Abstract
Bee venom is a complex mixture composed of peptides, proteins with enzymatic properties, and low-molecular-weight compounds. Although the carboxylesterase in bee venom has been identified as an allergen, the enzyme’s role as a venom component has not been previously elucidated. Here, we show the lipolytic activity of a bumblebee (Bombus ignitus) venom carboxylesterase (BivCaE). The presence of BivCaE in the venom secreted by B. ignitus worker bees was confirmed using an anti-BivCaE antibody raised against a recombinant BivCaE protein produced in baculovirus-infected insect cells. The enzymatic activity of the recombinant BivCaE protein was optimal at 40 °C and pH 8.5. Recombinant BivCaE protein degrades triglycerides and exhibits high lipolytic activity toward long-chain triglycerides, defining the role of BivCaE as a lipolytic agent. Bee venom phospholipase A2 binds to mammalian cells and induces apoptosis, whereas BivCaE does not affect mammalian cells. Collectively, our data demonstrate that BivCaE functions as a lipolytic agent in bee venom, suggesting that BivCaE will be involved in distributing the venom via degradation of blood triglycerides.
Key Contribution:
BivCaE functions as a lipolytic agent in bumblebee venom and mediates distribution of the venom via degradation of blood triglycerides.
1. Introduction
Carboxylesterases have a broad substrate specificity and are involved in energy homeostasis and lipid metabolism [1]. Carboxylesterases catalyze the hydrolysis of esters and thioesters, including lipids. Lipases catalyze the reactions of long-chain triacylglycerols, whereas carboxylesterases catalyze the hydrolysis of short-chain triacylglycerols [2,3]. Moreover, carboxylesterases have been demonstrated to play detoxifying roles against drugs and toxicants [1].
In honeybees, carboxylesterases reportedly mediate physiological activities owing to their involvement in the detoxification or metabolism of insecticides [4] as well as the processing of dietary nicotine [5] and regulation of oxidative stress [6]. In numerous insect species, carboxylesterases are identified as major enzymes responsible for detoxifying xenobiotics [4,5,7,8]. On the other hand, carboxylesterases are present in bee venom [9,10] and wasp venom [11].
Bee venom is a mixture of toxic components and functions as a defense against predator attacks while also having components with pharmacological and therapeutic value [12,13,14,15]. Thus, bee venom has been used in alternative therapeutic methods, such as in bee venom therapy (VT) [14] and bee venom immunotherapy (VIT) [16,17]. Various pharmacotherapeutic effects of bee venom have been reported in the treatment of several disorders, including arthritic rheumatism, pain, and cancer [13,14,15]. Furthermore, the proteomic analysis of honeybee and bumblebee venoms has unraveled components of bee venom, such as peptides, proteins with enzymatic properties, and low-molecular-weight compounds [9,10,18,19]. Many studies have determined the biological and pharmacological properties of bee venom components, such as enzymes and peptides [9,10,13,20,21,22,23]. Although carboxylesterases present in honeybee (Apis mellifera) venom and bumblebee (Bombus terrestris) venom were identified as allergens [9,10], the biological and toxicological characteristics of bee venom carboxylesterases remain incompletely understood.
In the present study, we show the lipolytic activity of a bumblebee (Bombus ignitus) venom carboxylesterase (BivCaE). Because phospholipase A2 (PLA2) in bee venom is a cytotoxic and lipolytic enzyme that cleaves the glycerol backbone of phospholipids [24,25], we hypothesized that the carboxylesterase in bee venom acts as a lipolytic agent that produces toxicological rather than beneficial outcomes. We investigated the lipolytic role of BivCaE against triglycerides because carboxylesterase degrades triglycerides [2,3].
2. Results
2.1. BivCaE Is a Bee Venom Component
We cloned a BivCaE-encoding cDNA in B. ignitus worker bees in our search for identifying bee venom components. BivCaE consists of a predicted 19-amino acid signal peptide and a 536-amino acid mature protein (Figure 1). BivCaE exhibited high similarity to bumblebee venom carboxylesterases (93–98% protein sequence identity) and showed 61–63% protein sequence identity with honeybee venom carboxylesterases (Figure 1). Also, the protein sequence analysis of BivCaE revealed typical features of carboxylesterases, including a catalytic triad composed of Ser–Glu–His and a consensus active site motif GXSXG (Figure 1). These features indicated that BivCaE is a carboxylesterase.
