Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Algal Material and Drying Methods
2.3. Extractions
2.4. Phenolic Composition
2.5. Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition
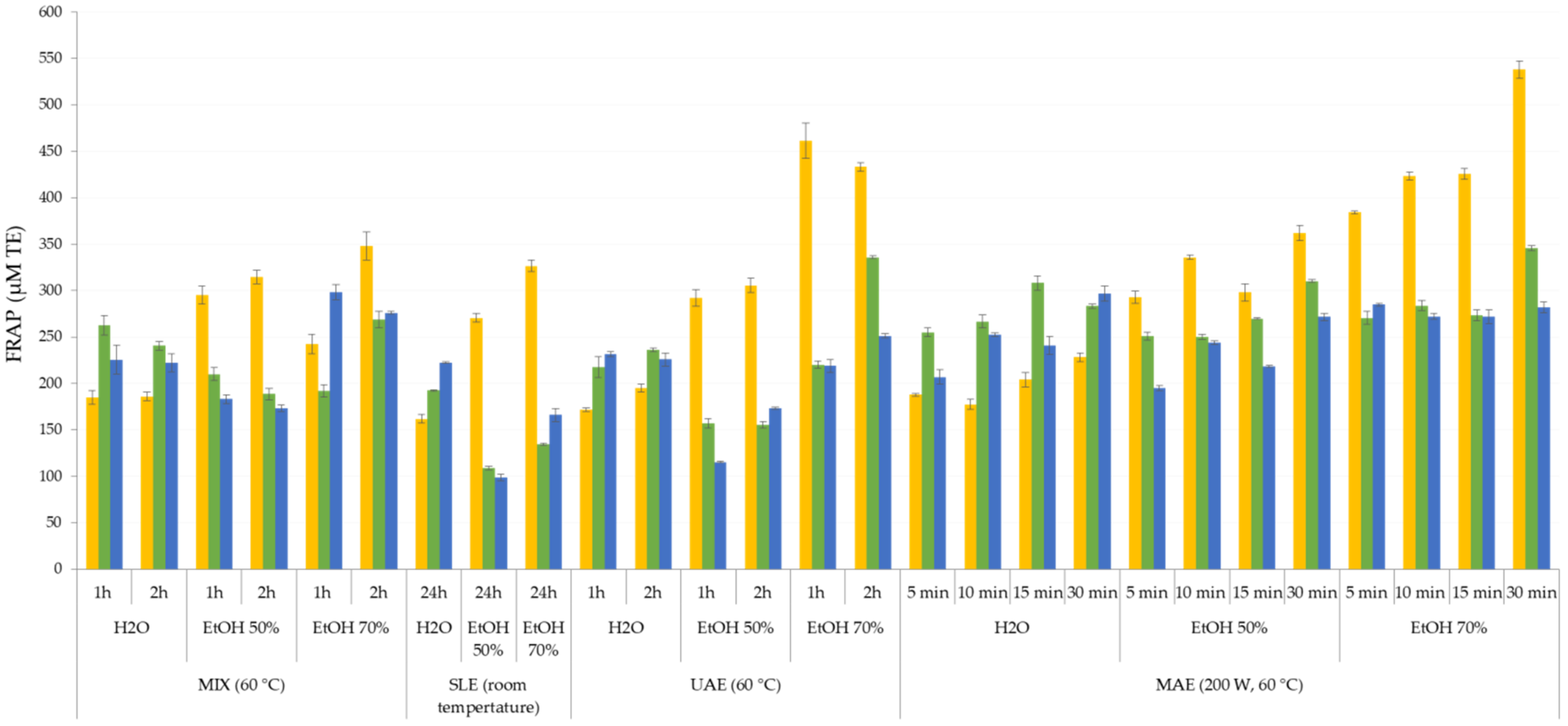
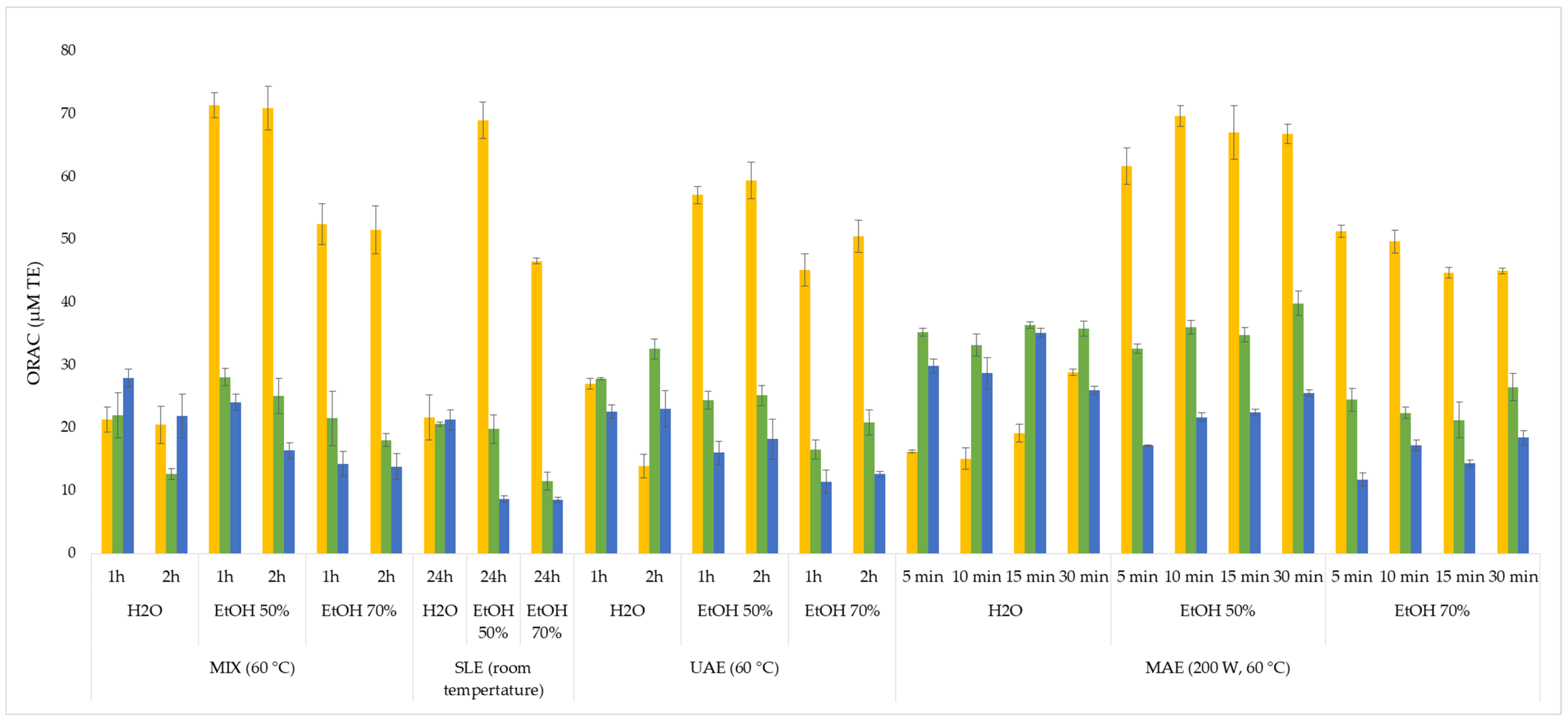
3.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silberfeld, T.; Bittner, L.; Fernández-García, C.; Cruaud, C.; Rousseau, F.; de Reviers, B.; Leliaert, F.; Payri, C.E.; De Clerck, O. Species Diversity, Phylogeny and Large Scale Biogeographic Patterns of the Genus Padina (Phaeophyceae, Dictyotales). J. Phycol. 2013, 49, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Ni-Ni-Win; Hanyuda, T.; Arai, S.; Uchimura, M.; Prathep, A.; Draisma, S.G.A.; Phang, S.M.; Abbott, I.A.; Millar, A.J.K.; Kawai, H. A Taxonomic Study of the Genus Padina (Dictyotales, Phaeophyceae) Including the Descriptions of Four New Species from Japan, Hawaii, and the Andaman Sea. J. Phycol. 2011, 47, 1193–1209. [Google Scholar] [CrossRef]
- Benita, M.; Dubinsky, Z.; Iluz, D. Padina Pavonica: Morphology and Calcification Functions and Mechanism. Am. J. Plant Sci. 2018, 9, 1156–1168. [Google Scholar] [CrossRef] [Green Version]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Bioactive Potential and Possible Health Effects of Edible Brown Seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Exploiting Biological Activities of Brown Seaweed Ecklonia Cava for Potential Industrial Applications: A Review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, G.; Minetti, M.; Polizzotto, G.; Biazzo, M.; Santucci, A. Pro-Apoptotic Activity of French Polynesian Padina Pavonica Extract on Human Osteosarcoma Cells. Mar. Drugs 2018, 16, 504. [Google Scholar] [CrossRef] [Green Version]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Kranl, K.; Schlesier, K.; Bitsch, R.; Hermann, H.; Rohe, M.; Böhm, V. Comparing Antioxidative Food Additives and Secondary Plant Products—Use of Different Assays. Food Chem. 2005, 93, 171–175. [Google Scholar] [CrossRef]
- Ling, A.L.M.; Yasir, S.; Matanjun, P.; Abu Bakar, M.F. Effect of Different Drying Techniques on the Phytochemical Content and Antioxidant Activity of Kappaphycus Alvarezii. J. Appl. Phycol. 2015, 27, 1717–1723. [Google Scholar] [CrossRef]
