Current Management and Therapeutic Strategies for Cerebral Amyloid Angiopathy
Abstract
1. Introduction
2. Neuropathology of CAA-Affected Vessels
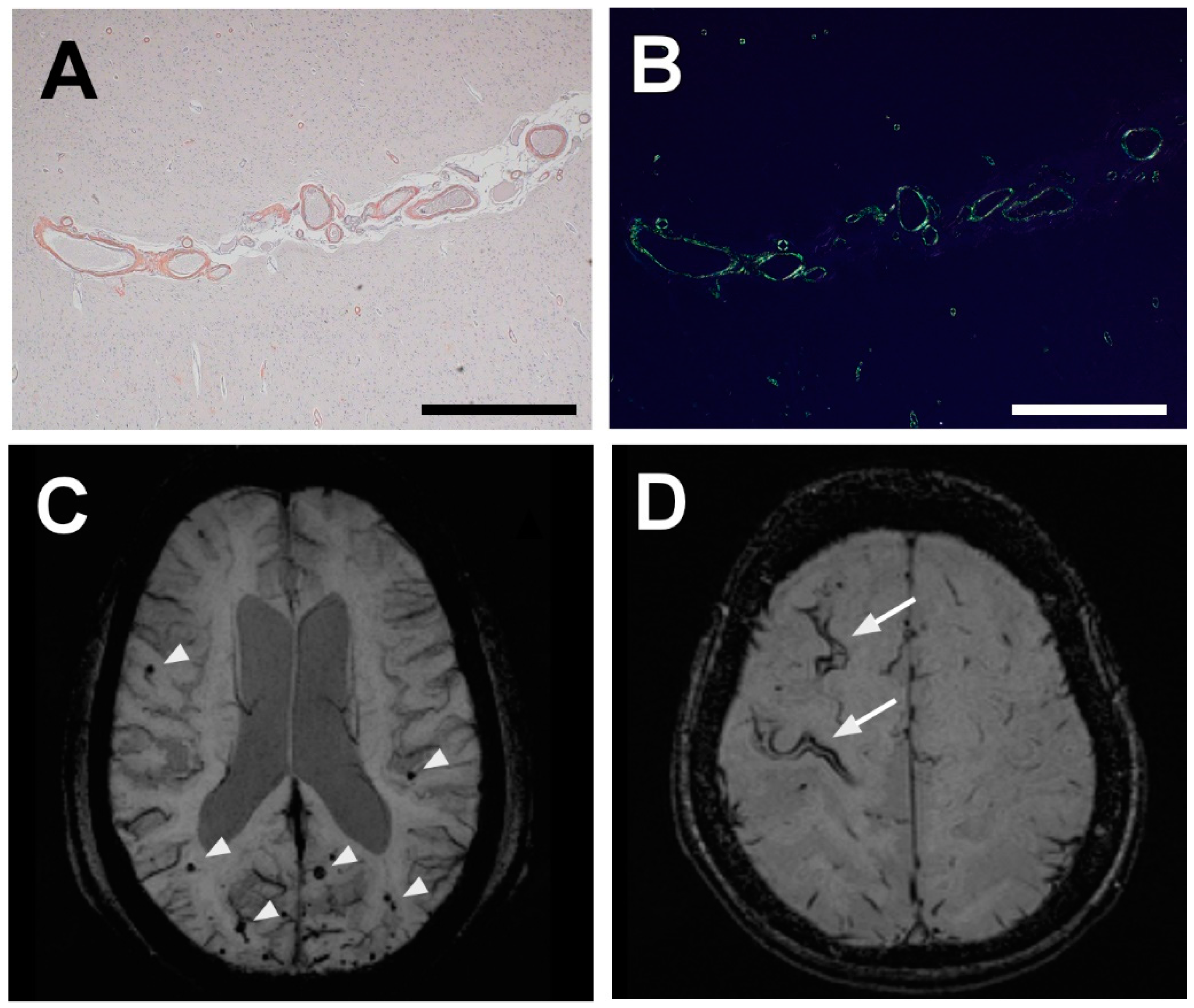
3. Diagnosis of CAA: Markers, Characteristic Features, and Techniques
4. Management of CAA, Af, and ICH in Clinical Settings
5. Promising Therapeutic Targets for CAA
6. Key Molecules in CAA Identified by Means of Proteomic Analyses
7. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAA | Cerebral amyloid angiopathy |
| ICH | Intracerebral hemorrhage |
| AD | Alzheimer’s disease |
| Aβ | Amyloid β |
| TFNEs | Transient focal neurological events |
| WMH | White matter hyperintensity |
| CAA-ri | CAA-related inflammation |
| PiB | 11C-labeled Pittsburgh compound B |
| PET | Positron emission tomography |
| AF | Atrial fibrillation |
| IPAD | Intramural periarterial drainage |
| CSF | Cerebrospinal fluid |
| SRPX1 | Sushi repeat-containing protein X-linked 1 |
| TIMP3 | Tissue inhibitor of metalloproteinases 3 |
| HTRA1 | HtrA serine peptidase 1 |
| LRP-1 | Low-density lipoprotein receptor-related protein 1 |
| ApoE | Apolipoprotein E |
| IDE | Insulin-degrading enzyme |
| BBB | Blood-brain barrier |
| ECM | Extracellular matrix |
| CMBs | Cerebral microbleeds |
| cSS | Cortical superficial siderosis |
| MRI | Magnetic resonance imaging |
| SWI | Susceptibility-weighted imaging |
| NMDAR | NMDA receptor |
| UPS | Ubiquitin-proteasome system |
References
- Viswanathan, A.; Greenberg, S.M. Cerebral amyloid angiopathy in the elderly. Ann. Neurol. 2011, 70, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Keage, H.A.; Carare, R.O.; Friedland, R.P.; Ince, P.G.; Love, S.; Nicoll, J.A.; Wharton, S.B.; Weller, R.O.; Brayne, C. Population studies of sporadic cerebral amyloid angiopathy and dementia: A systematic review. BMC Neurol. 2009, 9, 3. [Google Scholar] [CrossRef]
- Xuereb, J.H.; Brayne, C.; Dufouil, C.; Gertz, H.; Wischik, C.; Harrington, C.; Mukaetova-Ladinska, E.; McGee, M.A.; O’Sullivan, A.; O’Connor, D.; et al. Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann. N. Y. Acad. Sci. 2000, 903, 490–496. [Google Scholar] [CrossRef]
- Neuropathology Group: Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001, 357, 169–175. [Google Scholar] [CrossRef]
- Tanskanen, M.; Lindsberg, P.J.; Tienari, P.J.; Polvikoski, T.; Sulkava, R.; Verkkoniemi, A.; Rastas, S.; Paetau, A.; Kiuru-Enari, S. Cerebral amyloid angiopathy in a 95+ cohort: Complement activation and apolipoprotein E (ApoE) genotype. Neuropathol. Appl. Neurobiol. 2005, 31, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, A.; Strbian, D.; Putaala, J.; Curtze, S.; Haapaniemi, E.; Mustanoja, S.; Sairanen, T.; Satopää, J.; Silvennoinen, H.; Niemelä, M.; et al. SMASH-U: A proposal for etiologic classification of intracerebral hemorrhage. Stroke 2012, 43, 2592–2597. [Google Scholar] [CrossRef]
- Inoue, Y.; Miyashita, F.; Minematsu, K.; Toyoda, K. Clinical characteristics and outcomes of intracerebral hemorrhage in very elderly. J. Stroke Cerebrovasc. Dis. 2018, 27, 97–102. [Google Scholar] [CrossRef]
- Charidimou, A.; Imaizumi, T.; Moulin, S.; Biffi, A.; Samarasekera, N.; Yakushiji, Y.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.C.; et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology 2017, 89, 820–829. [Google Scholar] [CrossRef]
- Gravina, S.A.; Ho, L.; Eckman, C.B.; Long, K.E.; Otvos, L., Jr.; Younkin, L.H.; Suzuki, N.; Younkin, S.G. Amyloid β protein (Aβ) in Alzheimer’s disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ40 or Aβ42(43). J. Biol. Chem. 1995, 270, 7013–7016. [Google Scholar] [CrossRef]
- Miller, D.L.; Papayannopoulos, I.A.; Styles, J.; Bobin, S.A.; Lin, Y.Y.; Biemann, K.; Iqbal, K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer’s disease. Arch. Biochem. Biophys. 1993, 301, 41–52. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yamazaki, T.; Lemere, C.A.; Frosch, M.P.; Selkoe, D.J. Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease. An immunoelectron microscopic study. Am. J. Pathol. 1992, 141, 249–259. [Google Scholar] [PubMed]
