1. Introduction
The Korean pine breeding program remains among the largest coniferous tree improvement programs in Northeastern China that are carried out [
1].
Pinus koraiensis was primarily selected to increase its wood yield. A few years later, however, seed yield was taken into account, responding to users’ demand for the seeds and nuts of Korean pine and following the official decree in 2016 against the cutting of natural forests. Korean pine offers many economic benefits. Its wood is more appreciated in construction, and its seeds are variously used in diet and medicine [
2,
3].
P. koraiensis is much desired, being preferred in community reforestation projects following the services it provided in Northeastern China [
4]. However, the planting of stock with desirable genetic properties is still lower than the planting demand [
5]. Because of the limitation of asexual propagation techniques due to the difficulty of producing large amounts of planting materials by cuttings, tissue culture, somatic embryogenesis, and other asexual methods, seed orchards took an important place in improving Korean pine [
6].
The improvement of Korean pine dates back more than a half century [
7]. To date, significant gains have been realized, and several studies reported some improvements in growth rate, and seed and wood quality [
8,
9,
10,
11]. Facing multiple needs and a permanent global change, multi-objective approaches have been suggested for the sustainable management of forest resources [
12]. In tree improvement, selection for multiple characteristics has been introduced to meet multiple purposes.
From this perspective, the establishment of selection criteria in a tree improvement program remains a critical step for choosing appropriate individual trees on which future breeding objectives will focus [
13]. Several selection methods, for a particular trait or multiple traits, can be used for the purpose of improvement. Among these methods, the comprehensive evaluation by Qi value, for instance, assigns importance to the weighting of correlated characteristics when simultaneously enhancing various traits [
14,
15]. On another side, Leakey and Page [
16] referred to the breeding ideotypes approach, which consists in the combination of biomass-related traits to define a hypothetical model of suitable plants producing an economic yield.
Multiple traits selection has been applied to select superior Korean pine materials in different phases for its breeding, including clonal, provenance, and progeny tests [
17,
18]. The selection of multiple traits presents some advantages over tree selection for a single particular characteristic [
19], especially when the characteristics are positively correlated and display high heritability [
20]. However, correlation coefficients, although important for indirect selection, are not sufficient to designate more specific traits to be used as the selection index because of positive and negative effects between linked traits, due principally to uncontrollable and complex trait interaction [
21].
Pathway analysis, consisting of measurement of the weight of variance of dependent variables and independent ones [
22], involves the conversion of correlation coefficients into direct or indirect effects through a multiple regression equation that calculates the impact of correlated variables with precision. Pathway analysis has revealed associations between cause and effect among yield and yield-related traits of genotypes in agronomic plants [
23], as well as different growing environments or habitats [
24,
25]. By reducing a large set of variables into underlying correlated factors [
26], principal component analysis (PCA) appears to be a useful tool to highlight trait combinations that greatly contribute to the variation among tree populations under characterization [
27,
28].
Combining correlation, pathway analysis, and PCA in a multiple-trait comprehensive evaluation could be a powerful statistical method for selecting suitable values in multiple traits to achieve improvement goals.
Given that first generation Korean pine was selected for timber production, and that stem volume represents the major part of the above-ground tree biomass, knowledge of any effects of other growth characteristics on volume would allow the prediction of various gains from the orchard. As there has been little research previously on these aspects, especially on a multiple-usage tree species, this study tries to determine the effects of other growth traits on the volume of Korean pine. It specifically aims to (1) investigate the growth performances of different clones; (2) establish correlations between growth characteristics and their effects on wood yield; (3) highlight the optimum number of traits proper for a comprehensive evaluation index to select superior clones; and (4) select elite clones with high clonal values for future breeding purposes. This work is written from the perspective of setting up a basic theory for the efficient selection of trees for genetic improvement.
4. Discussion
Different ramets of cloned genotypes may have various phenotypic performances in different microenvironmental conditions [
46], which may lead to a certain amount of variation among trees within genotypes. Variation analysis helped to evaluate the magnitude of difference, and also to see if the difference was significant at the individual level, or at the level of the genotype, the blocks, and/or the interaction of blocks and genotypes [
47]. In this study, 11 growth characteristics of stem and crown were analyzed on 38-year-old Korean pine clones. Significant differences were found in variance sources (clone and blocks,
p ≤ 0.001) for most of the investigated phenotypic characteristics, indicating the feasible selection of clones. These results were almost similar to previous research on the growth, seed, and wood properties of Korean pine [
48], although the level of significance in this study seems slightly high, owing probably to different environmental conditions and different genotypes and ages of the studied materials.
When different clones are planted in a macro-site with approximately similar environmental conditions, the clonal average allows the selection of excellent genotypes by minimizing environmental effects at the individual level [
49]. Average performances of different genotypes helped to establish a clonal ranking to select the elite ones [
42]. In this study, average values of 14.22 m, 39.40 cm, 37.60 cm, 0.71 m
3, and 11.40 m in Ht, BD, DBH, DIAM3, Vol., and CH, respectively, and their annual increment (
Table 2), were greater than those reported previously for Norway spruce and
Casuarina junghuhniana [
50,
51], reflecting the good growing conditions for
Pinus koraiensis at the Naozhi seed orchard.
