.; Iwasaki, A.; Suenaga, K. Plant growth inhibitory activity of the extracts of Acmella oleracea and its growth inhibitory substances. Nat. Prod. Commun. 2019, 14, 1934578X19858805. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef]
- Rob, M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a novel compound involved in allelopathic activity of Garcinia Xanthochymus hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- El-Derek, M.H.; Hess, F.D. Inhibited mitotic entry is the cause of growth inhibition by cinmethylin. Weed Sci. 1986, 34, 684–688. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1, 2-isoxazolines: Putative inhibitors of plant tyrosine aminotransferase. Pest Manag. Sci. 2012, 68, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic activity of leaf and stem extracts and identification of a phytotoxic substance from Caesalpinia mimosoides Lamk. Theor. Exp. Plant Physiol. 2018, 30, 129–139. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Dalbergia cochinchinensis Pierre and its growth inhibitory substance. Emir. J. Food. Agric. 2020, 32, 513–521. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- El-Gawad, A.M.A.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018, 15, e1700438. [Google Scholar] [CrossRef]
- Quddus, M.S.; Bellairs, S.M.; Wurm, P.A. Acacia holosericea (Fabaceae) litter has allelopathic and physical effects on mission grass (Cenchrus pedicellatus and C. polystachios) (Poaceae) seedling establishment. Aust. J. Bot. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Pakkad, G.; Ueno, S.; Yoshimaru, H. Isolation and characterization of microsatellite loci in an endangered tree species, Afzelia xylocarpa (Kurz) Craib (Caesalpinioideae). Mol. Ecol. Resour. 2009, 9, 880–882. [Google Scholar] [CrossRef]
- Cai, X.; Liu, J.X.; Song, Q.S.; Chen, K.L.; Lu, Z.Y.; Zhang, Y.M. Chemical constituents of Afzelia xylocarpa. Chem. Nat. Compd. 2018, 54, 764–765. [Google Scholar] [CrossRef]
- Akah, P.A.; Okpi, O.; Okoli, C.O. Evaluation of the anti-inflammatory, analgesic and antimicrobial activities of bark of Afzelia africana. Niger. J. Nat. Prod. Med. 2007, 11, 48–52. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Adewusi, E.A.; Aiyegoro, O.A.; Akinpelu, D.A. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian. Pac. J. Trop. Med. 2011, 1, 353–358. [Google Scholar] [CrossRef]
- Akinpelu, D.A.; Aiyegoro, O.A.; Okoh, A.I. The in vitro antioxidant property of methanolic extract of Afzelia africana (Smith.). J. Med. Plant Res. 2010, 4, 2022–2027. [Google Scholar] [CrossRef]
- Pellissier, F.; Gallet, C.; Souto, X.C. Allelopathic interactions in forest ecosystems. In Allelopathy: From Molecules to Ecosystems; Reigosa, M.J., Nuria, P., Sanchez-Moreiras, A.M., Gonzales, L., Eds.; Science Publishers: Enfield, NH, USA, 2002; pp. 257–269. [Google Scholar]
- Kimura, F.; Sato, M.; Kato-Noguchi, H. Allelopathy of pine litter: Delivery of allelopathic substances into forest floor. J. Plant Biol. 2015, 58, 61–67. [Google Scholar] [CrossRef]
- Phuong, D.L.; Thuy, N.T.; Long, P.Q.; Kuo, P.C.; Thang, T.D. Composition of fatty acids, tocopherols, sterols, total phenolics, and antioxidant activity of seed oils of Afzelia xylocarpa and Cassia fistula. Chem. Nat. Compd. 2019, 55, 242–246. [Google Scholar] [CrossRef]
- Thanh, N.D.; Nghia, N.H. Genetic diversity of Afzelia xylocarpa (Kurz) Craib in Vietnam based on analyses of chloroplast markers and random amplified polymorphic DNA (RAPD). Afr. J. Biotechnol. 2012, 11, 14529–14535. [Google Scholar] [CrossRef]
- Yu, J.Q.; Shou, S.Y.; Qian, Y.R.; Zhu, Z.Z.; Hu, W.H. Autotoxic potential of cucurbit crops. Plant Soil. 2000, 223, 149–153. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils. 2018, 54, 683. [Google Scholar] [CrossRef]
- Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A potent phytotoxic substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. 2-Methoxystypandrone, a potent phytotoxic substance in Rumex maritimus L. Theor. Exp. Plant. Physiol. 2017, 29, 195–202. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichia, T.; Khanh, T.D.; Chung, M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Krumsri, R.; Boonmee, S.; Kato-Noguchi, H. Evaluation of the allelopathic potential of leaf extracts from Dischidia imbricata (Blume) Steud. on the seedling growth of six test plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1019–1024. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification of 6, 7-dimethoxychromone as a potent allelochemical from Jatropha podagrica. Nat. Prod. Commun. 2018, 13, 1934578X1801301126. [Google Scholar] [CrossRef]