- Gilbert, J.J.; Vinters, H.V. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke 1983, 14, 915–923. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Samarasekera, N.; Lerpiniere, C.; Humphreys, C.; McCarron, M.O.; White, P.M.; Nicoll, J.A.R.; Sudlow, C.L.M.; Cordonnier, C.; Wardlaw, J.M.; et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: Model development and diagnostic test accuracy study. Lancet Neurol. 2018, 17, 232–240. [Google Scholar] [CrossRef]
- Linn, J.; Halpin, A.; Demaerel, P.; Ruhland, J.; Giese, A.D.; Dichgans, M.; van Buchem, M.A.; Bruckmann, H.; Greenberg, S.M. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010, 74, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Kleinert, R.; Roob, G.; Kleinert, G.; Kapeller, P.; Schmidt, R.; Hartung, H.P. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am. J. Neuroradiol. 1999, 20, 637–642. [Google Scholar] [PubMed]
- Cordonnier, C.; Al-Shahi Salman, R.; Wardlaw, J. Spontaneous brain microbleeds: Systematic review, subgroup analyses and standards for study design and reporting. Brain 2007, 130, 1988–2003. [Google Scholar] [CrossRef]
- Vernooij, M.W.; van der Lugt, A.; Ikram, M.A.; Wielopolski, P.A.; Niessen, W.J.; Hofman, A.; Krestin, G.P.; Breteler, M.M. Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology 2008, 70, 1208–1214. [Google Scholar] [CrossRef]
- Martinez-Ramirez, S.; Romero, J.R.; Shoamanesh, A.; McKee, A.C.; Van Etten, E.; Pontes-Neto, O.; Macklin, E.A.; Ayres, A.; Auriel, E.; Himali, J.J.; et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015, 11, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Martola, J.; Charidimou, A.; Cavallin, L.; Granberg, T.; Shams, M.; Forslin, Y.; Aspelin, P.; Kristoffersen-Wiberg, M.; Wahlund, L.O. Cortical superficial siderosis: Prevalence and biomarker profile in a memory clinic population. Neurology 2016, 87, 1110–1117. [Google Scholar] [CrossRef]
- Inoue, Y.; Nakajima, M.; Uetani, H.; Hirai, T.; Ueda, M.; Kitajima, M.; Utsunomiya, D.; Watanabe, M.; Hashimoto, M.; Ikeda, M.; et al. Diagnostic significance of cortical superficial siderosis for Alzheimer disease in patients with cognitive impairment. AJNR Am. J. Neuroradiol 2016, 37, 223–227. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Greenberg, S.M.; Viswanathan, A. Cortical superficial siderosis and bleeding risk in cerebral amyloid angiopathy: A meta-analysis. Neurology 2019, 93, e2192–e2202. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Martinez-Ramirez, S.; Shoamanesh, A.; Oliveira-Filho, J.; Frosch, M.; Vashkevich, A.; Ayres, A.; Rosand, J.; Gurol, M.E.; Greenberg, S.M.; et al. Cerebral amyloid angiopathy with and without hemorrhage: Evidence for different disease phenotypes. Neurology 2015, 84, 1206–1212. [Google Scholar] [CrossRef]
- Charidimou, A.; Linn, J.; Vernooij, M.W.; Opherk, C.; Akoudad, S.; Baron, J.C.; Greenberg, S.M.; Jäger, H.R.; Werring, D.J. Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015, 138, 2126–2139. [Google Scholar] [CrossRef]
- Martínez-Lizana, E.; Carmona-Iragui, M.; Alcolea, D.; Gómez-Choco, M.; Vilaplana, E.; Sánchez-Saudinós, M.B.; Clarimón, J.; Hernández-Guillamon, M.; Munuera, J.; Gelpi, E.; et al. Cerebral amyloid angiopathy-related atraumatic convexal subarachnoid hemorrhage: An ARIA before the tsunami. J. Cereb. Blood Flow Metab. 2015, 35, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Peeters, A.; Fox, Z.; Gregoire, S.M.; Vandermeeren, Y.; Laloux, P.; Jäger, H.R.; Baron, J.C.; Werring, D.J. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: Multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012, 43, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Pasi, M.; Auriel, E.; van Etten, E.S.; Haley, K.