Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Elicit Better Preservation of the Intra-Renal Microvasculature Than Renal Revascularization in Pigs with Renovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Studies
2.2. Ex Vivo Studies
2.3. EV mRNA Cargo
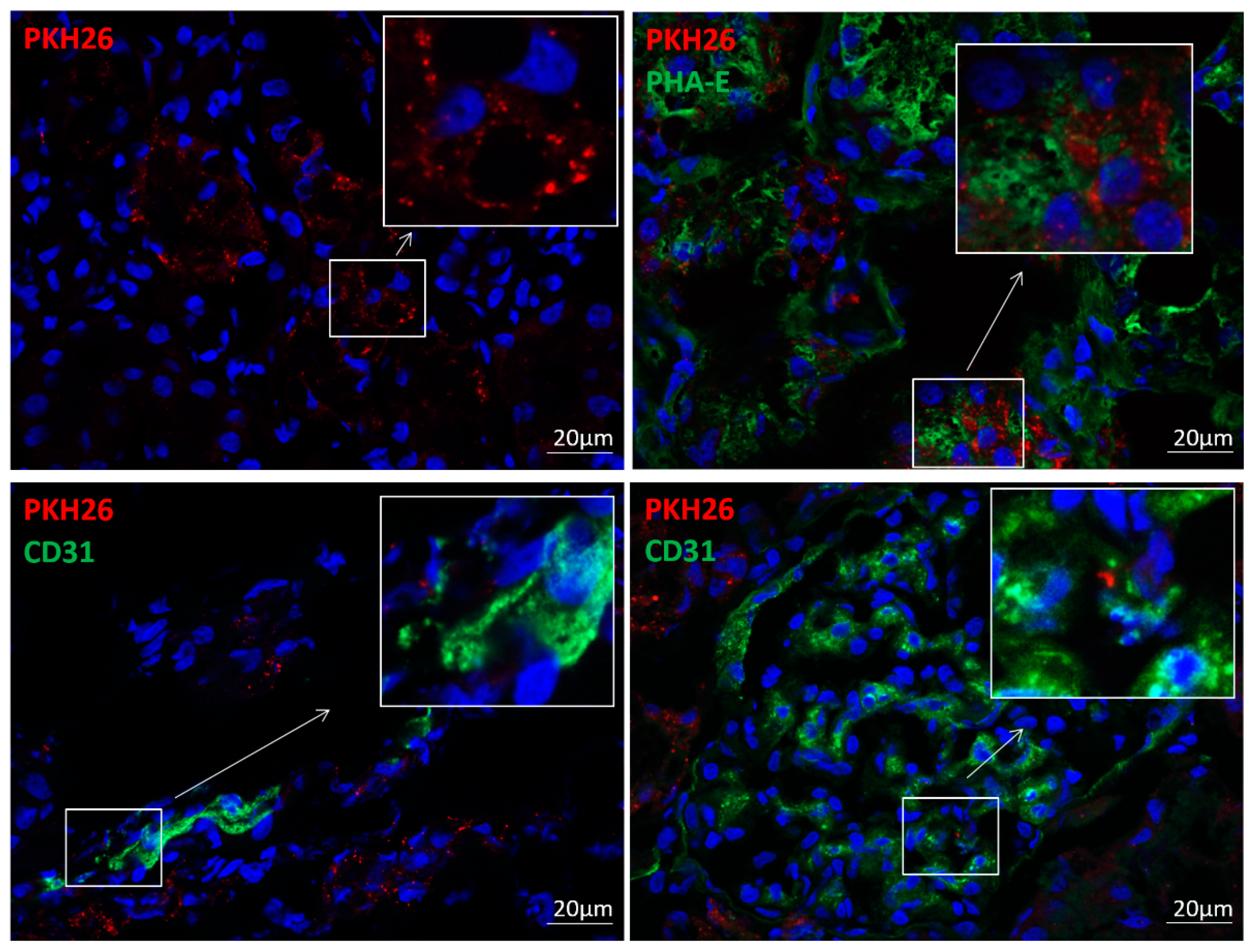
2.4. EV Tracking
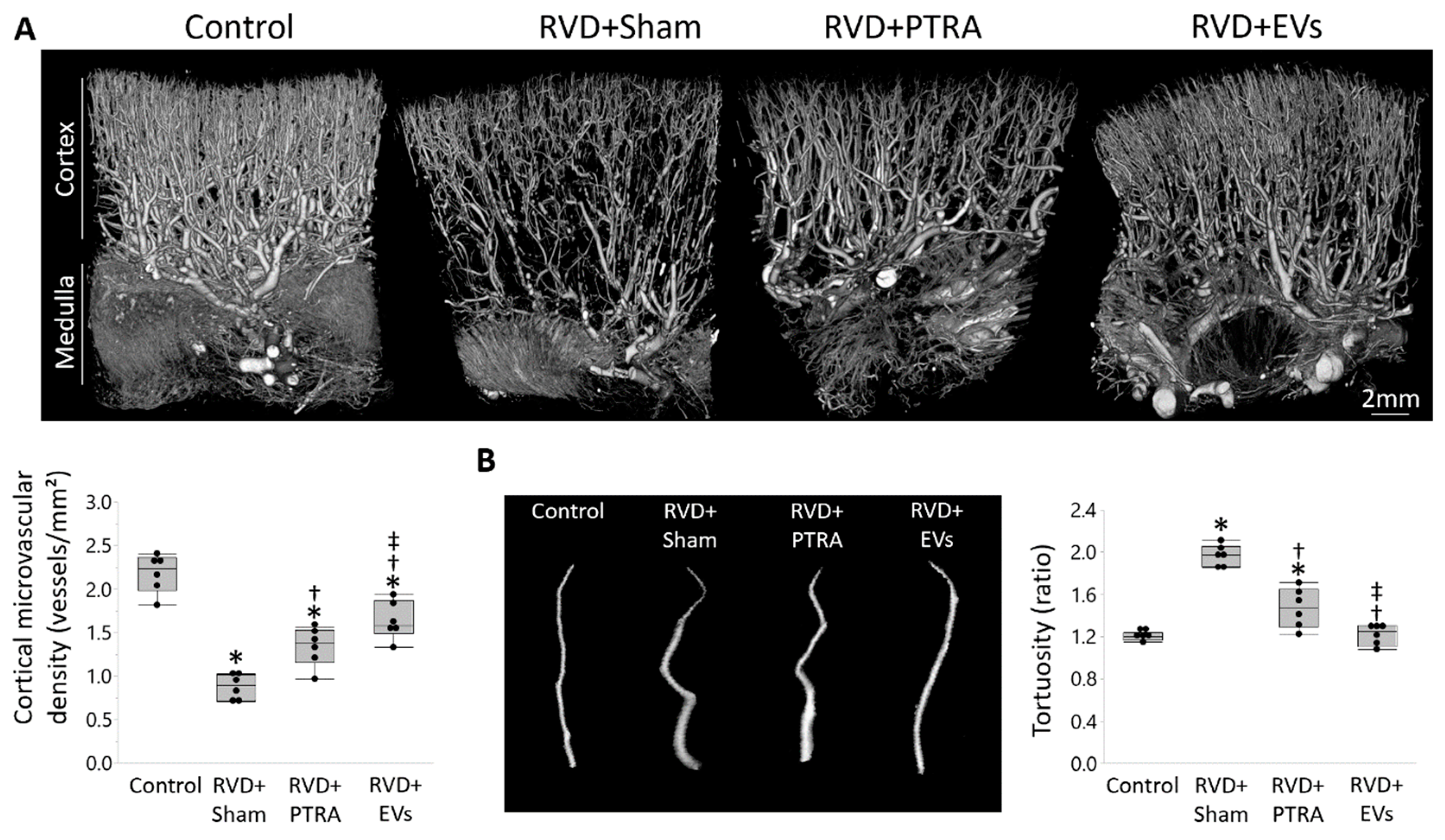
2.5. Renal Microvascular Density
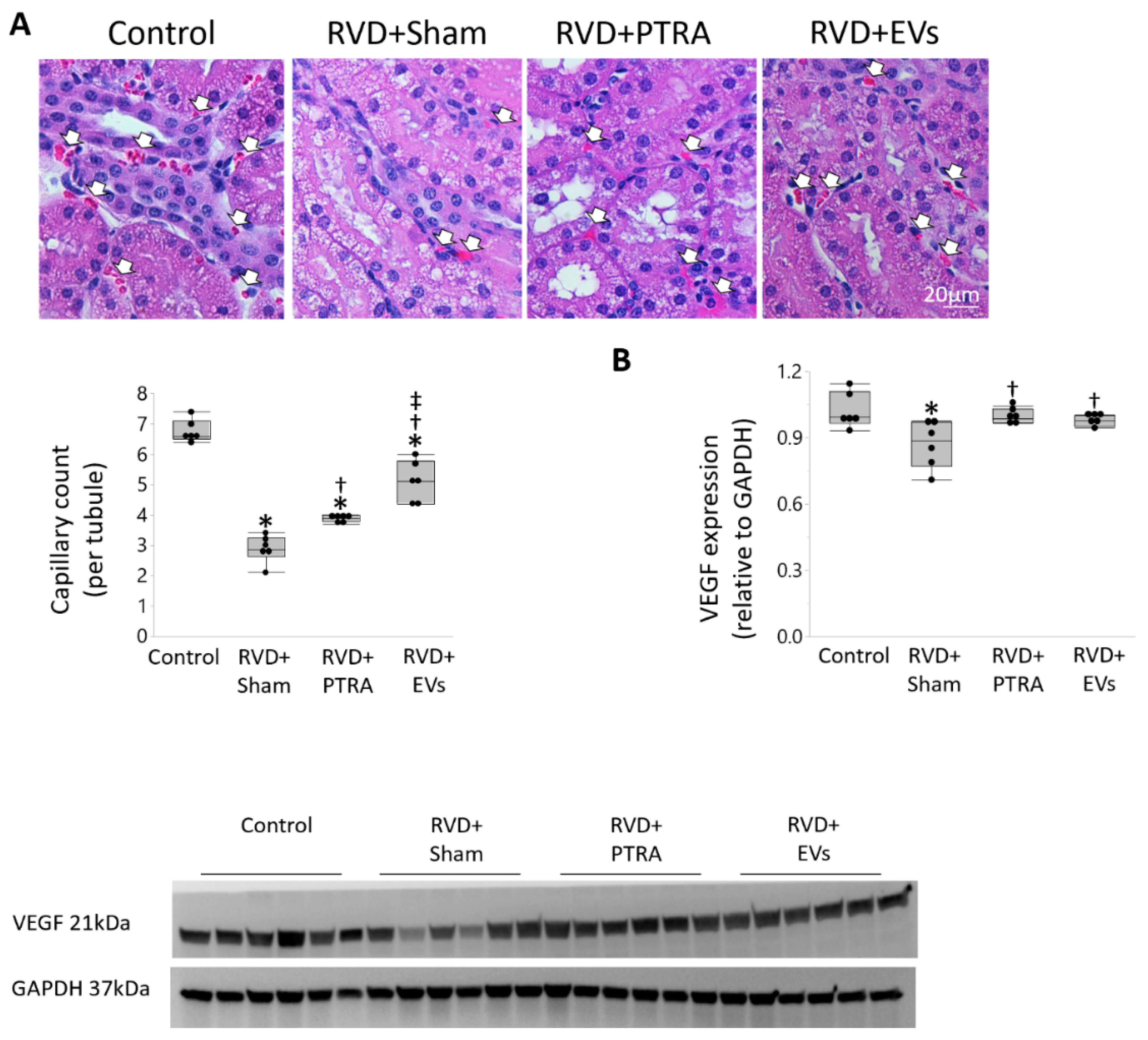
2.6. Renal Injury
2.7. Statistical Analysis
2.8. In Vitro Studies
3. Results
3.1. EVs Were Retained in the Stenotic Kidney
3.2. EVs Exerted Greater Improvement in the Intra-Renal Microvasculature Than PTRA
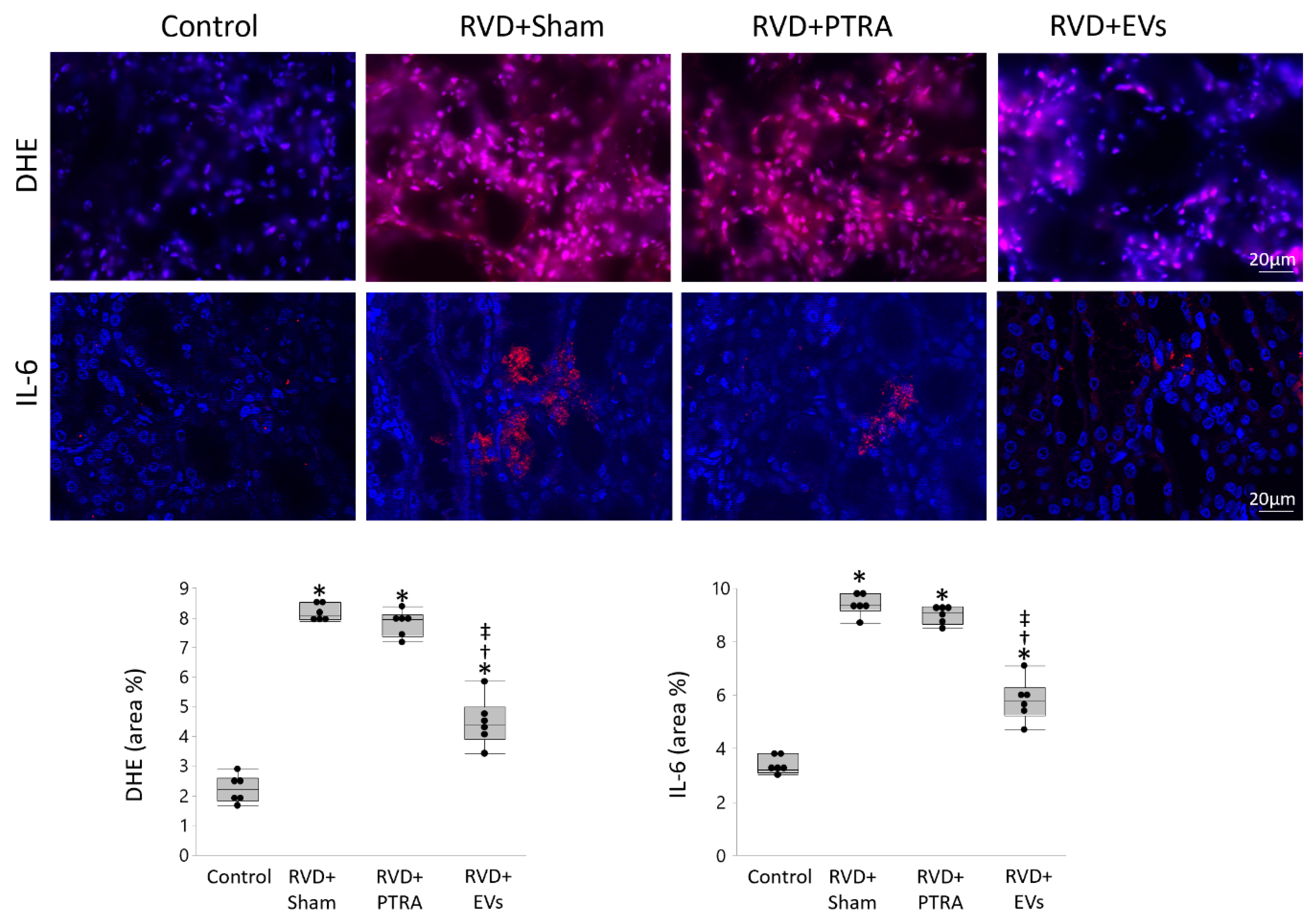
3.3. EVs Produced More Attenuation of Renal Injury Compared to PTRA
3.4. MSC-Derived EVs Were Enriched with Vasculo-Protective Genes
3.5. EVs Modulate Angiogenesis and Inflammation in Recipient Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, K.J.; Edwards, M.S.; Craven, T.E.; Cherr, G.S.; Jackson, S.A.; Appel, R.G.; Burke, G.L.; Dean, R.H. Prevalence of renovascular disease in the elderly: A population-based study. J. Vasc. Surg. 2002, 36, 443–451. [Google Scholar] [CrossRef]
- Textor, S.C.; Lerman, L.O. Paradigm Shifts in Atherosclerotic Renovascular Disease: Where Are We Now? J. Am. Soc Nephrol. 2015, 26, 2074–2080. [Google Scholar] [CrossRef]
- Eirin, A.; Lerman, L.O. Darkness at the end of the tunnel: Poststenotic kidney injury. Physiology 2013, 28, 245–253. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Saad, A.; Textor, S.C. Management of atherosclerotic renovascular disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL). Nephrol. Dial. Transplant. 2015, 30, 366–375. [Google Scholar] [CrossRef]