- Amorim, A.M.; Nardelli, A.E.; Chow, F. Effects of Drying Processes on Antioxidant Properties and Chemical Constituents of Four Tropical Macroalgae Suitable as Functional Bioproducts. J. Appl. Phycol. 2020, 32, 1495–1509. [Google Scholar] [CrossRef]
- Phasanasophon, K.; Kim, S.M. Antioxidant and Cosmeceutical Activities of Agarum Cribrosum Phlorotannin Extracted by Ultrasound Treatment. Nat. Prod. Commun. 2018, 13, 565–570. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Şahin, S.; Şamlı, R. Optimization of Olive Leaf Extract Obtained by Ultrasound-Assisted Extraction with Response Surface Methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Alghazeer, R.; Elmansori, A.; Sidati, M.; Gammoudi, F.; Azwai, S.; Naas, H.; Garbaj, A.; Eldaghayes, I. In Vitro Antibacterial Activity of Flavonoid Extracts of Two Selected Libyan Algae against Multi-Drug Resistant Bacteria Isolated from Food Products. J. Biosci. Med. 2017, 5, 26–48. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.M.; Vaglica, V.; Oddo, E. Interspecific Variation in Total Phenolic Content in Temperate Brown Algae. J. Biol. Res. 2017, 90, 26–29. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective Effect of Seaweeds with High Antioxidant Activity from the Peniche Coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Fayad, S.; Nehmé, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morin, P. Macroalga Padina Pavonica Water Extracts Obtained by Pressurized Liquid Extraction and Microwave-Assisted Extraction Inhibit Hyaluronidase Activity as Shown by Capillary Electrophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef]
- Hlila, M.B.; Hichri, A.O.; Mahjoub, M.A.; Mighri, Z.; Mastouri, M. Antioxidant and Antimicrobial Activities of Padina Pavonica and Enteromorpha Sp. from the Tunisian Mediterranean Coast. J. Coast. Life Med. 2017, 336–342. [Google Scholar] [CrossRef]
- Minetti, M.; Bernardini, G.; Biazzo, M.; Gutierrez, G.; Geminiani, M.; Petrucci, T.; Santucci, A. Padina Pavonica Extract Promotes In Vitro Differentiation and Functionality of Human Primary Osteoblasts. Mar. Drugs 2019, 17, 473. [Google Scholar] [CrossRef] [Green Version]