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Goldstein, J.N.; et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017, 88, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ramirez, S.; Pontes-Neto, O.M.; Dumas, A.P.; Auriel, E.; Halpin, A.; Quimby, M.; Gurol, M.E.; Greenberg, S.M.; Viswanathan, A. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013, 80, 1551–1556. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Standards for reporting vascular changes on neuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Haley, K.; Auriel, E.; van Etten, E.S.; Fotiadis, P.; Reijmer, Y.; Ayres, A.; Vashkevich, A.; Dipucchio, Z.Y.; et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016, 86, 505–511. [Google Scholar] [CrossRef]
- Thanprasertsuk, S.; Martinez-Ramirez, S.; Pontes-Neto, O.M.; Ni, J.; Ayres, A.; Reed, A.; Swords, K.; Gurol, M.E.; Greenberg, S.M.; Viswanathan, A. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology 2014, 83, 794–800. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Chabriat, H.; Godin, O.; Dufouil, C.; Rosand, J.; Greenberg, S.M.; Smith, E.E.; Tzourio, C.; Viswanathan, A. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J. Neurol. 2012, 259, 530–536. [Google Scholar] [CrossRef]
- Lou, M.; Al-Hazzani, A.; Goddeau, R.P., Jr.; Novak, V.; Selim, M. Relationship between white-matter hyperintensities and hematoma volume and growth in patients with intracerebral hemorrhage. Stroke 2010, 41, 34–40. [Google Scholar] [CrossRef]
- Charidimou, A.; Gang, Q.; Werring, D.J. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry 2012, 83, 124–137. [Google Scholar] [CrossRef]
- Smith, E.E.; Gurol, M.E.; Eng, J.A.; Engel, C.R.; Nguyen, T.N.; Rosand, J.; Greenberg, S.M. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004, 63, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Kinnecom, C.; Lev, M.H.; Wendell, L.; Smith, E.E.; Rosand, J.; Frosch, M.P.; Greenberg, S.M. Course of cerebral amyloid angiopathy-related inflammation. Neurology 2007, 68, 1411–1416. [Google Scholar] [CrossRef]
- Auriel, E.; Charidimou, A.; Gurol, M.E.; Ni, J.; Van Etten, E.S.; Martinez-Ramirez, S.; Boulouis, G.; Piazza, F.; DiFrancesco, J.C.; Frosch, M.P.; et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016, 73, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Scolding, N.J.; Joseph, F.; Kirby, P.A.; Mazanti, I.; Gray, F.; Mikol, J.; Ellison, D.; Hilton, D.A.; Williams, T.L.; MacKenzie, J.M.; et al. Aβ-related angiitis: Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005, 128, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Gilson, A.; Rost, N.S.; Rosand, J.; Viswanathan, A.; Smith, E.E.; Greenberg, S.M. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 2009, 72, 1230–1235. [Google Scholar] [CrossRef]
- Ter Telgte, A.; Scherlek, A.A.; Reijmer, Y.D.; van der Kouwe, A.J.; van Harten, T.; Duering, M.; Bacskai, B.J.; de Leeuw, F.E.; Frosch, M.P.; Greenberg, S.M.; et al. Histopathology of diffusion-weighted imaging-positive lesions in cerebral amyloid angiopathy. Acta Neuropathol. 2020, 139, 799–812. [Google Scholar] [CrossRef]
- Fotiadis, P.; van Rooden, S.; van der Grond, J.; Schultz, A.; Martinez-Ramirez, S.; Auriel, E.; Reijmer, Y.; van Opstal, A.M.; Ayres, A.; Schwab, K.M.; et al. Cortical atrophy in patients with cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2016, 15, 811–819. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Gurol, M.E.; Dierksen, G.; Betensky, R.; Gidicsin, C.; Halpin, A.; Becker, A.; Carmasin, J.; Ayres, A.; Schwab, K.; Viswanathan, A.; et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology 2012, 79, 320–326. [Google Scholar] [CrossRef]
- Farid, K.; Charidimou, A.; Baron, J.C. Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: A systematic critical update. Neuroimage Clin. 2017, 15, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.M.; Kremer, B.P.; Rikkert, M.O.; Van Domburg, P.H.; Skehan, M.E.; Greenberg, S.M. Cerebrospinal fluid amyloid β40 is decreased in cerebral amyloid angiopathy. Ann. Neurol. 2009, 66, 245–249. [Google Scholar] [CrossRef]