- Cooper, C.J.; Murphy, T.P.; Cutlip, D.E.; Jamerson, K.; Henrich, W.; Reid, D.M.; Cohen, D.J.; Matsumoto, A.H.; Steffes, M.; Jaff, M.R.; et al. Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis. N. Engl. J. Med. 2014, 370, 13–22. [Google Scholar] [CrossRef]
- Ritchie, J.; Green, D.; Chrysochou, C.; Chalmers, N.; Foley, R.N.; Kalra, P.A. High-Risk Clinical Presentations in Atherosclerotic Renovascular Disease: Prognosis and Response to Renal Artery Revascularization. Am. J. Kidney Dis. 2014, 63, 186–197. [Google Scholar] [CrossRef]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Herrmann, S.M.; Hickson, L.J.; Goksu, B.B.; Bendel, E.; Misra, S.; Glockner, J.; et al. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020, 97, 793–804. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Jonnada, S.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. Mesenchymal Stem Cell-Derived Extracellular Vesicles Improve the Renal Microvasculature in Metabolic Renovascular Disease in Swine. Cell Transplant. 2018, 27, 1080–1095. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Pawar, A.S.; Zhu, X.-Y.; Eirin, A.; Tang, H.; Jordan, K.L.; Woollard, J.R.; Lerman, A.; Lerman, L.O. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity 2015, 23, 399–407. [Google Scholar] [CrossRef]
- Lerman, L.O.; Schwartz, R.S.; Grande, J.P.; Sheedy, P.F.; Romero, J.C. Noninvasive evaluation of a novel swine model of renal artery stenosis. J. Am. Soc. Nephrol. 1999, 10, 1455–1465. [Google Scholar] [PubMed]
- Eirin, A.; Zhu, X.-Y.; Urbieta-Caceres, V.H.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am. J. Physiol. Physiol. 2011, 300, F1394–F1401. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Ferguson, C.M.; Zhu, X.-Y.; Saadiq, I.M.; Tang, H.; Lerman, A.; Lerman, L.O. Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem Cell Res. 2020, 47, 101877. [Google Scholar] [CrossRef]