- Ismail, G.A.; Gheda, S.F.; Abo-Shady, A.M.; Abdel-Karim, O.H. In Vitro Potential Activity of Some Seaweeds as Antioxidants and Inhibitors of Diabetic Enzymes. Food Sci. Technol. 2020, 40, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In Vitro Amoebicidal and Antioxidant Activities of Some Tunisian Seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Jouini, M.; Bel Haj Amor, H.; Mzoughi, Z.; Dridi, M.; Ben Said, R.; Bouraoui, A. Phytochemical Analysis and Evaluation of the Antioxidant, Anti-Inflammatory, and Antinociceptive Potential of Phlorotannin-Rich Fractions from Three Mediterranean Brown Seaweeds. Mar. Biotechnol. 2018, 20, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Al-Enazi, N.M.; Awaad, A.S.; Zain, M.E.; Alqasoumi, S.I. Antimicrobial, Antioxidant and Anticancer Activities of Laurencia Catarinensis, Laurencia Majuscula and Padina Pavonica Extracts. Saudi Pharm. J. 2018, 26, 44–52. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown Macroalgae from the Adriatic Sea as a Promising Source of Bioactive Nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; John Wiley & Sons: New York, NY, USA, 1980; pp. 181–200. [Google Scholar]
- Lovrić, V.; Putnik, P.; Bursać Kovačević, D.; Jukić, M.; Dragović-Uzelac, V. The Effect of Microwave-Assisted Extraction on the Phenolic Compounds and Antioxidant Capacity of Blackthorn Flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant Activity, Total Phenolics and Flavonoids Contents: Should We Ban in Vitro Screening Methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘“Antioxidant Power”’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of Oxidation and Browning of Macerated White Wine on Its Antioxidant and Direct Vasodilatory Activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase Inhibiting, Antioxidant, and Anti-Inflammatory Activity. J. Enzyme Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of Ambient Temperature on the Photosynthetic Activity and Phenolic Content of the Intertidal Cystoseira Compressa along the Italian Coastline. J. Appl. Phycol. 2019, 31, 3069–3076. [Google Scholar] [CrossRef]
