Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of the Bioadsorbent
2.3. Pb (II) Adsorption Experiments
2.4. Kinetics and Isotherms of Adsorption
3. Results
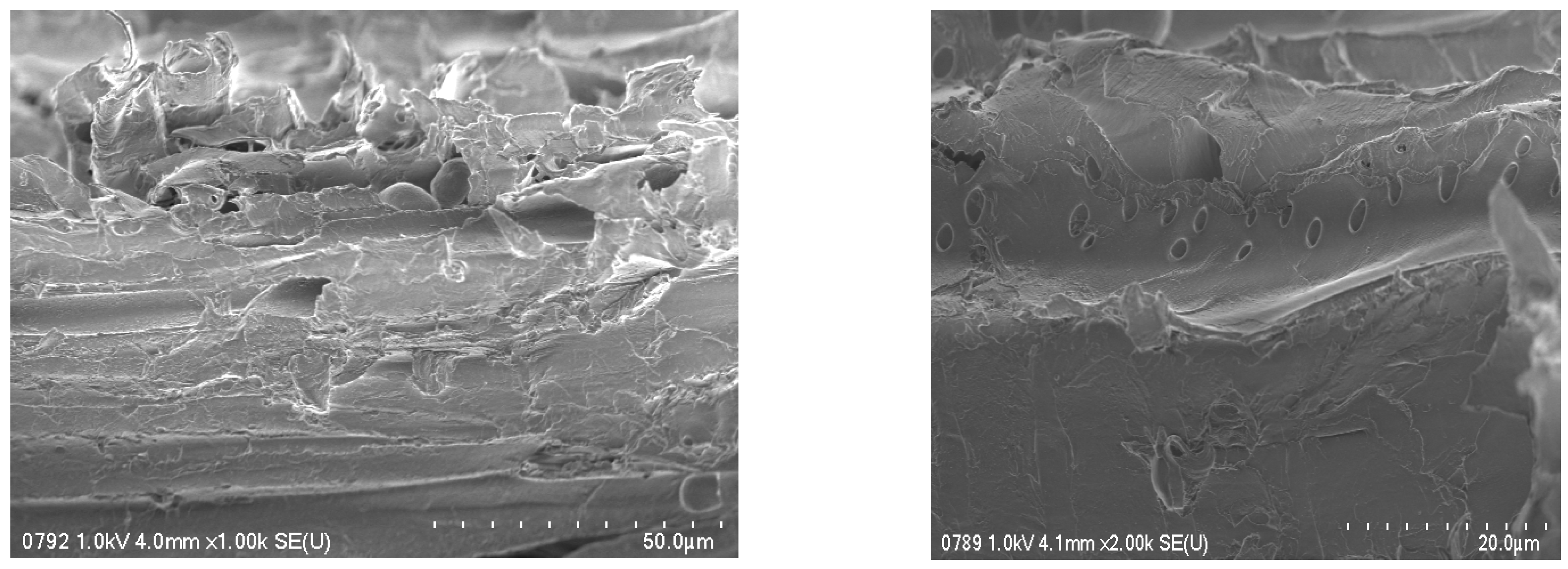
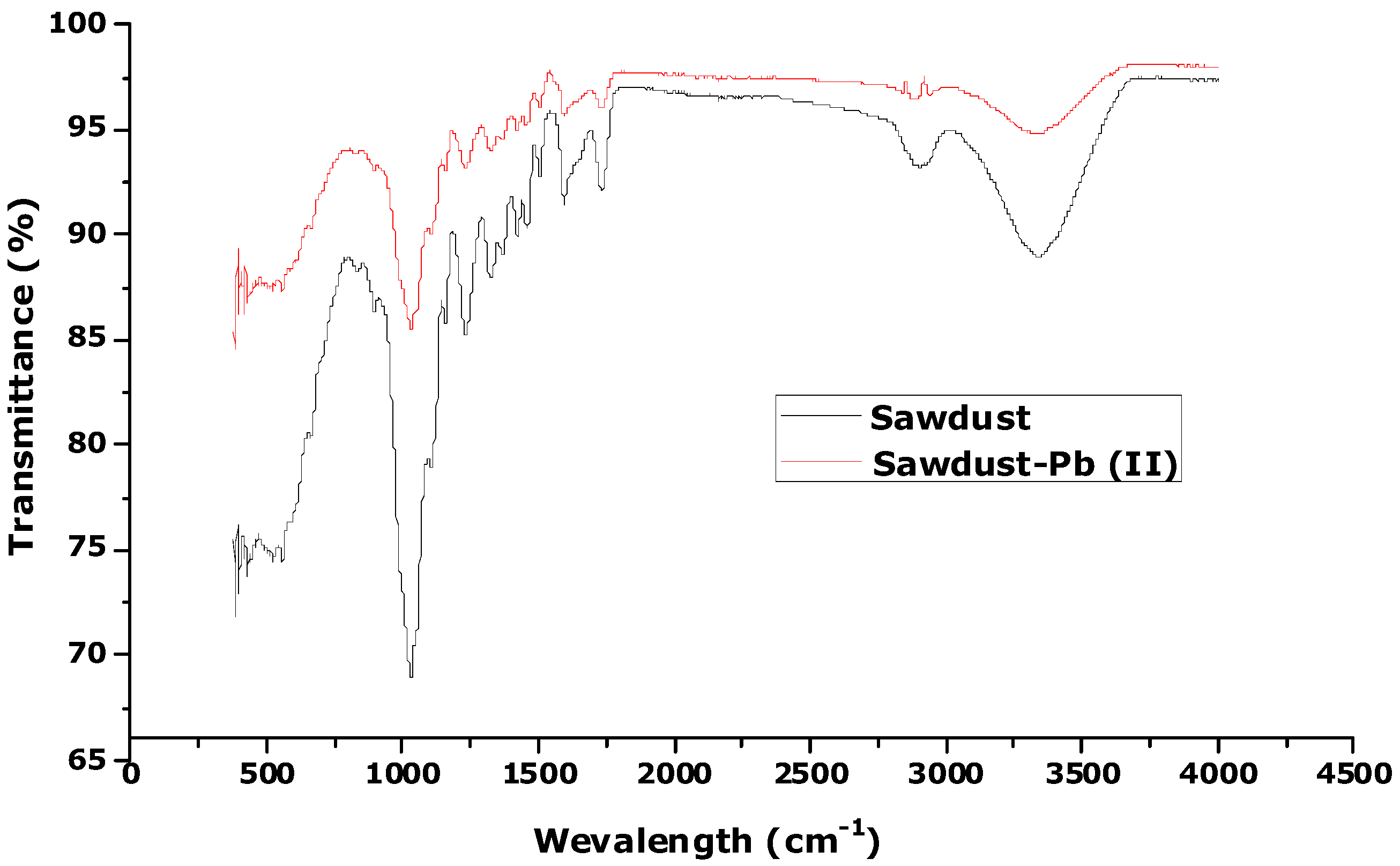
3.1. Characterization of Sawdust
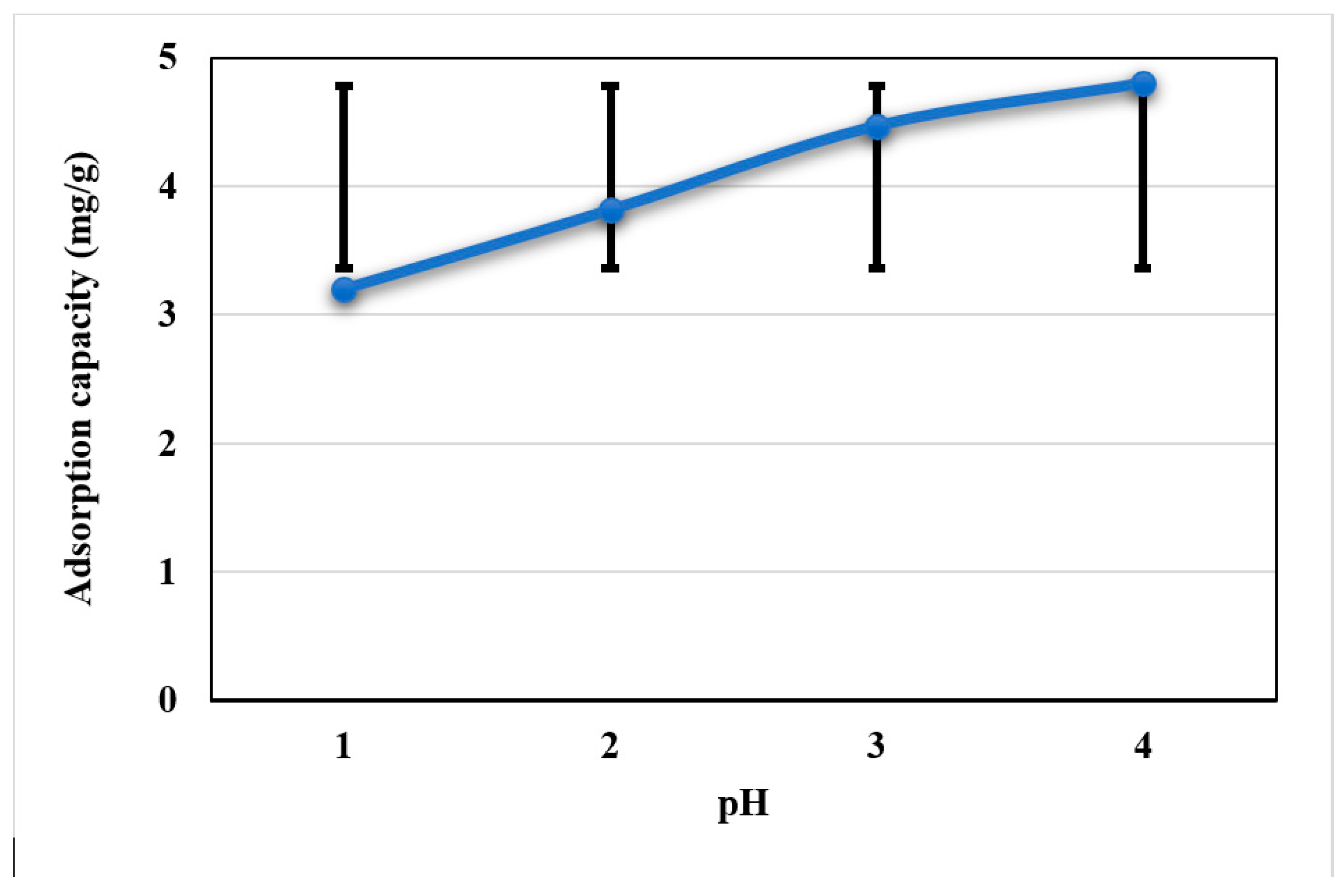
3.2. Effect of pH
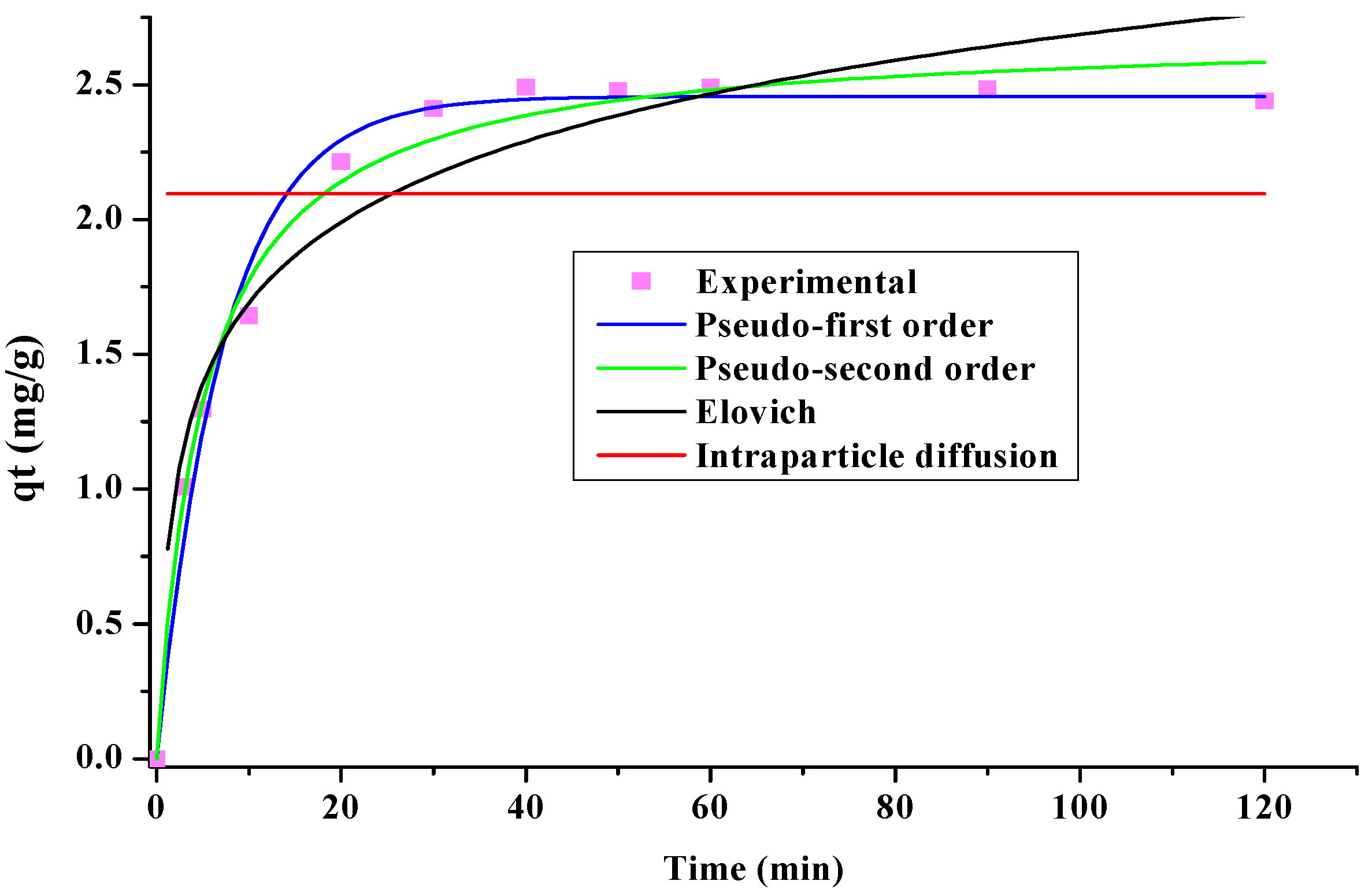
3.3. Adsorption Kinetics
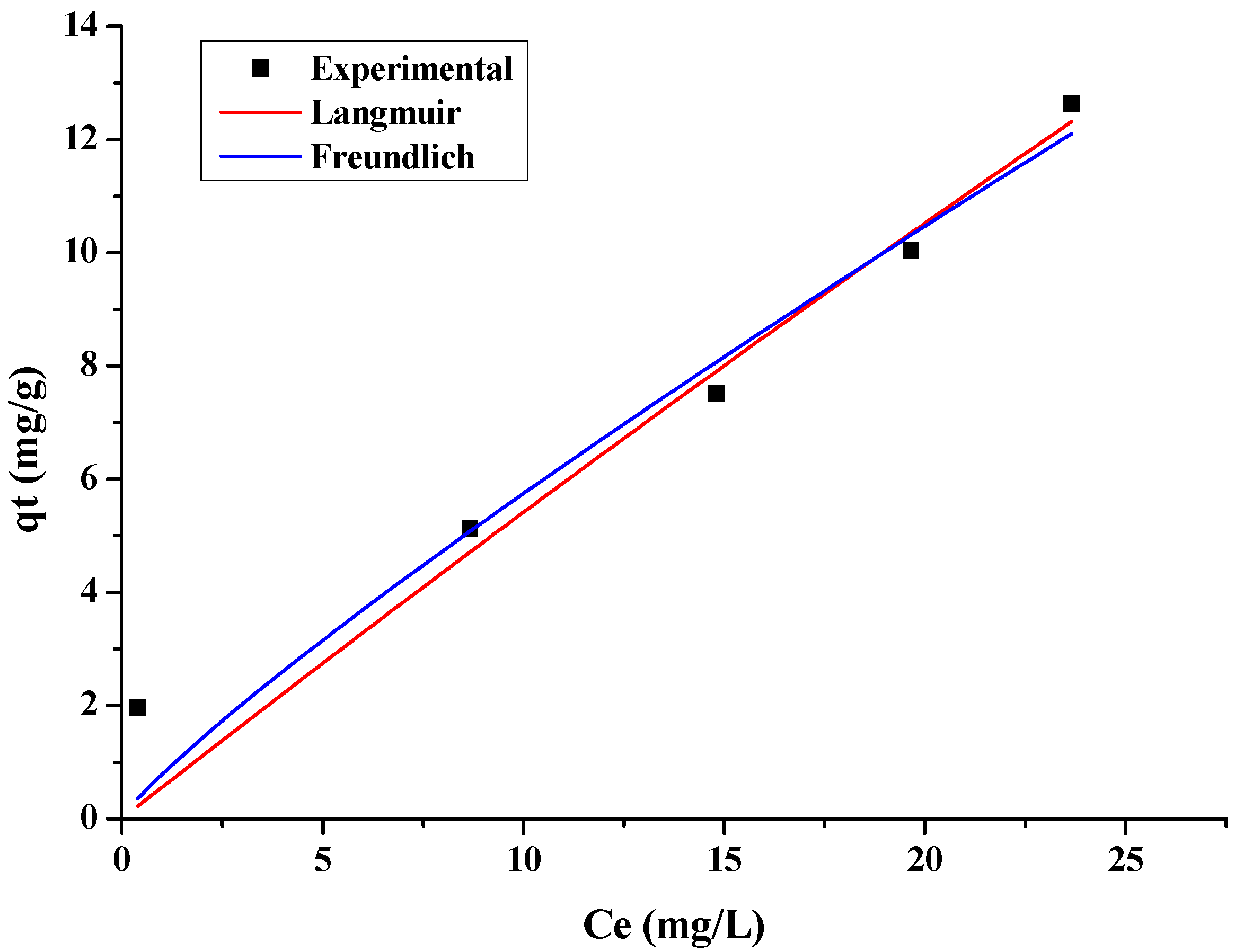
3.4. Adsorption Equilibrium
4. Conclusions
5. Novelty Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naseem, K.; Huma, R.; Shahbaz, A.; Jamal, J.; Rehman, M.Z.U.; Sharif, A.; Ahmed, E.; Begum, R.; Irfan, A.; Al-Sehemi, A.G.; et al. Extraction of Heavy Metals from Aqueous Medium by Husk Biomass: Adsorption Isotherm, Kinetic and Thermodynamic study. Z. Phys. Chem. 2018, 233, 201–223. [Google Scholar] [CrossRef]
- Okoli, C.P.; Diagboya, P.N.; Anigbogu, I.O.; Olu-Owolabi, B.I.; Adebowale, K.O. Competitive biosorption of Pb(II) and Cd(II) ions from aqueous solutions using chemically modified moss biomass (Barbula lambarenensis). Environ. Earth Sci. 2017, 76, 33. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Ugwekar, R.P.; Mankar, R.B. (Eds.) Novel Water Treatment and Separation Methods: Simulation of Chemical Processes; Apple Academic Press: Waretown, NJ, USA, 2017. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Allahdin, O.; Mabingui, J.; Wartel, M.; Boughriet, A. Removal of Pb 2+ ions from aqueous solutions by fixed-BED column using a modified brick: (Micro)structural, electrokinetic and mechanistic aspects. Appl. Clay Sci. 2017, 148, 56–67. [Google Scholar] [CrossRef]