- Krier, J.D.; Ritman, E.L.; Bajzer, Z.; Romero, J.C.; Lerman, A.; Lerman, L.O. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am. J. Physiol. Physiol. 2001, 281, F630–F638. [Google Scholar] [CrossRef]
- Eirin, A.; Ebrahimi, B.; Zhang, X.; Zhu, X.-Y.; Tang, H.; Crane, J.A.; Lerman, A.; Textor, S.C.; Lerman, L.O. Changes in Glomerular Filtration Rate After Renal Revascularization Correlate With Microvascular Hemodynamics and Inflammation in Swine Renal Artery Stenosis. Circ. Cardiovasc. Interv. 2012, 5, 720–728. [Google Scholar] [CrossRef]
- Eirin, A.; Saad, A.; Tang, H.; Herrmann, S.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Lerman, L.O. Urinary Mitochondrial DNA Copy Number Identifies Chronic Renal Injury in Hypertensive Patients. Hypertension 2016, 68, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Riester, S.M.; Zhu, X.-Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Adipose Tissue-Derived Mesenchymal Stem Cells Improve Revascularization Outcomes to Restore Renal Function in Swine Atherosclerotic Renal Artery Stenosis. Stem Cells 2012, 30, 1030–1041. [Google Scholar] [CrossRef]
- Eirin, A.; Zhang, X.; Zhu, X.-Y.; Tang, H.; Jordan, K.L.; Grande, J.P.; Dietz, A.B.; Lerman, A.; Textor, S.C.; Lerman, L.O. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol. Dial. Transplant. 2013, 29, 274–282. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Eirin, A.; Li, Z.; Zhu, X.-Y.; Zhang, X.; Lerman, A.; Textor, S.C.; Lerman, L.O. Mesenchymal Stem Cells Improve Medullary Inflammation and Fibrosis after Revascularization of Swine Atherosclerotic Renal Artery Stenosis. PLoS ONE 2013, 8, e67474. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Eirin, A.; Zhu, X.-Y.; Tang, H.; Chanana, P.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytom. Part A 2018, 93, 93–103. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef] [PubMed]