- Magnusson, M.; Yuen, A.K.L.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A Comparative Assessment of Microwave Assisted (MAE) and Conventional Solid-Liquid (SLE) Techniques for the Extraction of Phloroglucinol from Brown Seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the Use of Enzyme-Assisted Extraction in Combination with Pressurized Liquids to Recover Bioactive Compounds from Algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of Pressurized Liquid Extraction (PLE) to Obtain Bioactive Fatty Acids and Phenols from Laminaria Ochroleuca Collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of Chemical Profile and Antioxidant Properties of the Brown Algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Ismail-ben Ali, A.; Ktari, L.; Boudabbous, A.; El Bour, M. Seasonal Variation of Antibacterial Activity of The Brown Alga Padina Pavonica (L) Thivy Collected From Northern Coast Of Tunisia. Bull. Inst. Natn. Scien. Tech. Mer de Salammbô. 2010, 37. [Google Scholar]



| Extraction | Solvent | Targeted Compound/Activity | Reference |
|---|---|---|---|
| MAE | Ethanol and methanol 80% | Antibacterial activity, flavonoids | [17] |
| Extracted in centrifuge tubes in a 37 °C water bath for 2.5 h | 96% Ethanol | Phenolic compounds | [18] |
| Stirring 12 h | Methanol and dichloromethane | Antioxidant activity, cytoprotectivepotential | [19] |
| Soxhlet extraction | Acetone | Pro-apoptotic activity, antioxidant activity | [9] |
| PLE, MAE, SFE, electroporation extraction | Petroleum ether, ethanol, ethyl acetate and water | Anti-hyaluronidase activity | [20] |
| Extracted at 100 °C for 2 h using a reflux condenser under reduced pressure | Acetone and water | Antioxidant activity, antimicrobial activity | [21] |
| Soxhlet extraction | Acetone | Pro-osteogenic ability, antioxidant activity | [22] |
| Extracted for 72 h in shaking incubators | 80% Acetone, 80% ethanol, 80% methanol and water | Antioxidant activity, antidiabetic activity | [23] |
| Maceration at room temperature twice for 24 h | Hexane, ethyl acetate, and methanol | Antiparasitic activity, antioxidant activity | [24] |
| SLE (30 min) | Ethanol 50% | Antioxidant activity, anti-inflammatory activity, and antinociceptive activity | [25] |
| Percolation at room temperature for 2 days | 95% Ethanol | Antimicrobial activity, antioxidant activity and anticancer activity | [26] |
| Extraction Method * | Solvent | Time | FD ** | OD | SD | FD | OD | SD | FD | OD | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | TFC (mg QE/g) | TTC (mg CE/g) | |||||||||