Variation parameters are mostly considered as the main factors to select trees for breeding purposes [
52,
53]. In this study, the difference in variation of different traits as given by PCV values varied from 10.73% to 35.45%, which were higher (considering the stand age) in comparison to the study of Lee et al. [
54]. This demonstrates that the selection made on this population was well founded, although, compared to those values obtained with
Betula platyphylla [
43] and
Eucalyptus sp. [
55], the investigated traits here seem to be less varied. However, these results were close to those reported previously on Korean pine by Liang et al. [
11] and Wang et al. [
17].
BA, Ht, BD, DBH, DIAM3, CH, and Vol. were highly inherited (0.523 to 0.778) (
Table 2), meaning that these traits will be recognized by different clones than CB, BNN, and SSD, which showed lower repeatability of clones (R, 0.060 to 0.392), even for the unique macro site. The high repeatability in this study was in accordance with Yin et al. [
56], which reported repeatability of 0.849, 0.687, and 0.362 in Ht, DBH, and SSD, respectively, in
Larix olgensis clones.
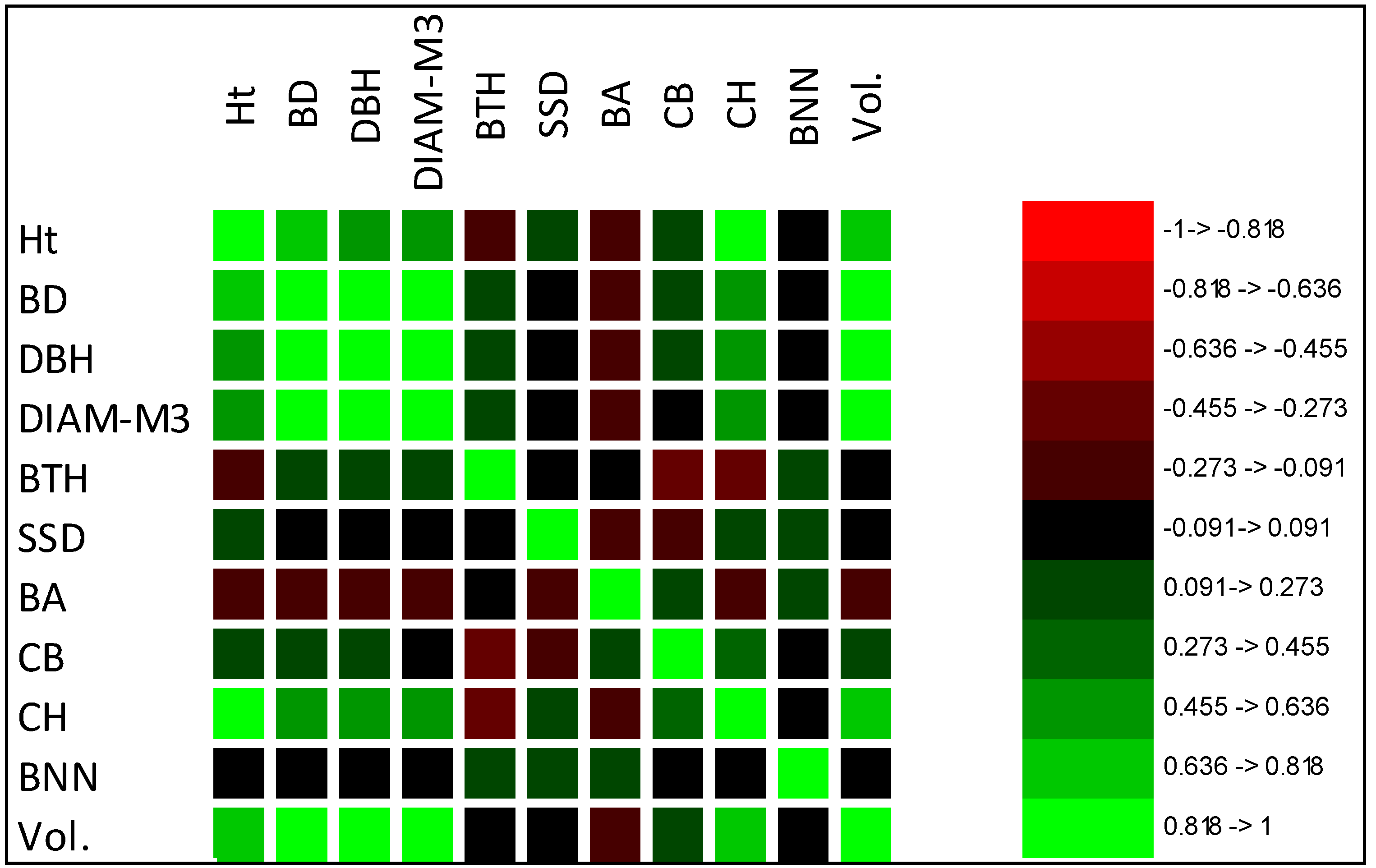
The interdependence of traits was revealed by correlation coefficients [
57]. This relationship is an important parameter in tree improvement when selecting for multiple characteristics [
58]. High genotypic correlation is a high degree of pleiotropy, signifying that many common gene loci affect several traits [
24]. In the present study, moderate to high phenotypic and genotypic correlation was found between Ht, BD, DBH, DIAM3, CH, and Vol., indicating that these traits would be improved simultaneously when selecting elite clones using tree volume as an index [
59], whereas SSD, CB, or AB would be slightly improved or would need to be selected independently, as they showed weak and negative correlations with stem volume [
59]. The correlation results were in agreement with previous studies by Zhao et al. [
41] and Hui et al. [
60], suggesting indirect selection of Ht, BD, and DBH by volume in
Betula platyphylla and
Larix olgensis families in Northeastern China.