- Renard, D.; Castelnovo, G.; Wacongne, A.; Le Floch, A.; Thouvenot, E.; Mas, J.; Gabelle, A.; Labauge, P.; Lehmann, S. Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria. J. Neurol. 2012, 259, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Renard, D.; Gabelle, A.; Hirtz, C.; Demattei, C.; Thouvenot, E.; Lehmann, S. Cerebrospinal fluid Alzheimer’s disease biomarkers in isolated supratentorial cortical superficial siderosis. J. Alzheimers Dis. 2016, 54, 1291–1295. [Google Scholar] [CrossRef]
- Goos, J.D.; Kester, M.I.; Barkhof, F.; Klein, M.; Blankenstein, M.A.; Scheltens, P.; van der Flier, W.M. Patients with Alzheimer disease with multiple microbleeds: Relation with cerebrospinal fluid biomarkers and cognition. Stroke 2009, 40, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yamamoto, T.; Kalaria, R.N.; Senzaki, H.; Maki, T.; Hase, Y.; Kitamura, A.; Washida, K.; Yamada, M.; Ito, H.; et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012, 123, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.T.; Poulet, R.; Di Pardo, A.; Cifelli, G.; Maffei, A.; Vecchione, C.; Passarelli, F.; Landolfi, A.; Carullo, P.; Lembo, G. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol. Aging 2009, 30, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Sato, N.; Uchio-Yamada, K.; Sawada, K.; Kunieda, T.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Rakugi, H.; Morishita, R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 7036–7041. [Google Scholar] [CrossRef] [PubMed]
- Eckman, M.H.; Rosand, J.; Knudsen, K.A.; Singer, D.E.; Greenberg, S.M. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003, 34, 1710–1716. [Google Scholar] [CrossRef]
- Murthy, S.B.; Gupta, A.; Merkler, A.E.; Navi, B.B.; Mandava, P.; Iadecola, C.; Sheth, K.N.; Hanley, D.F.; Ziai, W.C.; Kamel, H. Restarting anticoagulant therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke 2017, 48, 1594–1600. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Larsen, T.B.; Skjøth, F.; Gorst-Rasmussen, A.; Rasmussen, L.H.; Lip, G.Y. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A nationwide cohort study. Circulation 2015, 132, 517–525. [Google Scholar] [CrossRef]
- Kuramatsu, J.B.; Gerner, S.T.; Schellinger, P.D.; Glahn, J.; Endres, M.; Sobesky, J.; Flechsenhar, J.; Neugebauer, H.; Jüttler, E.; Grau, A.; et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015, 313, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar]
- Wilson, D.; Hostettler, I.C.; Ambler, G.; Banerjee, G.; Jäger, H.R.; Werring, D.J. Convexity subarachnoid haemorrhage has a high risk of intracerebral haemorrhage in suspected cerebral amyloid angiopathy. J. Neurol. 2017, 264, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, P.; Manjila, S.; Ostergard, T.; Eisele, S.; Cohen, M.L.; Sila, C.; Selman, W.R. Cerebral amyloid angiopathy-associated intracerebral hemorrhage: Pathology and management. Neurosurg. Focus 2012, 32, E7. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E.; Kawashima-Morishima, M.; Lee, H.J.; Hama, E.; Sekine-Aizawa, Y.; et al. Identification of the major Aβ1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.J.; Saido, T.C. Metabolic regulation of brain Aβ by neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef]
- Bourassa, P.; Tremblay, C.; Schneider, J.A.; Bennett, D.A.; Calon, F. Beta-amyloid pathology in human brain microvessel extracts from the parietal cortex: Relation with cerebral amyloid angiopathy and Alzheimer’s disease. Acta Neuropathol. 2019, 137, 801–823. [Google Scholar] [CrossRef]
