Human Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Therapy for Cerebellar Ataxia: A Case Report
Abstract
:1. Introduction
2. Case Presentation
2.1. Methodology
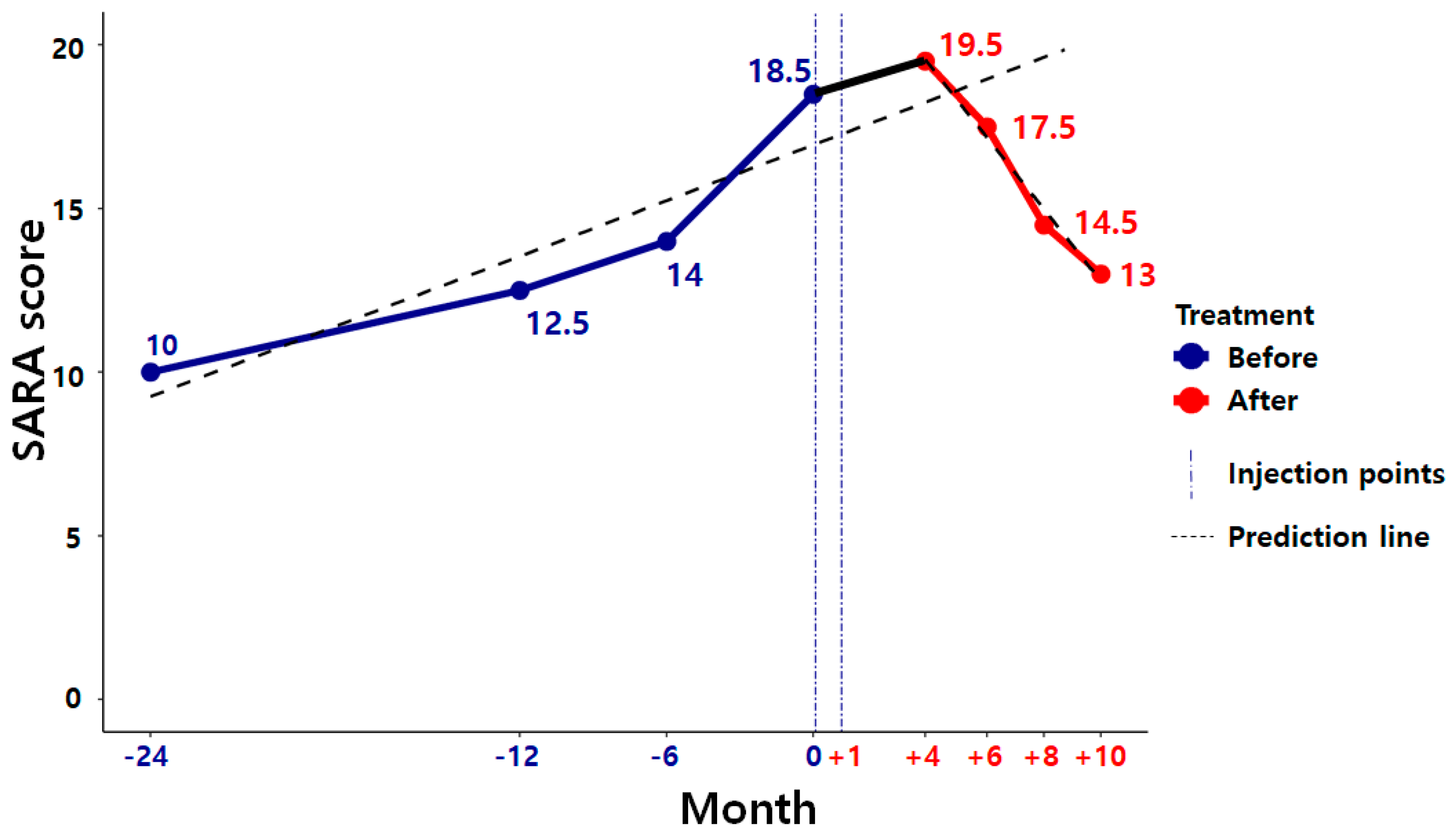
2.2. Case Study
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diener, H.C.; Dichgans, J. Pathophysiology of Cerebellar Ataxia. Mov. Disord. 1992, 7, 95–109. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.N.; Vallortigara, J.; Greenfield, J.; Hunt, B.; Giunti, P.; Hadjivassiliou, M. Diagnosis and management of progressive ataxia in adults. Pract. Neurol. 2019, 19, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Benvenuto, F.; Laroni, A.; Giuti, D. Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol. 2011, 24, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tropel, P.; Platet, N.; Platel, J.C.; Noel, D.; Albrieux, M.; Benabid, A.-L.; Berger, F. Fuctional Neuronal Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T.; Ludtke, R.; Kramer, B.; Abele, M.; Burk, K.; Schols, L.; Riess, O.; Laccone, F.; Boesch, S.; Lopes-Cendes, I.; et al. The natural history of degenerative ataxia: A retrospective study in 466 patients. Brain 1999, 121, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Edalatmanesh, M.A.; Bahrami, A.R.; Hosseini, E.; Hosseini, M.; Khatamsaz, S. Neuroprotective effects of mesenchymal stem cell transplantation in animal model of cerebellar degeneration. Neurol. Res. 2011, 33, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Lee, J.E.; Kim, H.S.; Song, S.K.; Lee, H.S.; Nam, H.S.; Cheong, J.-W.; Jeong, Y.; Park, H.-J.; Kim, D.J.; et al. A Randomized Trial of Mesenchymal Stem Cells in Multiple System Atrophy. Ann. Neurol. 2012, 72, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Dietz, A.B.; Zeller, A.D.; Gehrking, T.L.; Schmelzer, J.D.; Schmeichel, A.M.; Gehrking, J.A.; Suarez, M.D.; Sletten, D.M.; Minota Pacheco, K.V.; et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology 2019, 93, e77–e87. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Yoon, D.; Hong, J.; Kim, M.S.; Lee, T.Y.; Kim, K.S.; Lee, H.W.; Suk, K.; Kim, S.R. Therapeutic Effects of Human Mesenchymal Stem Cells in a Mouse Model of Cerebellar Ataxia with Neuroinflammation. J. Clin. Med. 2020, 9, 3654. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, J.; Seo, D.; Yoon, J.H.; Yoon, D.; Hong, J.; Kim, S.R.; Kim, M.S.; Lee, T.Y.; Kim, K.S.; et al. Characterization of Mesenchymal Stem Cells Derived from Patients with Cerebellar Ataxia: Downregulation of the Anti-Inflammatory Secretome Profile. Cells 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, P.-W.; Park, S.; Kang, K.; Lim, Y.-H.; Kim, S.R.; Suk, K.; Kim, K.S.; Lee, H.-W. Human Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Therapy for Cerebellar Ataxia: A Case Report. Medicina 2021, 57, 334. https://doi.org/10.3390/medicina57040334
Ko P-W, Park S, Kang K, Lim Y-H, Kim SR, Suk K, Kim KS, Lee H-W. Human Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Therapy for Cerebellar Ataxia: A Case Report. Medicina. 2021; 57(4):334. https://doi.org/10.3390/medicina57040334
Chicago/Turabian StyleKo, Pan-Woo, Sangmin Park, Kyunghun Kang, Yong-Hyun Lim, Sang Ryong Kim, Kyoungho Suk, Kyung Suk Kim, and Ho-Won Lee. 2021. "Human Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Therapy for Cerebellar Ataxia: A Case Report" Medicina 57, no. 4: 334. https://doi.org/10.3390/medicina57040334





