Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy
Abstract
:1. Introduction
2. Renovascular Disease and Microvascular Injury
3. Ischemic Nephropathy and Renal Oxygenation
4. Tissue Hypoxia and Inflammatory Injury
5. Stem Cells and Injury Pathways in the Kidney
6. Functional Characteristics of Human MSC Related to Age and Hypoxia
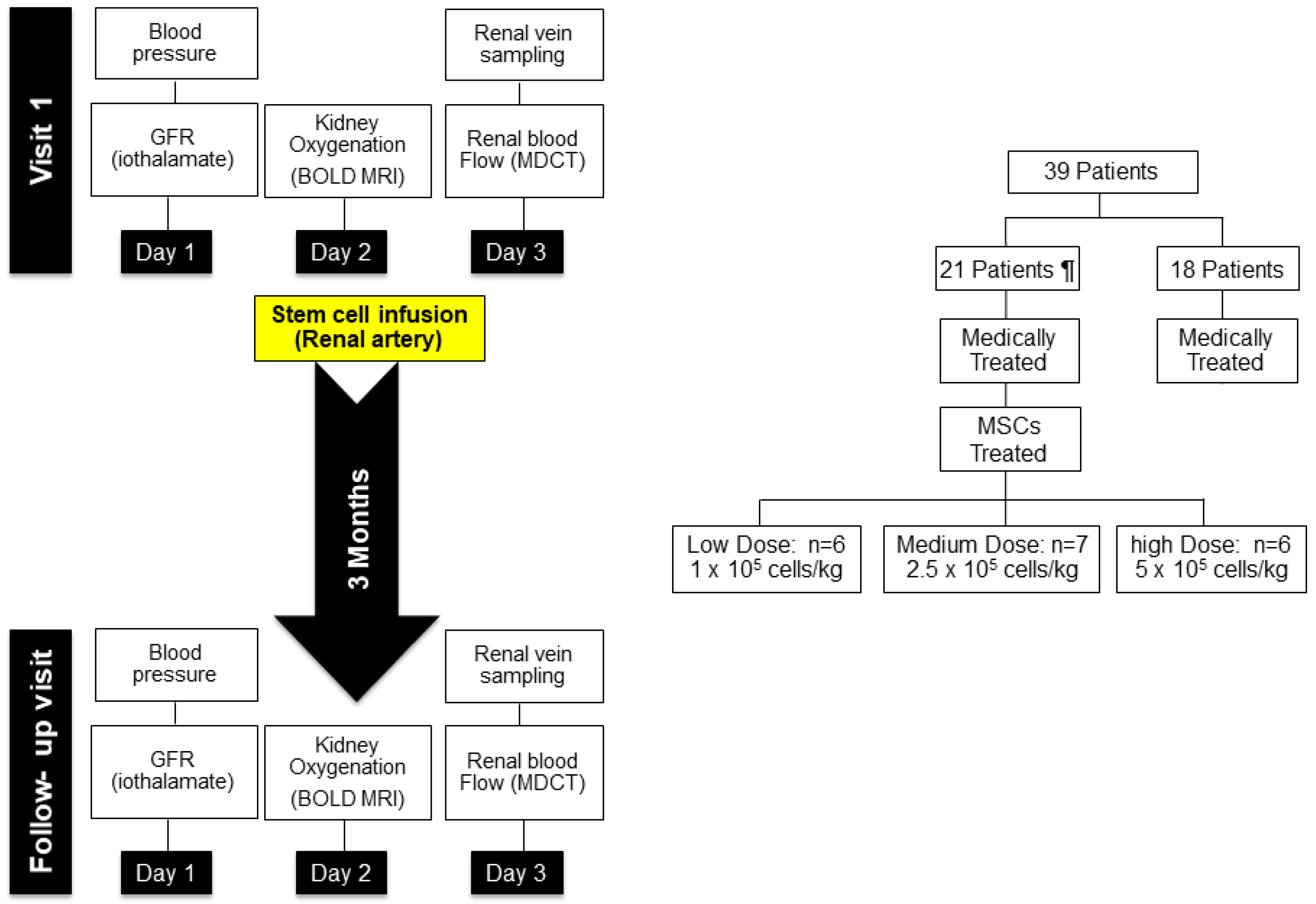
7. Mesenchymal Stem Cell Effects in Human Subjects with Ischemic Nephropathy
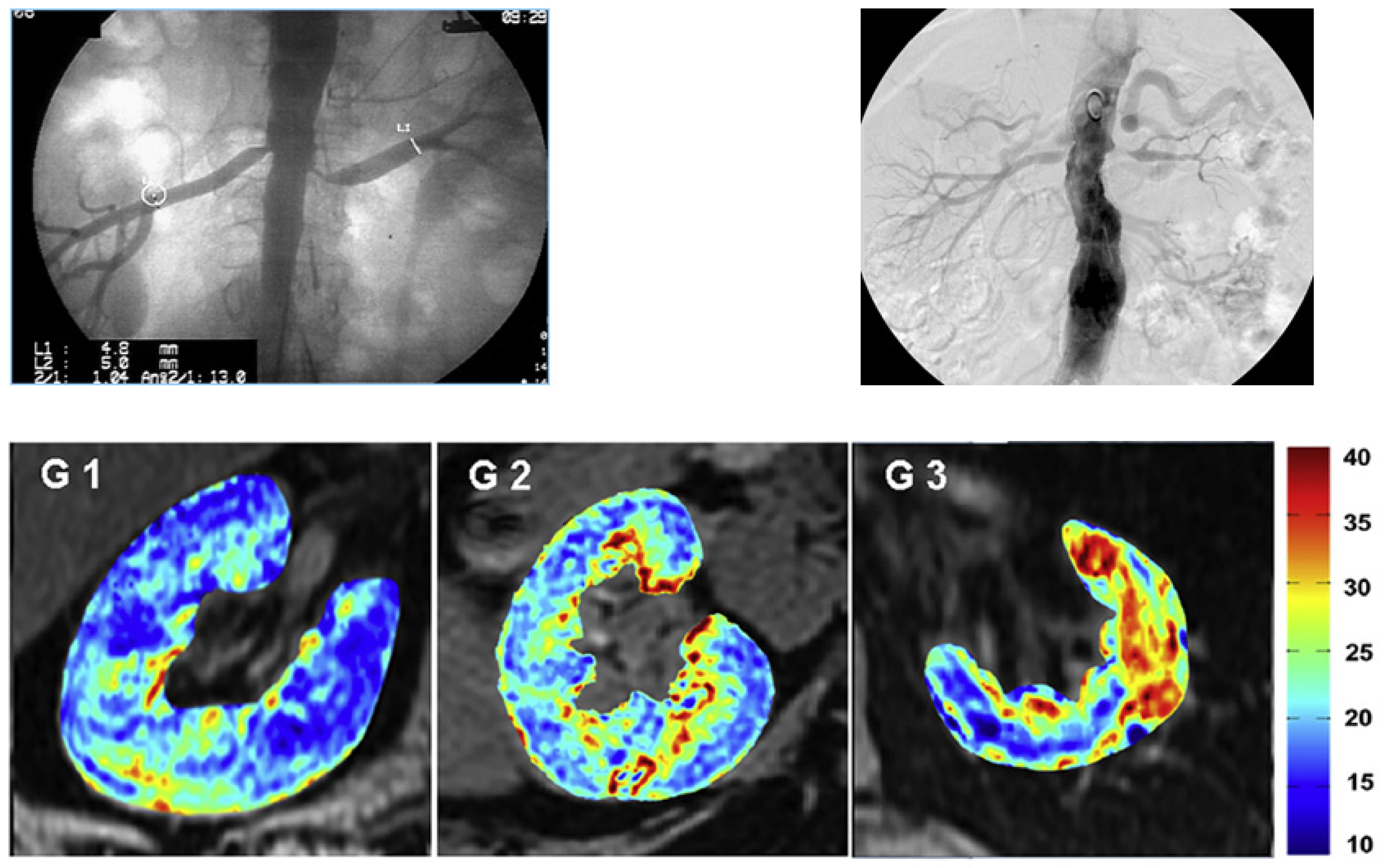
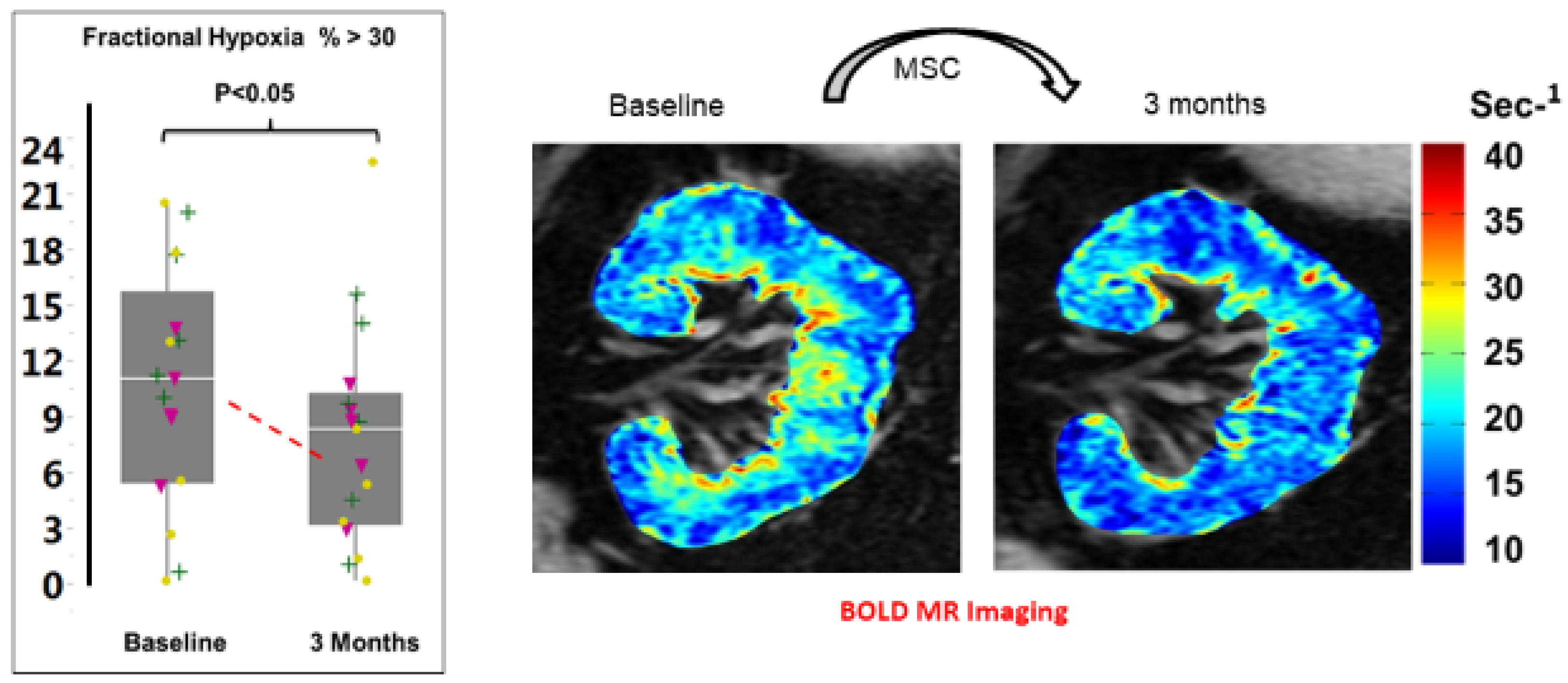
In Vivo Imaging Studies
8. Clinical Effects of MSC Administration
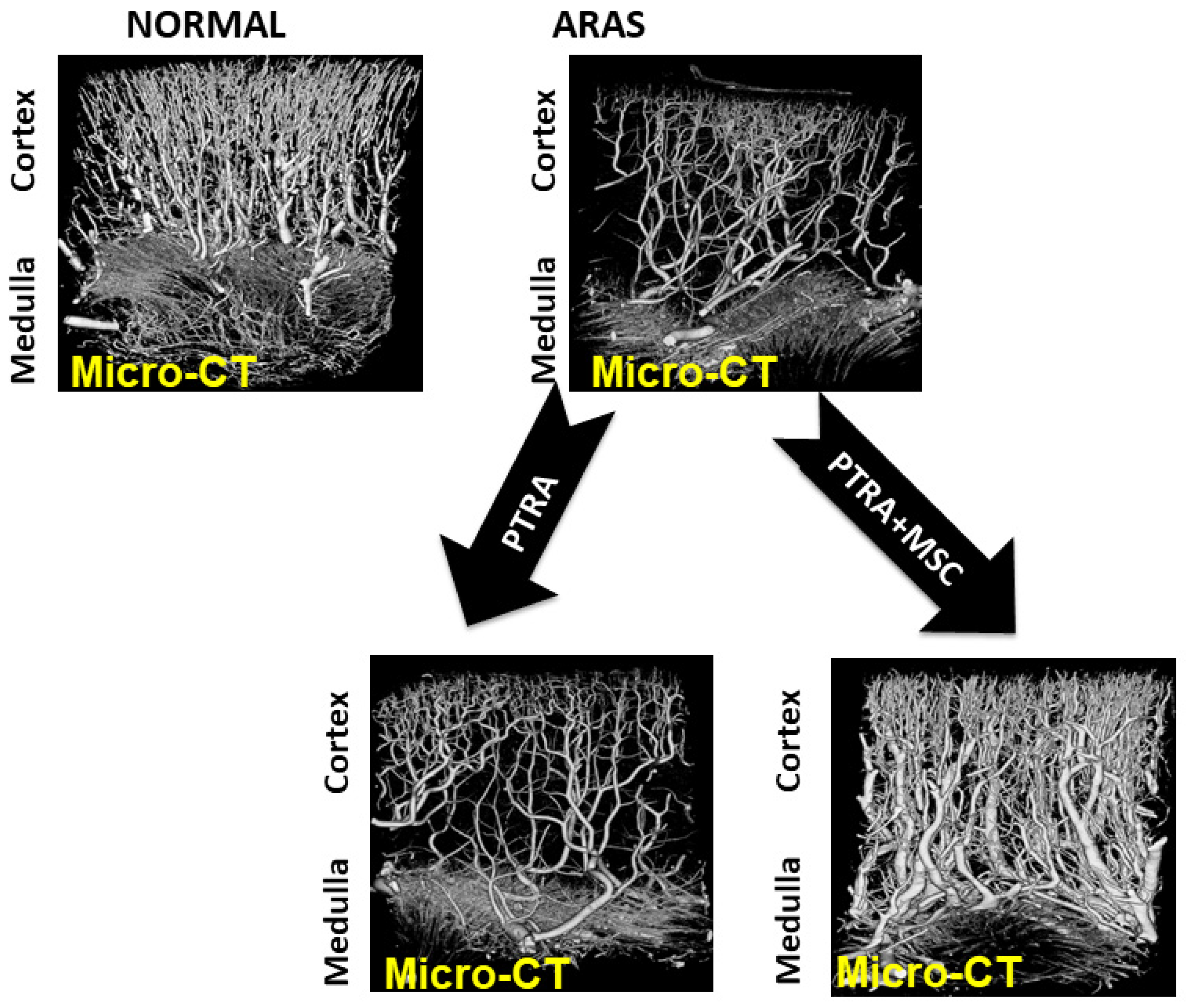
9. Microvascular Repair Associated with MSC Therapy in Humans
10. Caveats
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perico, N.; Casiraghi, F.; Remuzzi, G. Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J. Am. Soc. Nephrol. 2018, 29, 362–375. [Google Scholar] [CrossRef]
- Schoenberg, S.O.; Rieger, J.R.; Michaely, H.J.; Rupprecht, H.; Samtleben, W.; Reiser, M.F. Functional magnetic resonance imaging in renal artery stenosis. Abdom. Imaging 2006, 31, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.C. Renal Arterial Disease and Hypertension. Med. Clin. N. Am. 2017, 101, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Woollard, J.R.; Herrmann, S.M.; Hickson, L.J.; Bendel, E.C.; Misra, S.; Glockner, J.; et al. Tissue hypoxia, inflammation, and loss of glomerular filtration rate in human atherosclerotic renovascular disease. Kidney Int. 2019, 95, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Kelsen, S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ. Cardiovasc. Interv. 2010, 3, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Eirin, A.; Zhu, X.-Y.; Urbieta-Caceres, V.H.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am. J. Physiol. Ren. Physiol. 2011, 300, F1394–F1401. [Google Scholar] [CrossRef] [Green Version]
- Chade, A.R. Renal vascular structure and rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar]