Although correlation coefficients explained the inference for traits’ indirect selection, regression coefficients provided more precision to appreciate the influences of trait interactions and traits’ contributions to the related wood yield [
61]. Korean pine was firstly bred to improve timber production, and growth traits such as volume were primarily taken into account in the selection process [
4]. In this study, tree height, diameter at breast height, and branch number positively influenced the stem volume (
Figure S1). DBH was the most influential trait, followed by Ht. In fact, in artificial plantations, growth in tree height and diameter at breast height were reported to be greatly influenced by interline and tree spacing. Wide spacing, for example, promotes growth in diameter, and reduced spacing promotes growth in height [
62,
63]. Considering individual influence diagnostics, from three to nine genotypes—including PK105, PK104, PK87, PK86, PK84, PK83, PK74, PK69, PK59, and PK57—showed outlier values, taking observation values of the cloned genotypes as a predictor, stem volume in the studentized deleted residuals with threshold was equal to 3, Cook’s distances had a threshold of 0.039, DFFits (Std.) a threshold of 0.603, and DFBetas (Std.) a threshold of 0.191 (
Figure S2). Most of these clones were selected for their high growth performances (
Figure S3). Regarding the goodness of fit statistics parameters, the fitted regression model significantly explained the instructive variables’ contribution to the wood yield based on stem volume (
Table S1). The findings in this study were in agreement with Wang et al. [
63], who found that diameter at breast height and tree height were the traits that most affected the stem volume in Korean pine.
PCA is a valuable technique in the selection process because it reduces a large number of indicators to new principal factors (components) that effectively summarize much of the variation in a complex dataset [
64]. The method is very important in the selection of multiple traits, determining suitable traits that contribute most to the variation of the materials to be selected [
65]. PCA helped to determine the trait that displayed great contributions to the variations in growth, wood, and seed traits [
66]. In this study, variance contributions of Vol., DBH, BD, DIAM3, Ht, and CH were high in the PCI, demonstrating that these characteristics are the key traits accounting for most of the variations in these cloned genotypes, and further, they contribute more to wood yield production in the characterized clone population. The accumulating contribution of the first three principal axes accounted for over 82% of the total variation, which was higher compared to Singh et al. [
67] and Du et al. [
68]. This finding was likewise high compared to the cumulative variation of 78% found in the study of Wang et al. [
17], who used PCA to select suitable traits in growth and wood properties of Korean pine. However, the high cumulative amount of variation in PCA in this study may be due to high multicollinearity among traits (
Table S2).
Multiple-trait comprehensive evaluation has served as an accurate practice in multiple-trait selection referring to Qi value [
41]. Since multiple-trait selection may reduce the genetic gain for some uncorrelated or negatively correlated traits, it is crucial to select appropriate traits to be used as the selection index [
69]. Based on correlation coefficients and PCA results, six growth traits—including Ht, BD, DBH, DIAM3, CH, and Vol.—were retained and used to select superior clones in this study. The six elite clones (PK109, PK101, PK14, PK47, PK107, and PK27) displayed high Qi values using a selection rate of 5%. The generated genetic gain reached 42.96% in stem volume (
Table 11), which was higher compared to the study of Liang et al. [
11], possibly due to high variation (great PCV value) in stem volume in these materials.
Based on growth performance, the economic outcome of the Naozhi orchard was projected. In fact, estimating economic outcome from improved materials is of great importance to know whether outlay-related investments in tree improvement will be covered [
70]. The current price reported for
P. koraiensis logs was approximately 145.31 USD per cubic meter in Jilin Province [
18]. Considering the average volume of clones (0.418 m
3), and the genetic gain of 42.96% in volume for elite clones, the price of a single tree will be increased by 26.093 USD (i.e., 15% of the initial price) and the value of the stand volume per hectare will increase by 5325.10 USD with a plant spacing of 7.0 m × 7.0 m, or a density of 204.081 trees/hectare. This achievement was superior to that reported on improved Scots pine in northern Finland after clear-cutting [
71]. However, the engendered economic value was significantly higher than that reported on sib-families Korean pine [
18], which would be due to the difference in planting age and stand density per hectare, which has been mentioned as having an effect on wood yield per hectare in seed orchards [
72].