- Kalari, K.R.; A Nair, A.; Bhavsar, J.D.; O’Brien, D.R.; I Davila, J.; A Bockol, M.; Nie, J.; Tang, X.; Baheti, S.; Doughty, J.B.; et al. MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing. BMC Bioinform. 2014, 15, 224. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Chade, A.R.; Rodriguez-Porcel, M.; Bentley, M.D.; Ritman, E.L.; Lerman, A.; Lerman, L.O. Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler. Throm. Vasc. Biol. 2004, 24, 1854–1859. [Google Scholar] [CrossRef]
- Eirin, A.; Li, Z.; Zhang, X.; Krier, J.D.; Woollard, J.R.; Zhu, X.Y.; Tang, H.; Herrmann, S.M.; Lerman, A.; Textor, S.C.; et al. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 2012, 60, 1242–1249. [Google Scholar] [CrossRef]
- Nangaku, M.; E Alpers, C.; Pippin, J.; Shankland, S.J.; Kurokawa, K.; Adler, S.; Morgan, B.P.; Johnson, R.J.; Couser, W.G. CD59 protects glomerular endothelial cells from immune-mediated thrombotic microangiopathy in rats. J. Am. Soc. Nephrol. 1998, 9, 590–597. [Google Scholar]
- Eirin, A.; Ebrahimi, B.; Zhang, X.; Zhu, X.-Y.; Woollard, J.R.; He, Q.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc. Res. 2014, 103, 461–472. [Google Scholar] [CrossRef]
- Chade, A.R.; Zhu, X.; Lavi, R.; Krier, J.D.; Pislaru, S.; Simari, R.D.; Napoli, C.; Lerman, A.; Lerman, L.O. Endothelial Progenitor Cells Restore Renal Function in Chronic Experimental Renovascular Disease. Circulation 2009, 119, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, K.L.; Huh, W.; Kim, B.; Byun, J.; Suh, W.; Sung, J.; Jeon, E.-S.; Oh, H.-Y.; Kim, D.-K. Decreased Number and Impaired Angiogenic Function of Endothelial Progenitor Cells in Patients With Chronic Renal Failure. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1246–1252. [Google Scholar] [CrossRef]