- Meneguin, J.G.; Moisés, M.P.; Karchiyappan, T.; Faria, S.H.B.; Gimenes, M.L.; de Barros, M.A.S.; Venkatachalam, S. Preparation and characterization of calcium treated bentonite clay and its application for the removal of lead and cadmium ions: Adsorption and thermodynamic modeling. Process. Saf. Environ. Prot. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Rajoriya, S.; Singh, S.R.; Kumar, P. Adsorption of Cr (VI) ion from tannery wastewater on tea waste: Kinetics, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2019, 7, 103188. [Google Scholar] [CrossRef]
- Berhe, S.; Ayele, D.; Tadesse, A.; Mulu, A. Adsorption Efficiency of Coffee Husk for Removal of Lead (II)from Industrial Effluents: Equilibrium and Kinetic Study. Int. J. Sci. Res. Publ. 2015, 5, 753–859. [Google Scholar]
- Borna, M.O.; Pirsaheb, M.; Niri, M.V.; Mashizie, R.K.; Kakavandi, B.; Zare, M.R.; Asadi, A. Batch and column studies for the adsorption of chromium(VI) on low-cost Hibiscus Cannabinus kenaf, a green adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 68, 80–89. [Google Scholar] [CrossRef]
- Ijeamaka, E.C. Isotherm Studies of Adsorption of Cr (Vi) Ions onto Coconut Husk. Int. J. Biochem. Biophys. Mol. Biol. 2018, 3, 38. [Google Scholar] [CrossRef]
- Mushtaq, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Eriobotrya japonica seed biocomposite efficiency for copper adsorption: Isotherms, kinetics, thermodynamic and desorption studies. J. Environ. Manag. 2016, 176, 21–33. [Google Scholar] [CrossRef]
- Naik, R.M.; Ratan, S.; Singh, I. Use of orange peel as an adsorbent for the removal of Cr (VI) from its aqueous solution. Indian J. Chem. Technol. 2018, 25, 300–305. [Google Scholar]