- Zaman, F.; Islam, M.S.; Kato-Noguchi, H. Allelopathic activity of the Oxalis europea L. extracts on the growth of eight test plant species. Res Crop. 2018, 19, 304–309. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Jumpathong, J. Evaluating herbicidal potential of aqueous–ethanol extracts of local plant species against Echinochloa crus-galli and Raphanus sativus. Int. J. Agric. Biol. 2019, 21, 648–652. [Google Scholar] [CrossRef]
- Rob, M.M.; Kato-Noguchi, H. Study of the allelopathic activity of Garcinia pedunculata Roxb. Plant Omics 2019, 12, 31–36. [Google Scholar] [CrossRef]
- Boonmee, S.; Suwitchayanon, P.; Krumsri, R.; Kato-noguchi, H. Investigation of the allelopathic potential of Nephrolepis cordifolia (L.) C. Presl against dicotyledonous and monocotyledonous plant species. Environ. Control. Biol. 2020, 58, 71–78. [Google Scholar] [CrossRef]
- Krumsri, R.; Kato-Noguchi, H.; Poonpaiboonpipat, T. Allelopathic effect of Sphenoclea zeylanica Gaertn. on rice (Oryza sativa L.) germination and seedling growth. Aust. J. Crop Sci. 2020, 14, 1450–1455. [Google Scholar] [CrossRef]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Growth Inhibitory Substances from Albizia richardiana (Voigt.) King & Prain. Appl. Sci. 2021, 11, 1455. [Google Scholar] [CrossRef]
- Suwitchayanon, P.; Ohno, O.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic property of Piper retrofractum fruit extracts and compounds against the germination and seedling growth of weeds. Acta Physiol. Plant 2019, 41, 33. [Google Scholar] [CrossRef]
- Namkeleja, H.S.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effects of Argemone mexicana to growth of native plant species. Am. J. Plant Sci. 2014, 5, 1336–1344. [Google Scholar] [CrossRef]
- Pu, W.; Wang, D.; Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from Astragalus taipaishanensis and their structure-activity relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ghareib, H.R.A.; Abdelhamed, M.S.; Ibrahim, O.H. Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium muraleon tomato plants. Weed Biol. Manag. 2010, 10, 64–72. [Google Scholar] [CrossRef]
- Domínguez, C.R.; Dominguez Avila, J.A.; Pareek, S.; Villegas Ochoa, M.A.; Ayala Zavala, J.F.; Yahia, E. Content of bioactive compounds and their contribution to antioxidant capacity during ripening of pineapple (Ananas comosus L.) cv. Esmeralda. J. Appl. Bot. Food Qual. 2018, 91, 61–68. [Google Scholar] [CrossRef]
- Archivio, D.M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Zavala-López, M.; Flint-García, S.; García-Lara, S. Compositional variation in trans-ferulic, p-coumaric, and diferulic acids levels among kernels of modern and traditional maize (Zea mays L.) hybrids. Front. Nutr. 2020, 7, 600747. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.; Nigro, R.L.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Variation of phenolic acids in shoot tissues. J. Chem. Ecol. 2001, 27, 125–135. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) straw. J. Plant Physiol. 2006, 163, 837–846. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [PubMed]
- Jmii, G.; Molinillo, J.M.; Zorrilla, J.G.; Haouala, R. Allelopathic activity of Thapsia garganica L. leaves on lettuce and weeds, and identification of the active principles. S. Afr. J. Bot. 2020, 131, 188–194. [Google Scholar] [CrossRef]
- Zakaria, W.; Razak, A.R. Effects of groundnut plant residues on germination and radicle elongation of four crop species. Pertanika 1990, 13, 297–302. [Google Scholar]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The evolutionary ecology of seed size. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 31–57. [Google Scholar] [CrossRef]
- Gan, X.; Hu, D.; Wang, Y.; Yu, L.; Song, B. Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction. J. Agric. Food Chem. 2017, 65, 4367–4377. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ 1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Jinxiang, C.; Yang, J.; Lanlan, M.; Li, J.; Nasir, S.; Kyung, K.C. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259–267. [Google Scholar] [PubMed]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Physiol. Plant 2002, 114, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Kudsk, P.; Mathiassen, S.K. Joint action of benzoxazinone derivatives and phenolic acids. J. Agric. Food Chem. 2006, 54, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).