- Iwata, N.; Mizukami, H.; Shirotani, K.; Takaki, Y.; Muramatsu, S.; Lu, B.; Gerard, N.P.; Gerard, C.; Ozawa, K.; Saido, T.C. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-β peptide in mouse brain. J. Neurosci. 2004, 24, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Ansorge, S.; Riederer, P.; Reiser, M.; Frölich, L.; Bogerts, B. Insulin-degrading enzyme in the Alzheimer’s disease brain: Prominent localization in neurons and senile plaques. Neurosci. Lett. 1999, 263, 161–164. [Google Scholar] [CrossRef]
- Cook, D.G.; Leverenz, J.B.; McMillan, P.J.; Kulstad, J.J.; Ericksen, S.; Roth, R.A.; Schellenberg, G.D.; Jin, L.W.; Kovacina, K.S.; Craft, S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-ε4 allele. Am. J. Pathol. 2003, 162, 313–319. [Google Scholar] [CrossRef]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.C.; Eckman, E.A.; Sambamurti, K.; Dobbs, N.; Chow, K.M.; Eckman, C.B.; Hersh, L.B.; Thiele, D.L. Amyloid-β peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 6221–6226. [Google Scholar] [CrossRef] [PubMed]
- Stargardt, A.; Gillis, J.; Kamphuis, W.; Wiemhoefer, A.; Kooijman, L.; Raspe, M.; Benckhuijsen, W.; Drijfhout, J.W.; Hol, E.M.; Reits, E. Reduced amyloid-β degradation in early Alzheimer’s disease but not in the APPswePS1dE9 and 3xTg-AD mouse models. Aging Cell 2013, 12, 499–507. [Google Scholar] [CrossRef]
- Inoue, Y.; Ueda, M.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Memantine, a noncompetitive N-methyl-D-aspartate receptor antagonist, attenuates cerebral amyloid angiopathy by increasing insulin-degrading enzyme expression. Mol. Neurobiol. 2019, 56, 8573–8588. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chang, J.; Guo, S.; Zhang, Q.; Wang, Z. ApoE 4 reduces the expression of Aβ degrading enzyme IDE by activating the NMDA receptor in hippocampal neurons. Neurosci. Lett. 2009, 464, 140–145. [Google Scholar] [CrossRef]
- Lopez Salon, M.; Pasquini, L.; Besio Moreno, M.; Pasquini, J.M.; Soto, E. Relationship between beta-amyloid degradation and the 26S proteasome in neural cells. Exp. Neurol. 2003, 180, 131–143. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Liu, C.C.; Shinohara, M.; Li, J.; Bu, G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J. Neurosci. 2012, 32, 16458–16465. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal clearance of amyloid-β by endocytic receptor LRP1. J. Neurosci. 2013, 33, 19276–19283. [Google Scholar] [CrossRef]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal enhances amyloid-β clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Jeffrey, M.; Kalaria, R.N.; Weller, R.O. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 2013, 39, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Hawkes, C.A.; Kalaria, R.N.; Werring, D.J.; Carare, R.O. White matter changes in dementia: Role of impaired drainage of interstitial fluid. Brain Pathol. 2015, 25, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, J.A.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nat. Med. 2003, 9, 448–452. [Google Scholar] [CrossRef]
- Bakker, E.N.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.; Weller, R.O.; Carare, R.O. Lymphatic clearance of the brain: Perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef]
- Weller, R.O.; Subash, M.; Preston, S.D.; Mazanti, I.; Carare, R.O. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008, 18, 253–266. [Google Scholar] [CrossRef]
- Schley, D.; Carare-Nnadi, R.; Please, C.P.; Perry, V.H.; Weller, R.O. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006, 238, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Yao, X.; Dix, J.A.; Jin, B.J.; Verkman, A.S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 2017, 6, e27679. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Vitek, M.P.; Colton, C.A. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 2009, 159, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Okamoto, Y.; Carare, R.O.; Hase, Y.; Hattori, Y.; Hawkes, C.A.; Saito, S.; Yamamoto, Y.; Terasaki, Y.; Ishibashi-Ueda, H.; et al. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular β-amyloid. Ann. Clin. Transl. Neurol. 2014, 1, 519–533. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R.; et al. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017, 5, 26. [Google Scholar] [CrossRef]