- Peng, S.-H.; Wang, R.; Yang, L.-Z.; He, L.; He, X.; Liu, X. Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicol. Environ. Saf. 2018, 165, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mahmood-Ul-Hassan, M.; Yasin, M.; Yousra, M.; Ahmad, R.; Sarwar, S. Kinetics, isotherms, and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust. Environ. Sci. Pollut. Res. 2018, 25, 12570–12578. [Google Scholar] [CrossRef]
- Priyantha, N.; Lim, L.B.L.; Mansor, N.H.M.; Liyandeniya, A.B. Irreversible sorption of Pb(II) from aqueous solution on breadfruit peel to mitigate environmental pollution problems. Water Sci. Technol. 2019, 80, 2241–2249. [Google Scholar] [CrossRef]
- Semerjian, L. Removal of heavy metals (Cu, Pb) from aqueous solutions using pine (Pinus halepensis) sawdust: Equilibrium, kinetic, and thermodynamic studies. Environ. Technol. Innov. 2018, 12, 91–103. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.; Leyva-Ramos, R. Novel biosorbent with high adsorption capacity prepared by chemical modification of white pine (Pinus durangensis) sawdust. Adsorption of Pb(II) from aqueous solutions. J. Environ. Manag. 2016, 169, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Demcak, S.; Balintova, M.; Hurakova, M.; Frontasyeva, M.V.; Zinicovscaia, I.; Yushin, N. Utilization of poplar wood sawdust for heavy metals removal from model solutions. Nova Biotechnol. Chim. 2017, 16, 26–31. [Google Scholar] [CrossRef]