| MIX (60 °C) | H2O | 1 h | 1.85 ± 0.11 | 1.79 ± 0.07 | 1.57 ± 0.03 | 1.06 ± 0.08 | 1.59 ± 0.04 | 1.64 ± 0.11 | 8.07 ± 0.79 | 10.02 ± 0.51 | 6.77 ± 0.52 |
| 2 h | 1.94 ± 0.05 | 1.74 ± 0.01 | 1.56 ± 0.03 | 1.01 ± 0.04 | 2.01 ± 0.08 | 1.68 ± 0.07 | 9.02 ± 0.77 | 9.79 ± 0.85 | 8.67 ± 0.35 | ||
| EtOH 50% | 1 h | 4.25 ± 0.12 | 1.29 ± 0.04 | 0.79 ± 0.06 | 0.81 ± 0.02 | 0.71 ± 0.05 | 0.69 ± 0.01 | 4.64 ± 0.46 | 4.06 ± 0.35 | 3.02 ± 0.28 | |
| 2 h | 3.90 ± 0.12 | 1.01 ± 0.04 | 0.65 ± 0.03 | 0.80 ± 0.05 | 0.62 ± 0.04 | 0.67 ± 0.00 | 3.25 ± 0.38 | 1.95 ± 0.12 | 1.63 ± 0.16 | ||
| EtOH 70% | 1 h | 3.84 ± 0.09 | 1.01 ± 0.03 | 0.71 ± 0.04 | 2.79 ± 0.07 | 2.21 ± 0.11 | 1.92 ± 0.08 | 7.43 ± 0.27 | 3.00 ± 0.24 | 2.18 ± 0.09 | |
| 2 h | 3.48 ± 0.19 | 0.89 ± 0.01 | 0.66 ± 0.05 | 2.21 ± 0.08 | 2.00 ± 0.06 | 2.07 ± 0.10 | 1.88 ± 0.02 | 2.86 ± 0.05 | 1.30 ± 0.08 | ||
| SLE (room temperature) | H2O | 24 h | 1.51 ± 0.11 | 1.31 ± 0.06 | 1.48 ± 0.05 | 0.84 ± 0.02 | 0.49 ± 0.02 | 0.36 ± 0.02 | 8.14 ± 0.64 | 6.23 ± 0.05 | 3.92 ± 0.35 |
| EtOH 50% | 24 h | 3.41 ± 0.12 | 0.63 ± 0.02 | 0.49 ± 0.09 | 0.84 ± 0.02 | 0.31 ± 0.01 | 0.43 ± 0.03 | 2.27 ± 0.10 | 2.30 ± 0.22 | 2.22 ± 0.19 | |
| EtOH 70% | 24 h | 3.31 ± 0.13 | 0.53 ± 0.06 | 0.44 ± 0.03 | 2.22 ± 0.09 | 0.73 ± 0.05 | 1.15 ± 0.06 | 2.19 ± 0.14 | 1.44 ± 0.06 | 1.53 ± 0.04 | |
| UAE (60 °C) | H2O | 1 h | 1.72 ± 0.03 | 1.62 ± 0.12 | 1.43 ± 0.03 | 0.63 ± 0.02 | 0.32 ± 0.01 | 0.45 ± 0.01 | 5.34 ± 0.17 | 7.05 ± 0.46 | 5.96 ± 0.26 |
| 2 h | 1.70 ± 0.02 | 1.59 ± 0.12 | 1.31 ± 0.08 | 0.78 ± 0.01 | 0.38 ± 0.02 | 0.61 ± 0.02 | 10.41 ± 0.62 | 7.67 ± 0.03 | 6.16 ± 0.27 | ||
| EtOH 50% | 1 h | 3.94 ± 0.08 | 0.96 ± 0.08 | 0.64 ± 0.08 | 0.89 ± 0.04 | 0.48 ± 0.03 | 0.63 ± 0.02 | 2.90 ± 0.10 | 0.62 ± 0.03 | 0.94 ± 0.14 | |
| 2 h | 3.97 ± 0.12 | 0.92 ± 0.00 | 0.77 ± 0.09 | 0.92 ± 0.01 | 0.50 ± 0.04 | 0.65 ± 0.04 | 3.63 ± 0.05 | 1.16 ± 0.08 | 2.03 ± 0.19 | ||
| EtOH 70% | 1 h | 3.51 ± 0.09 | 0.82 ± 0.07 | 0.66 ± 0.06 | 2.83 ± 0.11 | 1.31 ± 0.01 | 1.83 ± 0.08 | 2.62 ± 0.20 | 2.12 ± 0.13 | 2.08 ± 0.07 | |
| 2 h | 3.45 ± 0.05 | 1.04 ± 0.05 | 0.61 ± 0.01 | 2.75 ± 0.02 | 2.25 ± 0.12 | 1.57 ± 0.05 | 2.41 ± 0.23 | 2.46 ± 0.08 | 2.07 ± 0.16 | ||
| MAE (200 W, 60 °C) | H2O | 5 min | 2.06 ± 0.01 | 1.56 ± 0.04 | 1.38 ± 0.05 | 0.51 ± 0.02 | 0.68 ± 0.02 | 0.47 ± 0.02 | 9.03 ± 0.79 | 0.32 ± 0.02 | 5.20 ± 0.41 |