- Chen, Z.; Herrmann, S.M.S.; Zhu, X.; Jordan, K.L.; Gloviczki, M.L.; Lerman, A.; Textor, S.C.; Lerman, L.O. Preserved Function of Late-Outgrowth Endothelial Cells in Medically Treated Hypertensive Patients Under Well-Controlled Conditions. Hypertension 2014, 64, 808–814. [Google Scholar] [CrossRef][Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ivanovic, V.; McKusick, M.A.; Johnson, C.M.; Sabater, E.A.; Andrews, J.C.; Breen, J.F.; Bjarnason, H.; Misra, S.; Stanson, A.W. Renal artery stent placement: Complications at a single tertiary care center. J. Vasc. Interv. Radiol. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Chade, A.R.; Kelsen, S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ. Cardiovasc. Interv. 2010, 3, 376–383. [Google Scholar] [CrossRef]
- Gerwins, P.; Sköldenberg, E.; Claesson-Welsh, L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit. Rev. Oncol. 2000, 34, 185–194. [Google Scholar] [CrossRef]
- Chade, A.R. Renal Vascular Structure and Rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar] [CrossRef]
- Chade, A.R.; Kelsen, S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: A novel potential therapeutic approach. Am. J. Physiol. Renal. Physiol. 2012, 302, 1342–1350. [Google Scholar] [CrossRef]
- Stewart, N.; Chade, A.R. Renoprotective effects of hepatocyte growth factor in the stenotic kidney. Am. J. Physiol Renal Physiol. 2013, 304, 625–633. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Pitulescu, M.E.; Schmidt, I.; Giaimo, B.D.; Antoine, T.; Berkenfeld, F.; Ferrante, F.; Park, H.; Ehling, M.; Biljes, D.; Rocha, S.F.; et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 2017, 19, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Wang, X.; Wang, Y.; Niu, A.; Wang, S.; Zou, C.; Harris, R.C. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017, 91, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 induces macrophages to M2-like phenotype via NF-kappaB. Cancer Manag. Res. 2018, 10, 4217–4228. [Google Scholar] [CrossRef]