- Malwade, K.; Lataye, D.; Mhaisalkar, V.; Kurwadkar, S.; Ramirez, D. Adsorption of hexavalent chromium onto activated carbon derived from Leucaena leucocephala waste sawdust: Kinetics, equilibrium and thermodynamics. Int. J. Environ. Sci. Technol. 2016, 13, 2107–2116. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Yeddou, A.R. Potential of sawdust materials for the removal of dyes and heavy metals: Examination of isotherms and kinetics. Desalination Water Treat. 2016, 57, 24019–24034. [Google Scholar] [CrossRef]
- Valverde, J.C.; Barrena, V.M.; Guillén, R. Estimación de la biomasa aérea de Eucalyptus globulus Labill plantado en cercos vivos, distrito Huertas, Junín (Perú). Rev. For. Perú 2019, 34, 52–65. [Google Scholar] [CrossRef]
- FAO. FAO of the U.N. and Instituto Tecnológico de la Producción (ITP)-CITEmadera La Industria de la Madera en el Perú. Lima. 2018. [Online]. Available online: http://www.fao.org/3/I8335ES/i8335es.pdf (accessed on 20 March 2021).
- Villabona-Ortíz, A.; De Cartagena, U.; Tejada-Tovar, C.N.; Ortega-Toro, R. Modelling of the Adsorption Kinetics of Chromium (vi) Using Waste Biomaterials. Rev. Mex. Ing. Química 2019, 19, 401–408. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Competitive adsorption of heavy metal ions (Pb2+, Cu2+, and Ni2+) onto date seed biochar: Batch and fixed bed experiments. Sep. Sci. Technol. 2018, 54, 888–901. [Google Scholar] [CrossRef]
- Singh, Y.D.; Mahanta, P.; Bora, U. Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew. Energy 2017, 103, 490–500. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of Lead and Cadmium from Aqueous Solution in Single and Binary Systems Using Avocado Pear Exocarp: Effects of Competing Ions. Anal. Lett. 2020, 53, 2868–2885. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato Peels as an Adsorbent for Heavy Metals from Aqueous Solutions: Eco-Structuring of a Green Adsorbent Operating Plackett–Burman Design. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Gonzalez-Delgado, A.D.; Villabona-Ortiz, A. Characterization of Residual Biomasses and Its Application for the Removal of Lead Ions from Aqueous Solution. Appl. Sci. 2019, 9, 4486. [Google Scholar] [CrossRef]
- Al-Lagtah, N.M.; Al-Muhtaseb, A.H.; Ahmad, M.N.; Salameh, Y. Chemical and physical characteristics of optimal synthesised activated carbons from grass-derived sulfonated lignin versus commercial activated carbons. Microporous Mesoporous Mater. 2016, 225, 504–514. [Google Scholar] [CrossRef]
- Manirethan, V.; Gupta, N.; Balakrishnan, R.M.; Raval, K. Batch and continuous studies on the removal of heavy metals from aqueous solution using biosynthesised melanin-coated PVDF membranes. Environ. Sci. Pollut. Res. 2019, 27, 24723–24737. [Google Scholar] [CrossRef]
- Rinaldi, R.; Yasdi, Y.; Hutagalung, W.L.C. Removal of Ni (II) and Cu (II) ions from aqueous solution using rambutan fruit peels (Nephelium lappaceum L.) as adsorbent. In Proceedings of the Fourth Huntsville Gamma-Ray Burst Symposium; AIP Publishing: Yogyakarta, Indonesia August 14 and 15. , 2018; Volume 2026, p. 020098. [Google Scholar]
- Amro, A.N.; Abhary, M.K.; Shaikh, M.M.; Ali, S. Removal of Lead and Cadmium Ions from Aqueous Solution by Adsorption on a Low-Cost Phragmites Biomass. Processes 2019, 7, 406. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 4207–4228. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, Y.; Tan, R.; Ke, X.; Zhou, X.; Geng, J.; Hou, H.; Zhou, M. Calcium Sulfate Hemihydrate Whiskers Obtained from Flue Gas Desulfurization Gypsum and Used for the Adsorption Removal of Lead. Crystals 2017, 7, 270. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Leuz, A.-K.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands. Part 3: The Pb2+ + OH–, Cl–, CO32–, SO42–, and PO43– systems (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 2425–2476. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Saad, A.A.; Amer, R.A.; Tayeb, E.H.; Nady, N.; Mohamed, R.G. Unmodified Rice Straw for The Lead Removal Approach from Synthetic Lead Solution. Alex. Sci. Exch. J. 2020, 41, 43–52. [Google Scholar] [CrossRef]
- Alhogbi, B.G.; Salam, M.A.; Ibrahim, O. Environmental remediation of toxic lead ions from aqueous solution using palm tree waste fibers biosorbent. Desalination Water Treat. 2019, 145, 179–188. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu(II), Pb(II) and Zn(II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. J. Encapsul. Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef]
- Olasehinde, E.F.; Adegunloye, A.V.; Adebayo, M.A.; Oshodi, A.A. Sequestration of Aqueous Lead(II) Using Modified and Unmodified Red Onion Skin. Anal. Lett. 2018, 51, 2710–2732. [Google Scholar] [CrossRef]
- Kariuki, Z.; Kiptoo, J.; Onyancha, D. Biosorption studies of lead and copper using rogers mushroom biomass ‘Lepiota hystrix’. S. Afr. J. Chem. Eng. 2017, 23, 62–70. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Alharthi, F.A.; Azizi, M.; Al-Zaqri, N.; El-Marghany, A.; Abdelshafeek, K.A. Removal of Lead(II) from Synthetic Wastewater by Lavandula pubescens Decne Biosorbent: Insight into Composition–Adsorption Relationship. Appl. Sci. 2020, 10, 7450. [Google Scholar] [CrossRef]