- Tuttle, K.R.; Dworkin, L.D.; Henrich, W.; Greco, B.A.; Steffes, M.; Tobe, S.; Shapiro, J.I.; Jamerson, K.; Lyass, A.; Pencina, K.; et al. Effects of Stenting for Atherosclerotic Renal Artery Stenosis on eGFR and Predictors of Clinical Events in the CORAL Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Herrmann, S.M.S.; Crane, J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Eirin, A.; Ebrahimi, B.; Lerman, L.O.; Textor, S.C. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ. Cardiovasc. Interv. 2013, 6, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, E.A.; Harmsen, W.S.; Misra, S. Impact of Renal Function Trajectory on Renal Replacement Therapy and Mortality Risk after Renal Artery Revascularization. J. Vasc. Interv. Radiol. 2020, 31, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Fatica, R.A.; Port, F.K.; Young, E.W. Incidence trends and mortality in end-stage renal disease attributed to renovascular disease in the United States. Am. J. Kidney Dis. 2001, 37, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.A.; Guo, H.; Gilbertson, D.T.; Liu, J.C.S.; Ishani, A.; Collins, A.J.; Foley, R.N. Atherosclerotic renovascular disease in the United States. Kidney Int. 2010, 77, 37–43. [Google Scholar] [CrossRef]
- Epstein, F.H. Oxygen and renal metabolism. Kidney Int. 1997, 51, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, L.; Gomez, S.I.; Bolterman, R.; Haas, J.A.; Bentley, M.D.; Lerman, L.O.; Romero, J.C. Regional decreases in renal oxygenation during graded acute renal arterial stenosis: A case for renal ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R67–R71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal oxygenation: Unique challenges and the biophysical basis of homeostasis. Am. J. Physiol. Ren. Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, C.S.; Palm, F.; Welch, W.J. Renal oxygenation and function of the rat kidney: Effects of inspired oxygen and preglomerular oxygen shunting. Adv. Exp. Med. Biol. 2013, 765, 329–334. [Google Scholar] [PubMed]