- Yan, P.; Zhu, A.; Liao, F.; Xiao, Q.; Kraft, A.; Gonzales, E.; Perez, R.; Greenberg, S.M.; Holtzman, D.; Lee, J.M. Minocycline reduces spontaneous hemorrhage in mouse models of cerebral amyloid angiopathy. Stroke 2015, 46, 1633–1640. [Google Scholar] [CrossRef]
- Arima, H.; Tzourio, C.; Anderson, C.; Woodward, M.; Bousser, M.G.; MacMahon, S.; Neal, B.; Chalmers, J.; PROGRESS Collaborative Group. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: The PROGRESS trial. Stroke 2010, 41, 394–396. [Google Scholar] [CrossRef]
- Inoue, Y.; Ueda, M.; Tasaki, M.; Takeshima, A.; Nagatoshi, A.; Masuda, T.; Misumi, Y.; Kosaka, T.; Nomura, T.; Mizukami, M.; et al. Sushi repeat-containing protein 1: A novel disease-associated molecule in cerebral amyloid angiopathy. Acta Neuropathol. 2017, 134, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, K.; Muszewska, A.; Lenart, A.; Szczepińska, T.; Godzik, A.; Grynberg, M. A widespread peroxiredoxin-like domain present in tumor suppression- and progression-implicated proteins. BMC Genom. 2010, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Shimakage, M.; Kawahara, K.; Kikkawa, N.; Sasagawa, T.; Yutsudo, M.; Inoue, H. Down-regulation of drs mRNA in human colon adenocarcinomas. Int. J. Cancer 2000, 87, 5–11. [Google Scholar] [CrossRef]
- Tambe, Y.; Isono, T.; Haraguchi, S.; Yoshioka-Yamashita, A.; Yutsudo, M.; Inoue, H. A novel apoptotic pathway induced by the drs tumor suppressor gene. Oncogene 2004, 23, 2977–2987. [Google Scholar] [CrossRef]
- Meindl, A.; Carvalho, M.R.; Herrmann, K.; Lorenz, B.; Achatz, H.; Lorenz, B.; Apfelstedt-Sylla, E.; Wittwer, B.; Ross, M.; Meitinger, T. A gene (SRPX) encoding a sushi-repeat-containing protein is deleted in patients with X-linked retinitis pigmentosa. Hum. Mol. Genet. 1995, 4, 2339–2346. [Google Scholar] [CrossRef]
- Kim, C.J.; Shimakage, M.; Kushima, R.; Mukaisho, K.; Shinka, T.; Okada, Y.; Inoue, H. Down-regulation of drs mRNA in human prostate carcinomas. Hum. Pathol. 2003, 34, 654–657. [Google Scholar] [CrossRef]
- Endo, Y.; Hasegawa, K.; Nomura, R.; Arishima, H.; Kikuta, K.I.; Yamashita, T.; Inoue, Y.; Ueda, M.; Ando, Y.; Wilson, M.R.; et al. Apolipoprotein E and clusterin inhibit the early phase of amyloid-β aggregation in an in vitro model of cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Ozawa, D.; Ookoshi, T.; Naiki, H. Surface-bound basement membrane components accelerate amyloid-β peptide nucleation in air-free wells: An in vitro model of cerebral amyloid angiopathy. Biochim. Biophys. Acta 2013, 1834, 1624–1631. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Gatherer, M.; Smith, C.; Nicoll, J.A.R.; Woelk, C.H.; Johnson, M.; Kalaria, R.; Attems, J.; Garbis, S.D.; Carare, R.O. Systems proteomic analysis reveals that clusterin and tissue inhibitor of metalloproteinases 3 increase in leptomeningeal arteries affected by cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2017, 43, 492–504. [Google Scholar] [CrossRef]
- Hondius, D.C.; Eigenhuis, K.N.; Morrema, T.H.J.; van der Schors, R.C.; van Nierop, P.; Bugiani, M.; Li, K.W.; Hoozemans, J.J.M.; Smit, A.B.; Rozemuller, A.J.M. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 46. [Google Scholar] [CrossRef]
- Woo, D.; Falcone, G.J.; Devan, W.J.; Brown, W.M.; Biffi, A.; Howard, T.D.; Anderson, C.D.; Brouwers, H.B.; Valant, V.; Battey, T.W.; et al. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am. J. Hum. Genet. 2014, 94, 511–521. [Google Scholar] [CrossRef]
- Marini, S.; Crawford, K.; Morotti, A.; Lee, M.J.; Pezzini, A.; Moomaw, C.J.; Flaherty, M.L.; Montaner, J.; Roquer, J.; Jimenez-Conde, J.; et al. Association of apolipoprotein E with intracerebral hemorrhage risk by race/ethnicity: A meta-analysis. JAMA Neurol. 2019, 76, 480–491. [Google Scholar] [CrossRef]