- Rozman, U.; Kalčíková, G.; Marolt, G.; Skalar, T.; Gotvajn, A. Žgajnar Potential of waste fungal biomass for lead and cadmium removal: Characterization, biosorption kinetic and isotherm studies. Environ. Technol. Innov. 2020, 18, 100742. [Google Scholar] [CrossRef]
- Kaur, M.; Kumari, S.; Sharma, P. Removal of Pb (II) from aqueous solution using nanoadsorbent of Oryza sativa husk: Isotherm, kinetic and thermodynamic studies. Biotechnol. Rep. 2020, 25, e00410. [Google Scholar] [CrossRef] [PubMed]
- Obike, A.; Igwe, J.; Emeruwa, C.; Uwakwe, K. Equilibrium and kinetic studies of Cu (II), Cd (II), Pb (II) and Fe (II) adsorption from aqueous solution using cocoa (Theobroma cacao) pod husk. J. Appl. Sci. Environ. Manag. 2018, 22, 182. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef]
- Haq, A.U.; Saeed, M.; Anjum, S.; Bokhari, T.H.; Usman, M.; Tubbsum, S. Evaluation of Sorption Mechanism of Pb (II) and Ni (II) onto Pea (Pisum sativum) Peels. J. Oleo Sci. 2017, 66, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Raikar, R.V.; Correa, S.; Ghorpade, P. Removal of lead (II) from aqueous solution using natural and activated rice husk. Int. Res. J. Eng. Technol. 2015, 2, 1677–1686. [Google Scholar]
- Perez, T.; Pasquini, D.; Lima, A.D.F.; Rosa, E.V.; Sousa, M.H.; Cerqueira, D.A.; De Morais, L.C. Efficient removal of lead ions from water by magnetic nanosorbents based on manganese ferrite nanoparticles capped with thin layers of modified biopolymers. J. Environ. Chem. Eng. 2019, 7, 102892. [Google Scholar] [CrossRef]
- Ibisi, N.; Asoluka, C. Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem. Int. 2018, 4, 52–59. Available online: http://bosaljournals.com/chemint/images/pdffiles/18-7.pdf (accessed on 20 March 2021).