- Saad, A.; Herrmann, S.M.; Crane, J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Eirin, A.; Ebrahimi, B.; Lerman, L.O.; Textor, S.C. Stent Revascularization Restores Cortical Blood Flow and Reverses Tissue Hypoxia in Atherosclerotic Renal Artery Stenosis but Fails to Reverse Inflammatory Pathways or Glomerular Filtration Rate. Circ. Cardiovasc. Interv. 2013, 6, 428–435. [Google Scholar] [CrossRef]
- Wang, W.; Saad, A.; Herrmann, S.M.; Massat, A.E.; McKusick, M.A.; Misra, S.; Lerman, L.O.; Textor, S.C. Changes in inflammatory biomarkers after renal revascularization in atherosclerotic renal artery stenosis. Nephrol. Dial. Transplant. 2016, 31, 1437–1443. [Google Scholar] [CrossRef]
- Lerman, L.O.; Textor, S.C.; Grande, J.P. Mechanisms of Tissue Injury in Renal Artery Stenosis: Ischemia and Beyond. Prog. Cardiovasc. Dis. 2009, 52, 196–203. [Google Scholar] [CrossRef]
- Chade, A.R.; Rodriguez-Porcel, M.; Grande, J.P.; Zhu, X.; Sica, V.; Napoli, C.; Sawamura, T.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mechanisms of Renal Structural Alterations in Combined Hypercholesterolemia and Renal Artery Stenosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1295–1301. [Google Scholar] [CrossRef]
- Cervenka, L.; Wang, C.-T.; Mitchell, K.D.; Navar, L.G. Proximal Tubular Angiotensin II Levels and Renal Functional Responses to AT 1 Receptor Blockade in Nonclipped Kidneys of Goldblatt Hypertensive Rats. Hypertension 1999, 33, 102–107. [Google Scholar] [CrossRef]
- Wolf, G.; Mueller, E.; A Stahl, R.; Ziyadeh, F.N. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J. Clin. Investig. 1993, 92, 1366–1372. [Google Scholar] [CrossRef]