- Rosenberger, C.; Rosen, S.; Heyman, S.N. Renal parenchymal oxygenation and hypoxia adaptation in acute kidney injury. Clin. Exp. Pharm. Physiol. 2006, 33, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, H.R. Ischemic renal disease: An overlooked clinical entity. Kidney Int. 1988, 34, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Breyer, J.A.; Jacobson, H.R. Ischemic Nephropathy. Curr. Opin. Nephrol. Hyper. 1993, 2, 216–224. [Google Scholar] [CrossRef]
- Prasad, P.V.; Edelman, R.R.; Epstein, F.H. Non-invasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 1996, 94, 3271–3275. [Google Scholar] [CrossRef]
- Pruijm, M.; Mendichovszky, I.A.; Liss, P.; Van der Niepen, P.; Textor, S.C.; Lerman, L.O.; Krediet, C.T.P.; Caroli, A.; Burnier, M.; Prasad, P.V. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: A statement paper and systematic review. Nephrol. Dial. Transpl. 2018, 33, ii22–ii28. [Google Scholar] [CrossRef] [Green Version]
- Li, L.P.; Franklin, T.; Du, H.; Papadopoulou-Rosenzweig, M.; Carbray, J.; Solomon, R.; Prasad, P.V. Intrarenal oxygenation by blood oxygenation level-dependent MRI in contrast nephropathy model: Effect of the viscosity and dose. J. Magn. Reason. Imaging 2012, 36, 1162–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.; Epstein, F.H. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: Effects of aging and cyclooxygenase inhibition. Kidney Int. 1999, 55, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruijm, M.; Hofmann, L.; Maillard, M.; Tremblay, S.; Glatz, N.; Wuerzner, G.; Burnier, M.; Vogt, B. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension 2010, 55, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Pruijm, M.; Milani, B.; Pivin, E.; Podhajska, A.; Vogt, B.; Stuber, M.; Burnier, M. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018, 93, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.; Li, L.P.; Thacker, J.M.; Li, W.; Hack, B.; Kohn, O.; Sprague, S.M. Cortical Perfusion and Tubular Function as Evaluated by Magnetic Resonance Imaging Correlates with Annual Loss in Renal Function in Moderate Chronic Kidney Disease. Am. J. Nephrol. 2019, 49, 114–124. [Google Scholar] [CrossRef]