| 10 min | 1.88 ± 0.19 | 1.95 ± 0.01 | 1.77 ± 0.07 | 0.52 ± 0.02 | 0.66 ± 0.01 | 0.54 ± 0.05 | 7.77 ± 0.45 | 3.45 ± 0.17 | 5.48 ± 0.46 | ||
| 15 min | 1.90 ± 0.05 | 1.90 ± 0.18 | 1.84 ± 0.05 | 0.50 ± 0.03 | 0.68 ± 0.01 | 0.61 ± 0.04 | 6.25 ± 0.44 | 5.60 ± 0.19 | 3.12 ± 0.22 | ||
| 30 min | 2.11 ± 0.08 | 2.09 ± 0.01 | 1.75 ± 0.12 | 0.62 ± 0.03 | 0.69 ± 0.02 | 0.61 ± 0.02 | 5.86 ± 0.30 | 6.82 ± 0.64 | 5.14 ± 0.48 | ||
| EtOH 50% | 5 min | 4.32 ± 0.15 | 1.41 ± 0.05 | 1.01 ± 0.06 | 0.91 ± 0.04 | 1.06 ± 0.05 | 0.77 ± 0.04 | 2.19 ± 0.13 | 1.09 ± 0.08 | 1.06 ± 0.10 | |
| 10 min | 4.32 ± 0.13 | 1.46 ± 0.07 | 1.13 ± 0.05 | 1.48 ± 0.03 | 0.77 ± 0.05 | 0.82 ± 0.03 | 1.89 ± 0.18 | 0.92 ± 0.06 | 0.94 ± 0.09 | ||
| 15 min | 3.79 ± 0.07 | 1.51 ± 0.06 | 1.06 ± 0.05 | 1.12 ± 0.04 | 0.81 ± 0.02 | 0.77 ± 0.03 | 2.79 ± 0.13 | 1.38 ± 0.04 | 1.40 ± 0.06 | ||
| 30 min | 4.10 ± 0.13 | 1.64 ± 0.03 | 1.19 ± 0.05 | 1.27 ± 0.07 | 0.92 ± 0.06 | 0.86 ± 0.00 | 3.13 ± 0.26 | 0.93 ± 0.08 | 1.49 ± 0.13 | ||
| EtOH 70% | 5 min | 3.28 ± 0.02 | 1.12 ± 0.05 | 0.82 ± 0.05 | 2.87 ± 0.01 | 1.84 ± 0.01 | 1.79 ± 0.05 | 3.51 ± 0.16 | 1.56 ± 0.06 | 1.76 ± 0.08 | |
| 10 min | 3.48 ± 0.06 | 1.17 ± 0.10 | 0.87 ± 0.08 | 2.09 ± 0.12 | 1.82 ± 0.12 | 1.71 ± 0.05 | 2.45 ± 0.22 | 1.14 ± 0.11 | 1.22 ± 0.11 | ||
| 15 min | 3.46 ± 0.01 | 1.14 ± 0.05 | 0.89 ± 0.03 | 2.16 ± 0.08 | 1.82 ± 0.13 | 1.86 ± 0.02 | 3.30 ± 0.32 | 1.44 ± 0.04 | 1.86 ± 0.13 | ||
| 30 min | 2.98 ± 0.04 | 1.33 ± 0.05 | 0.95 ± 0.04 | 1.76 ± 0.03 | 2.11 ± 0.11 | 1.97 ± 0.12 | 3.51 ± 0.24 | 2.29 ± 0.19 | 1.87 ± 0.15 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. https://doi.org/10.3390/pr9040587
Čagalj M, Skroza D, Tabanelli G, Özogul F, Šimat V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes. 2021; 9(4):587. https://doi.org/10.3390/pr9040587
Chicago/Turabian StyleČagalj, Martina, Danijela Skroza, Giulia Tabanelli, Fatih Özogul, and Vida Šimat. 2021. "Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods" Processes 9, no. 4: 587. https://doi.org/10.3390/pr9040587
APA StyleČagalj, M., Skroza, D., Tabanelli, G., Özogul, F., & Šimat, V. (2021). Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes, 9(4), 587. https://doi.org/10.3390/pr9040587