- Verghese, P.B.; Castellano, J.M.; Garai, K.; Wang, Y.; Jiang, H.; Shah, A.; Bu, G.; Frieden, C.; Holtzman, D.M. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 1807–1816. [Google Scholar] [CrossRef]
- Huynh, T.V.; Davis, A.A.; Ulrich, J.D.; Holtzman, D.M. Apolipoprotein E and Alzheimer’s disease: The influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 2017, 58, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J.T.; Wang, H.F.; Han, P.R.; Tan, C.C.; Wang, C.; Meng, X.F.; Risacher, S.L.; Saykin, A.J.; Tan, L. APOE genotype and neuroimaging markers of Alzheimer’s disease: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Rebeck, G.W.; Vonsattel, J.P.; Gomez-Isla, T.; Hyman, B.T. Apolipoprotein E ε4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol. 1995, 38, 254–259. [Google Scholar] [CrossRef]
- O’Donnell, H.C.; Rosand, J.; Knudsen, K.A.; Furie, K.L.; Segal, A.Z.; Chiu, R.I.; Ikeda, D.; Greenberg, S.M. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N. Engl. J. Med. 2000, 342, 240–245. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.; Thal, D.R.; Van Nostrand, W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2011, 37, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Jeynes, B.; Provias, J. The possible role of capillary cerebral amyloid angiopathy in Alzheimer lesion development: A regional comparison. Acta Neuropathol. 2006, 112, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Cerebral amyloid angiopathy: Emerging concepts. J. Stroke 2015, 17, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Ghebremedhin, E.; Rüb, U.; Yamaguchi, H.; Del Tredici, K.; Braak, H. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2002, 61, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Papassotiropoulos, A.; Saido, T.C.; Griffin, W.S.; Mrak, R.E.; Kölsch, H.; Del Tredici, K.; Attems, J.; Ghebremedhin, E. Capillary cerebral amyloid angiopathy identifies a distinct APOE ε4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol. 2010, 120, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mesker, D.J.; Poels, M.M.; Ikram, M.A.; Vernooij, M.W.; Hofman, A.; Vrooman, H.A.; van der Lugt, A.; Breteler, M.M. Lobar distribution of cerebral microbleeds: The Rotterdam Scan Study. Arch. Neurol. 2011, 68, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, J.A.; Sathiyamoorthy, G.; Gao, F.Q.; Szilagyi, G.; Nadkarni, N.K.; St George-Hyslop, P.; Rogaeva, E.; Black, S.E. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch. Neurol. 2008, 65, 790–795. [Google Scholar] [CrossRef]
- Biffi, A.; Anderson, C.D.; Jagiella, J.M.; Schmidt, H.; Kissela, B.; Hansen, B.M.; Jimenez-Conde, J.; Pires, C.R.; Ayres, A.M.; Schwab, K.; et al. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: A genetic association study. Lancet Neurol. 2011, 10, 702–709. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vonsattel, J.P.; Segal, A.Z.; Chiu, R.I.; Clatworthy, A.E.; Liao, A.; Hyman, B.T.; Rebeck, G.W. Association of apolipoprotein E ε2 and vasculopathy in cerebral amyloid angiopathy. Neurology 1998, 50, 961–965. [Google Scholar] [CrossRef]
- McCarron, M.O.; Nicoll, J.A.; Stewart, J.; Ironside, J.W.; Mann, D.M.; Love, S.; Graham, D.I.; Dewar, D. The apolipoprotein E ε2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J. Neuropathol. Exp. Neurol. 1999, 58, 711–718. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Fagan, A.M.; Mackey, B.; Tenkova, T.; Sartorius, L.; Paul, S.M.; Bales, K.; Ashe, K.H.; Irizarry, M.C.; Hyman, B.T. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann. Neurol. 2000, 47, 739–747. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Bales, K.R.; Tenkova, T.; Fagan, A.M.; Parsadanian, M.; Sartorius, L.J.; Mackey, B.; Olney, J.; McKeel, D.; Wozniak, D.; et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2892–2897. [Google Scholar] [CrossRef]