- Siedek, F.; Persigehl, T.; Mueller, R.U.; Burst, V.; Benzing, T.; Maintz, D.; Haneder, S. Assessing renal changes after remote ischemic preconditioning (RIPC) of the upper extremity using BOLD imaging at 3T. MAGMA 2018, 31, 367–374. [Google Scholar] [CrossRef]
- Saad, A.; Crane, J.; Glockner, J.F.; Herrmann, S.M.S.; Friedman, H.; Ebrahimi, B.; Lerman, L.O.; Textor, S.C. Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology 2013, 268, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Lerman, L.O.; Taler, S.J.; Textor, S.C.; Sheedy, P.F., 2nd; Stanson, A.W.; Romero, J.C. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996, 49, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Textor, S.C.; Glockner, J.F.; Lerman, L.O.; Misra, S.; McKusick, M.A.; Riederer, S.J.; Grande, J.P.; Gomez, S.I.; Romero, J.C. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J. Am. Soc. Nephrol. 2008, 19, 780–788. [Google Scholar] [CrossRef]
- Gloviczki, M.L.; Glockner, J.F.; Lerman, L.O.; McKusick, M.A.; Misra, S.; Grande, J.P.; Textor, S.C. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 2010, 55, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Gloviczki, M.L.; Keddis, M.T.; Garovic, V.D.; Friedman, H.; Herrmann, S.; McKusick, M.A.; Misra, S.; Grande, J.P.; Lerman, L.O.; Textor, S.C. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.L.; Xie, Y.; Nguyen, H.; Brewster, P.S.; Sholl, H.; Sharrett, M.; Ren, K.; Chen, T.; Tuttle, K.R.; Haller, S.T.; et al. Early Rapid Decline in Kidney Function in Medically Managed Patients with Atherosclerotic Renal Artery Stenosis. J. Am. Heart Assoc. 2019, 8, e012366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloviczki, M.L.; Glockner, J.F.; Crane, J.A.; McKusick, M.A.; Misra, S.; Grande, J.P.; Lerman, L.O.; Textor, S.C. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 2011, 58, 1066–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Ebrahimi, B.; Zhang, X.; Zhu, X.-Y.; Tang, H.; Crane, J.A.; Lerman, A.; Textor, S.C.; Lerman, L.O. Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in Swine renal artery stenosis. Circ. Cardiovasc. Interv. 2012, 5, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Textor, S.C.; Wilcox, C.S. Ischemic nephropathy/azotemic renovascular disease. Semin. Nephrol. 2000, 20, 489–502. [Google Scholar] [PubMed]
- Kotliar, C.; Juncos, L.; Inserra, F.; de Cavanagh, E.M.V.; Chuluyan, E.; Aquino, J.B.; Hita, A.; Navari, C.; Sanchez, R. Local and systemic cellular immunity in early renal artery atherosclerosis. Clin. J. Am. Soc. Nephrol. 2012, 7, 224–230. [Google Scholar] [CrossRef]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Duffield, J.S. Macrophages in kidney repair and regeneration. J. Am. Soc. Nephrol. 2011, 22, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Puranik, A.S.; Leaf, I.A.; Jensen, M.A.; Hedayat, A.F.; Saad, A.; Kim, K.W.; Saadalla, A.M.; Woollard, J.R.; Kashyap, S.; Textor, S.C.; et al. Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci. Rep. 2018, 8, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keddis, M.; Garovic, V.; Bailey, K.; Wood, C.; Raissian, Y.; Grande, J. Ischemic nephropathy secondary to atherosclerotic renal artery stenosis: Clinical and histopathological correlates. Nephrol. Dial. Transpl. 2010, 25, 3615–3622. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Wang, W.; Herrmann, S.M.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Bjarnason, H.; Lerman, L.O.; Textor, S.C. Atherosclerotic renal artery stenosis is associated with elevated cell cycle arrest markers related to reduced renal blood flow and postcontrast hypoxia. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2016, 31, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Eirin, A.; Gloviczki, M.L.; Tang, H.; Gossl, M.; Jordan, K.L.; Woollard, J.R.; Lerman, A.; Grande, J.P.; Textor, S.C.; Lerman, L.O. Inflammatory and injury signals released from the post-stenotic human kidney. Eur. Heart J. 2013, 34, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chade, A.R.; Zhu, X.; Lavi, R.; Krier, J.D.; Pislaru, S.; Simari, R.D.; Napoli, C.; Lerman, A.; Lerman, L.O. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 2009, 119, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lim, J.; Yu, H.Y.; Park, J.; Chun, J.Y.; Jeong, J.; Heo, J.; Kang, H.; Kim, Y.; Cho, Y.M.; et al. Mesenchymal Stem Cell Therapy Alleviates Interstitial Cystitis by Activating Wnt Signaling Pathway. Stem Cells Dev. 2015, 24, 1648–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribot, J.; Caliaperoumal, G.; Paquet, J.; Boisson-Vidal, C.; Petite, H.; Anagnostou, F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J. Cell Mol. Med. 2017, 21, 349–363. [Google Scholar] [CrossRef]
- Jiang, C.M.; Liu, J.; Zhao, J.Y.; Xiao, L.; An, S.; Gou, Y.C.; Quan, H.X.; Cheng, Q.; Zhang, Y.L.; He, W.; et al. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J. Dent. Res. 2015, 94, 69–77. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, L.; Fu, B.; Zhang, J.; Hong, Q.; Hu, J.; Li, D.; Luo, C.; Cui, S.; Zhu, F.; et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res. Ther. 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Oliveira-Sales, E.B.; Boim, M.A. Mesenchymal stem cells and chronic renal artery stenosis. Am. J. Physiol. Ren. Physiol. 2016, 310, F6–F9. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Diaz, R.; Behfar, A.; Butler, G.W.; Padley, D.J.; Sarr, M.G.; Bartunek, J.; Dietz, A.B.; Terzic, J. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011, 20, 797–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, M.; Mehta, K.; Luther, J.; Baruah, A.; Dietz, A.B.; Faubion, W.A., Jr. Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Griffin, M.D. Back from the brink: A mesenchymal stem cell infusion rescues kidney function in acute experimental rhabdomyolysis. Stem Cell Res. Ther. 2014, 5, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, A.; Zhu, X.Y.; Herrmann, S.; Hickson, L.; Tang, H.; Dietz, A.B.; van Wijnen, A.J.; Lerman, L.; Textor, S. Adipose-derived mesenchymal stem cells from patients with atherosclerotic renovascular disease have increased DNA damage and reduced angiogenesis that can be modified by hypoxia. Stem Cell Res. Ther. 2016, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [Green Version]
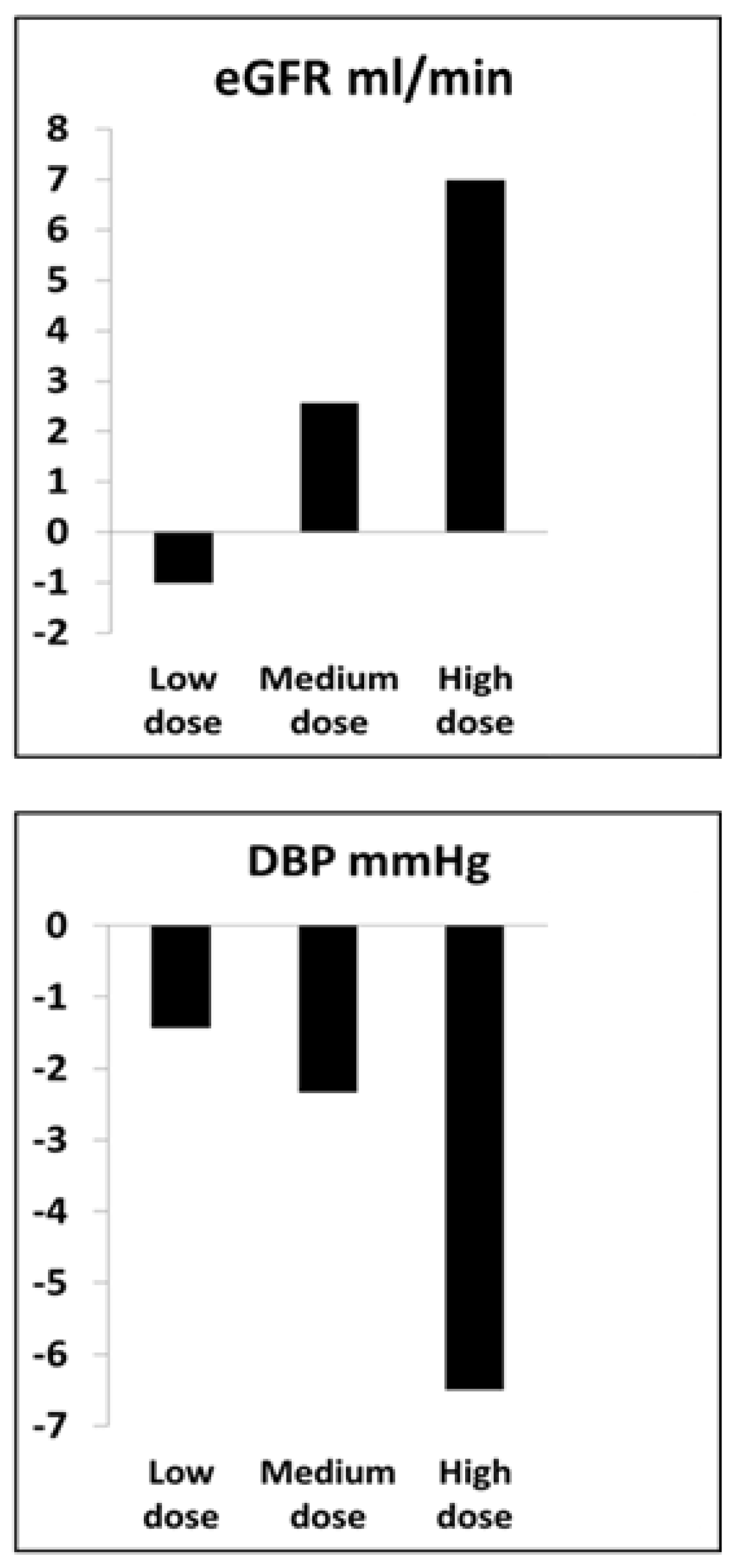
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Herrmann, S.M.; Hickson, L.J.; Goksu, B.B.; Bendel, E.; Misra, S.; Glockner, J.; et al. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020, 97, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, S.M.; Saad, A.; Eirin, A.; Woollard, J.; Tang, H.; McKusick, M.A.; Misra, S.; Glockner, J.F.; Lerman, L.O.; Textor, S.C. Differences in GFR and Tissue Oxygenation, and Interactions between Stenotic and Contralateral Kidneys in Unilateral Atherosclerotic Renovascular Disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Westenfelder, C.; Togel, F.E. Protective actions of administered mesenchymal stem cells in acute kidney injury: Relevance to clinical trials. Kidney Int. Suppl. 2011, 1, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Togel, F.E.; Westenfelder, C. Kidney protection and regeneration following acute injury: Progress through stem cell therapy. Am. J. Kidney Dis. 2012, 60, 1012–1022. [Google Scholar] [CrossRef]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2018, 29, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.; Griffin, T.P.; Zhu, X.; Islam, M.N.; Conley, S.M.; Eirin, A.; Tang, H.; O’Shea, P.M.; Palmer, A.K.; McCoy, R.G.; et al. Senescence marker activin A is increased in human diabetic kidney disease: Association with kidney function and potential implications for therapy. BMJ Open Diabetes Res. Care 2019, 7, e000720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.R.; Zou, X.; Tang, H.; Puranik, A.S.; Abumoawad, A.M.; Zhu, X.Y.; Hickson, L.J.; Tchkonia, T.; Textor, S.C.; Kirkland, J.L.; et al. Increased cellular senescence in the murine and human stenotic kidney: Effect of mesenchymal stem cells. J. Cell Physiol. 