- Garovic, V.D. and Textor, S.C. Renovascular hypertension and ischemic nephropathy. Circulation 2005, 112, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Jiang, K.; Puranik, A.S.; Jordan, K.L.; Tang, H.; Zhu, X.; Lerman, L.O. Targeting Murine Mesenchymal Stem Cells to Kidney Injury Molecule-1 Improves Their Therapeutic Efficacy in Chronic Ischemic Kidney Injury. Stem Cells Transl. Med. 2018, 7, 394–403. [Google Scholar] [CrossRef]
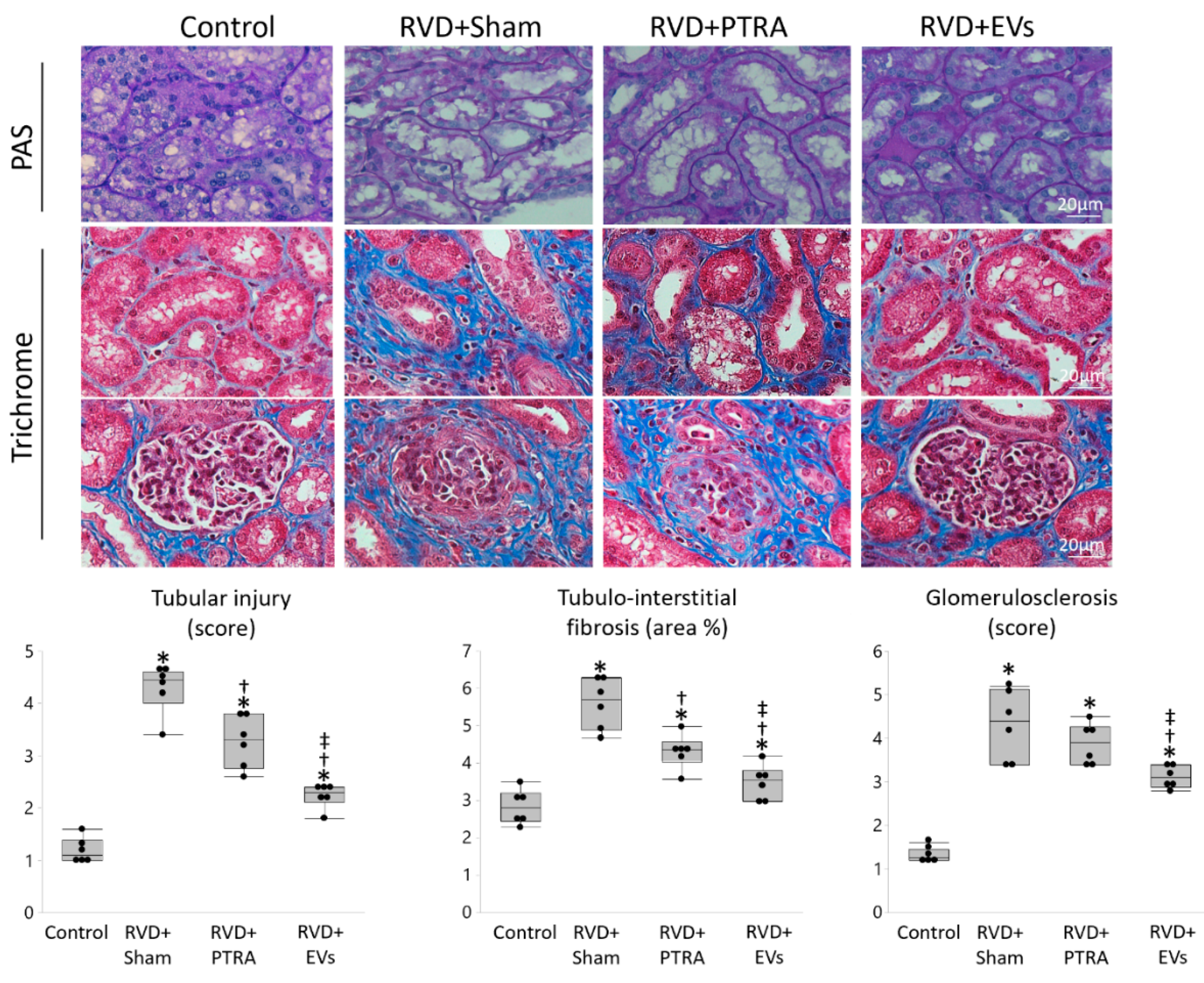
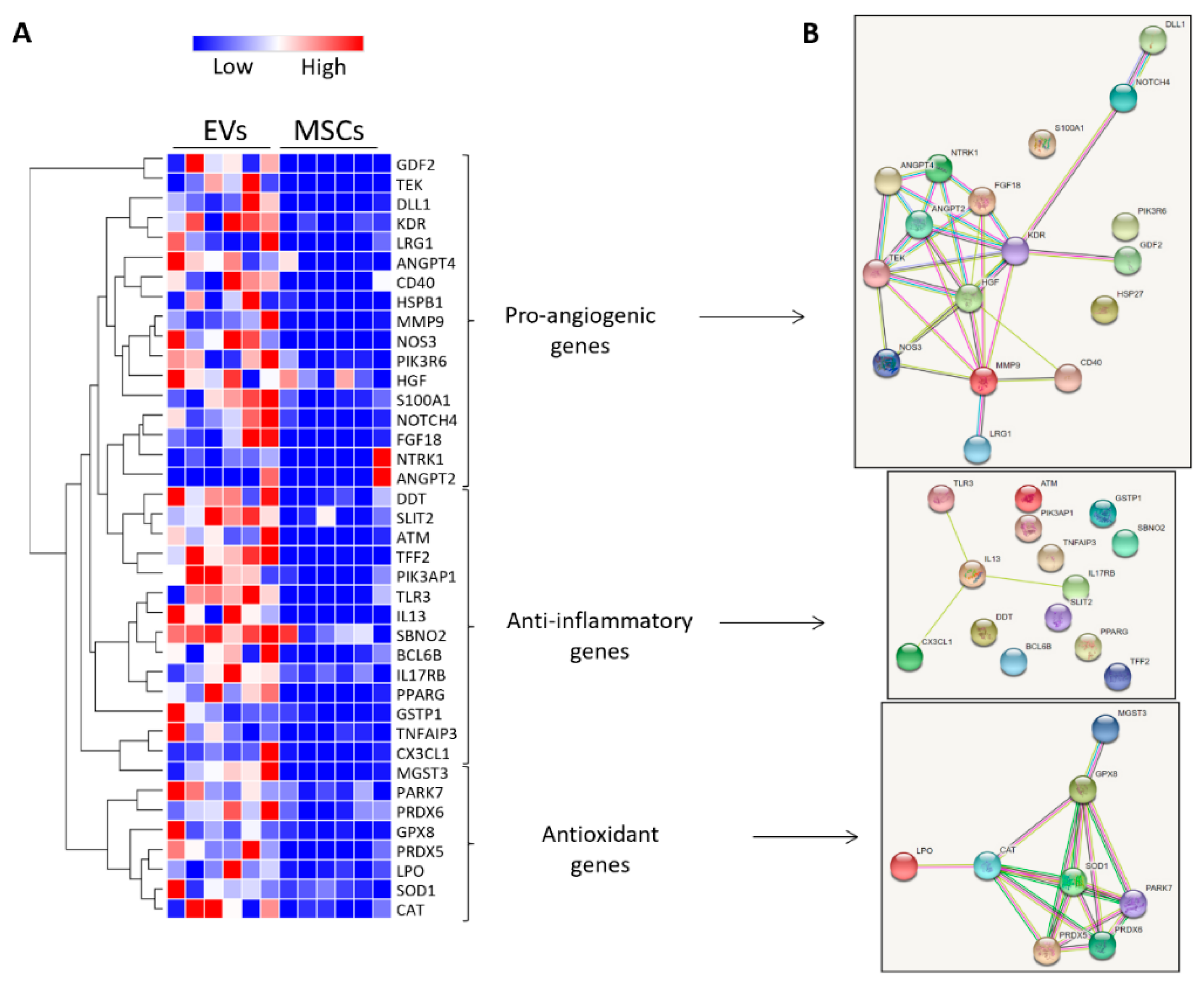
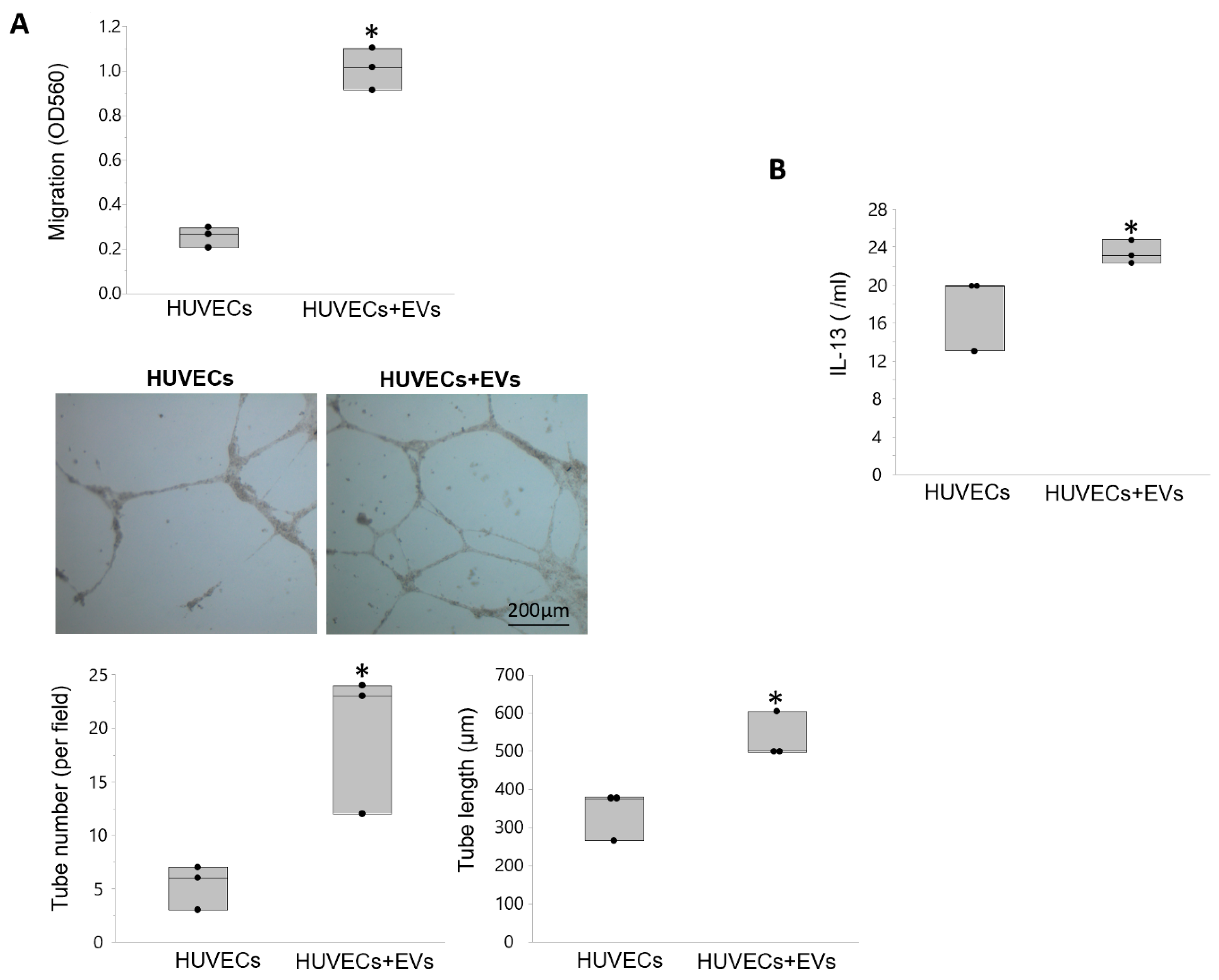
| Control | RVD + Sham | RVD + PTRA | RVD + EVs | |
|---|---|---|---|---|
| Body weight (kg) | 72.3± 5.9 | 92.2 ± 6.7 * | 84.9 ± 11.1 * | 86.7 ± 6.3 * |
| Degree of stenosis (%) | 0.0 ± 0.0 | 82.5 ± 10.4 * | 0.0 ± 0.0† | 75.5 ± 6.9 *,‡ |
| SBP (mmHg) | 129.2 ± 6.5 | 177.0 ± 9.9 * | 156.2± 16.5 *,† | 176.0 ± 10.5 *,‡ |
| DBP (mmHg) | 81.7 ± 3.7 | 116.0 ± 16.3 * | 97.5 ± 9.9 *,† | 112.2 ± 8.3 *,‡ |
| MAP (mmHg) | 97.5 ± 3.6 | 136.3 ± 12.7 * | 117.1 ± 11.3 *,† | 133.4 ± 7.4 *,‡ |
| 8-isoprostane (pg/mL) | 78.4 (75.4–95.3) | 306.8 (243.6–426.9) * | 356.6 (255.5–441.4) * | 111.0 (95.2–150.6) *,†,‡ |
| RV renin (ng/mL) | 175.1 ± 11.8 | 287.1 ± 22.5 * | 249.2 ± 15.0 *,† | 279.7 ± 23.8 *,‡ |
| PRA (ng/mL/h) | 0.15 ± 0.13 | 0.17 ± 0.07 | 0.16 ± 0.05 | 0.18 ± 0.11 |
| Serum creatinine (mg/dL) | 1.22 (1.18–1.23) | 1.93 (1.89–2.02) * | 1.67 (1.53–1.87) *,† | 1.62 (1.54–1.77) *,† |
| RBF (mL/min/kg) | 7.0 ± 0.6 | 6.3 ± 0.8 * | 9.1 ± 1.7 *,† | 8.8 ± 0.4 *,† |
| GFR (mL/min/kg) | 1.1 ± 0.1 | 1.0 ± 0.1 * | 1.5 ± 0.2 *,† | 1.5 ± 0.1 *,† |
| Intervention | RVD + PTRA | RVD + EVs |
|---|---|---|
| Blood pressure | ↓ | = |
| RBF | ↑ | ↑ |
| GFR | ↑ | ↑ |
| Serum creatinine | ↓ | ↓ |
| Microvascular density | ↑ | ↑↑ |