- Wilson, M.R.; Zoubeidi, A. Clusterin as a therapeutic target. Expert Opin. Ther. Targets 2017, 21, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Easterbrook-Smith, S.B. Clusterin is a secreted mammalian chaperone. Trends Biochem. Sci. 2000, 25, 95–98. [Google Scholar] [CrossRef]
- Humphreys, D.T.; Carver, J.A.; Easterbrook-Smith, S.B.; Wilson, M.R. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J. Biol. Chem. 1999, 274, 6875–6881. [Google Scholar] [CrossRef]
- Narayan, P.; Meehan, S.; Carver, J.A.; Wilson, M.R.; Dobson, C.M.; Klenerman, D. Amyloid-β oligomers are sequestered by both intracellular and extracellular chaperones. Biochemistry 2012, 51, 9270–9276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G.; et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015, 18, 978–987. [Google Scholar] [CrossRef]
- Wojtas, A.M.; Kang, S.S.; Olley, B.M.; Gatherer, M.; Shinohara, M.; Lozano, P.A.; Liu, C.C.; Kurti, A.; Baker, K.E.; Dickson, D.W.; et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc. Natl. Acad. Sci. USA 2017, 114, E6962–E6971. [Google Scholar] [CrossRef]
- Kishnani, N.S.; Staskus, P.W.; Yang, T.T.; Masiarz, F.R.; Hawkes, S.P. Identification and characterization of human tissue inhibitor of metalloproteinase-3 and detection of three additional metalloproteinase inhibitor activities in extracellular matrix. Matrix Biol. 1995, 14, 479–488. [Google Scholar] [CrossRef]
- Basu, R.; Lee, J.; Morton, J.S.; Takawale, A.; Fan, D.; Kandalam, V.; Wang, X.; Davidge, S.T.; Kassiri, Z. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc. Res. 2013, 98, 360–371. [Google Scholar] [CrossRef]
- Chintalgattu, V.; Greenberg, J.; Singh, S.; Chiueh, V.; Gilbert, A.; O’Neill, J.W.; Smith, S.; Jackson, S.; Khakoo, A.Y.; Lee, T. Utility of glycosylated TIMP3 molecules: Inhibition of MMPs and TACE to improve cardiac function in rat myocardial infarct model. Pharmacol. Res. Perspect. 2018, 6, e00442. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.; Kaiser, M.; Huber, R.; Ehrmann, M. HTRA proteases: Regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 2011, 12, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Baldi, A.; Bussani, R.; Tian, X.; Stefanescu, R.; Przybylski, M.; Richards, P.; Jones, S.A.; Shridhar, V.; Clausen, T.; et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 2005, 102, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Shiga, A.; Fukutake, T.; Nozaki, H.; Miyashita, A.; Yokoseki, A.; Kawata, H.; Koyama, A.; Arima, K.; Takahashi, T.; et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N. Engl. J. Med. 2009, 360, 1729–1739. [Google Scholar] [CrossRef]
| Diagnosis | Description |
|---|---|
| Definite CAA | Full postmortem examination demonstrating: |
| |
| |
| |
| Probable CAA with supporting pathology | Clinical data and pathological tissue (evacuated hematoma or cortical biopsy) demonstrating: |
| |
| |
| |
| Probable CAA | Clinical data and MRI or CT demonstrating: |
| |
| |
| |
| Possible CAA | Clinical data and MRI or CT demonstrating: |
| |
| |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Ando, Y.; Misumi, Y.; Ueda, M. Current Management and Therapeutic Strategies for Cerebral Amyloid Angiopathy. Int. J. Mol. Sci. 2021, 22, 3869. https://doi.org/10.3390/ijms22083869
Inoue Y, Ando Y, Misumi Y, Ueda M. Current Management and Therapeutic Strategies for Cerebral Amyloid Angiopathy. International Journal of Molecular Sciences. 2021; 22(8):3869. https://doi.org/10.3390/ijms22083869
Chicago/Turabian StyleInoue, Yasuteru, Yukio Ando, Yohei Misumi, and Mitsuharu Ueda. 2021. "Current Management and Therapeutic Strategies for Cerebral Amyloid Angiopathy" International Journal of Molecular Sciences 22, no. 8: 3869. https://doi.org/10.3390/ijms22083869
APA StyleInoue, Y., Ando, Y., Misumi, Y., & Ueda, M. (2021). Current Management and Therapeutic Strategies for Cerebral Amyloid Angiopathy. International Journal of Molecular Sciences, 22(8), 3869. https://doi.org/10.3390/ijms22083869