- Abiaziem, C.V.; Williams, A.B.; Inegbenebor, A.I.; Onwordi, C.T.; Ehi-Eromosele, C.O.; Petrik, L.F. Adsorption of lead ion from aqueous solution unto cellulose nanocrystal from cassava peel. J. Phys. Conf. Ser. 2019, 1299, 012122. [Google Scholar] [CrossRef]
- Yu, X.-L.; He, Y. Optimal ranges of variables for an effective adsorption of lead (II) by the agricultural waste pomelo (Citrus grandis) peels using Doehlert designs. Sci. Rep. 2018, 8, 729. [Google Scholar] [CrossRef] [PubMed]
| Model | Equation | Parameter |
|---|---|---|
| Pseudo-first-order | ×(1) | qe and qt (mg/g): adsorption capacities in equilibrium and at a certain time. k1 (min−1): Constant of Lagergren. |
| Pseudo-second-order | (2) | k2 (g−1 min−1): pseudo-second-order adsorption constant. |
| Elovich | (3) | α (mg·g−1·min−1): initial speed of adsorption. β (g·mg−1): desorption constant related to surface range, and activation energy for chemisorption. qt (mg/g): the amount of chemisorbed metal. |
| Intraparticle diffusion | (4) | qt (mg/g): quantity of metal adsorbed per mass unit of adsorbent in a time t. t (min): is the time. k3 (mg·g−1·min−1/2): constant intraparticular diffusion. |
| Model | Equation | Parameters |
|---|---|---|
| Langmuir | qt (mg/g): is the amount of metal adsorbed on the bioadsorbent. Cf (mg/L): residual metal concentration in solution. qmax (mg/g): maximum adsorption capacity of the Langmuir model. KL: is Langmuir’s constant and can be correlated with the variation of the adsorption area and the porosity of the adsorbent. | |
| Freundlich | kf (L/g): Freundlich’s constant and represents the distribution coefficient. n (mg/g): represents the adsorption intensity and indicates the heterogeneity of the active sites. qt (mg/g): the amount of metal adsorbed at equilibrium. Ce (mg/L): residual concentration of the metal in solution. |
| Source | GL | Sum of Squares | Mean Square | F-Value | p Value |
|---|---|---|---|---|---|
| pH | 3 | 24.2654 | 8.0885 | 49.98 | 0.000 |
| Error | 12 | 1.9472 | 0.1637 | ||
| Total | 15 | 26.2301 |
| Model | Parameters | Value |
|---|---|---|
| Pseudo-first-order | qe (mg/g) | 2.4561 |
| k1 (min−1) | 0.1364 | |
| R2 | 0.9859 | |
| Pseudo-second-order | qe (mg/g) | 2.69 |
| k2 (g/mg×min) | 0.07 | |
| R2 | 0.9879 | |
| Elovich | α (mg/g×min) | 2.311 |
| β (g/mg) | 2.154 | |
| R2 | 0.469 | |
| Intraparticle diffusion | k3 (mg/g×s1/2) | 2.09 |
| R2 | 0.5819 |
| Model | Parameter | Value |
|---|---|---|
| Langmuir | qmax (mg/g) | 145.5417 |
| KL (L/mg) | 0.0039 | |
| R2 | 0.9317 | |
| Freundlich | Kf (mg/g) | 1.019 |
| N | 1.288 | |
| R2 | 0.7764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortíz, A.; Ortega-Toro, R.; Mancilla-Bonilla, H.; Espinoza-León, F. Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium. Appl. Sci. 2021, 11, 3125. https://doi.org/10.3390/app11073125
Tejada-Tovar C, Villabona-Ortíz A, Ortega-Toro R, Mancilla-Bonilla H, Espinoza-León F. Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium. Applied Sciences. 2021; 11(7):3125. https://doi.org/10.3390/app11073125
Chicago/Turabian StyleTejada-Tovar, Candelaria, Angel Villabona-Ortíz, Rodrigo Ortega-Toro, Humberto Mancilla-Bonilla, and Fran Espinoza-León. 2021. "Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium" Applied Sciences 11, no. 7: 3125. https://doi.org/10.3390/app11073125
APA StyleTejada-Tovar, C., Villabona-Ortíz, A., Ortega-Toro, R., Mancilla-Bonilla, H., & Espinoza-León, F. (2021). Potential Use of Residual Sawdust of Eucalyptus globulus Labill in Pb (II) Adsorption: Modelling of the Kinetics and Equilibrium. Applied Sciences, 11(7), 3125. https://doi.org/10.3390/app11073125