| Microvascular maturity | ↑ | ↑↑ |
| Peritubular capillary density | ↑ | ↑↑ |
| Renal oxidative stress | = | ↓ |
| Renal Inflammation | = | ↓ |
| Tubular injury | ↓ | ↓↓ |
| Tubulo-interstitial fibrosis | ↓ | ↓↓ |
| Glomerulosclerosis | = | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferguson, C.M.; Farahani, R.A.; Zhu, X.-Y.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Lerman, A.; Lerman, L.O.; Eirin, A. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Elicit Better Preservation of the Intra-Renal Microvasculature Than Renal Revascularization in Pigs with Renovascular Disease. Cells 2021, 10, 763. https://doi.org/10.3390/cells10040763
Ferguson CM, Farahani RA, Zhu X-Y, Tang H, Jordan KL, Saadiq IM, Lerman A, Lerman LO, Eirin A. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Elicit Better Preservation of the Intra-Renal Microvasculature Than Renal Revascularization in Pigs with Renovascular Disease. Cells. 2021; 10(4):763. https://doi.org/10.3390/cells10040763
Chicago/Turabian StyleFerguson, Christopher M., Rahele A. Farahani, Xiang-Yang Zhu, Hui Tang, Kyra L. Jordan, Ishran M. Saadiq, Amir Lerman, Lilach O. Lerman, and Alfonso Eirin. 2021. "Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Elicit Better Preservation of the Intra-Renal Microvasculature Than Renal Revascularization in Pigs with Renovascular Disease" Cells 10, no. 4: 763. https://doi.org/10.3390/cells10040763
APA StyleFerguson, C. M., Farahani, R. A., Zhu, X.-Y., Tang, H., Jordan, K. L., Saadiq, I. M., Lerman, A., Lerman, L. O., & Eirin, A. (2021). Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Elicit Better Preservation of the Intra-Renal Microvasculature Than Renal Revascularization in Pigs with Renovascular Disease. Cells, 10(4), 763. https://doi.org/10.3390/cells10040763