2021, 236, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Griffin, M.D. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells 2020, 38, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Engel, J.E.; Williams, E.; Williams, M.L.; Bidwell, G.L., 3rd; Chade, A.R. Targeted VEGF (Vascular Endothelial Growth Factor) Therapy Induces Long-Term Renal Recovery in Chronic Kidney Disease via Macrophage Polarization. Hypertension 2019, 74, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Tullos, N.A.; Harvey, T.W.; Mahdi, F.; Bidwell, G.L., 3rd. Renal Therapeutic Angiogenesis Using a Bioengineered Polymer-Stabilized Vascular Endothelial Growth Factor Construct. J. Am. Soc. Nephrol. 2016, 27, 1741–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eirin, A.; Ebrahimi, B.; Zhang, X.; Zhu, X.-Y.; Woollard, J.R.; He, Q.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc. Res. 2014, 103, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Eirin, A.; Zhu, X.-Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 2012, 30, 1030–1041. [Google Scholar] [CrossRef] [Green Version]

Cell preparation and conditioning
|
|
|
|
|
Cell administration
|
|
| Potential development as adjunctive maneuvers |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Textor, S.C.; Abumoawad, A.; Saad, A.; Ferguson, C.; Dietz, A. Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy. Cells 2021, 10, 765. https://doi.org/10.3390/cells10040765
Textor SC, Abumoawad A, Saad A, Ferguson C, Dietz A. Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy. Cells. 2021; 10(4):765. https://doi.org/10.3390/cells10040765
Chicago/Turabian StyleTextor, Stephen C., Abdu Abumoawad, Ahmed Saad, Christopher Ferguson, and Allan Dietz. 2021. "Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy" Cells 10, no. 4: 765. https://doi.org/10.3390/cells10040765