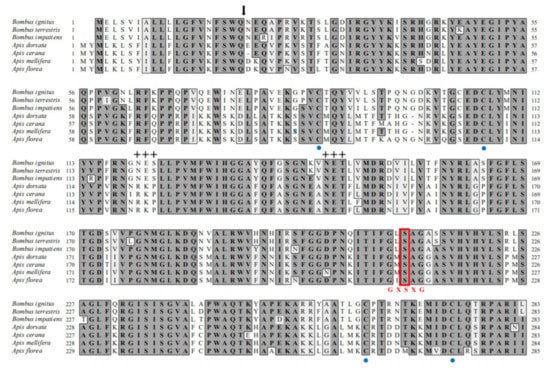
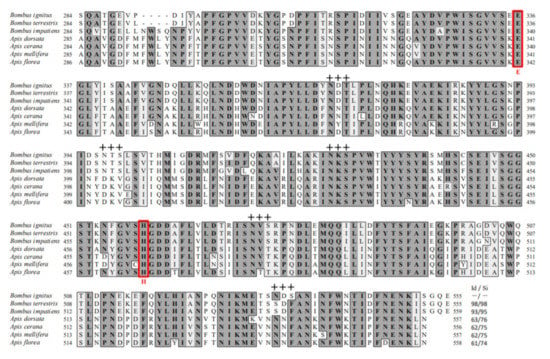
Figure 1.
Alignment of the protein sequences of BivCaE and venom carboxylesterases from bee species. Predicted signal sequence (arrow), conserved cysteine residues (blue circles), and potential N-glycosylation sites (crosses) are indicated. The Ser–Glu–His catalytic triads and the classical GXSXG pentapeptide motif containing Ser residue are indicated with red letters. The protein sequences were aligned using BivCaE (this study, GenBank accession no. MW699017), B. terrestris venom carboxylesterase (XP_003394675), B. impatiens venom carboxylesterase (XP_012241172), A. dorsata venom carboxylesterase (XP_031367211), A. cerana venom carboxylesterase (XP_016912910), A. mellifera venom carboxylesterase (NP_001119716), and A. florea venom carboxylesterase (XP_031776417). Identity/similarity (Id/Si) values were determined using BivCaE sequence as a reference.
To characterize BivCaE, we produced recombinant BivCaE protein in baculovirus-infected insect cells and generated an anti-BivCaE antibody against recombinant BivCaE protein (Figure 2A). As shown in Figure 1, the protein sequence of BivCaE revealed several N-linked glycosylation sites. Glycoprotein staining analysis indicated that recombinant BivCaE protein was produced as a 71.4 kDa protein, which was 9.4 kDa greater than the predicted molecular mass of 62 kDa, thereby demonstrating that recombinant BivCaE protein was N-linked glycosylated (Figure 2B).
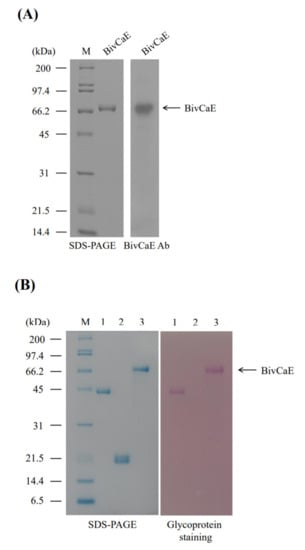
Figure 2.
Production of recombinant BivCaE protein. (A) Recombinant BivCaE protein purified from baculovirus-infected insect cells was analyzed using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (left) and Western blot carried out by employing anti-BivCaE antibody against recombinant BivCaE (right). A molecular weight standard (M) is indicated. (B) Glycoprotein staining of recombinant BivCaE protein. Lane 1, horseradish peroxidase (positive control); lane 2, soybean trypsin inhibitor (negative control); and lane 3, recombinant BivCaE protein. The molecular weight standard (M) and recombinant BivCaE (arrow) are shown.
The expression profile of BivCaE in B. ignitus worker bees was examined to confirm that BivCaE is a component of B. ignitus venom. Northern blot analysis revealed BivCaE transcripts in all the tissues investigated in this study (Figure 3A). Western blot analysis indicated that BivCaE proteins were detected in all the tissues, consistent with the Northern blot data, and demonstrated that BivCaE is present in the venom secreted by B. ignitus worker bees (Figure 3B); therefore, this result confirms that BivCaE is a venom component.
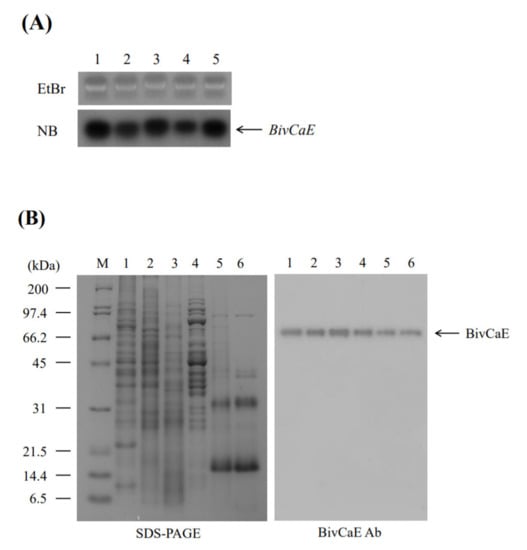
Figure 3.
Expression of BivCaE in B. ignitus. (A) Expression of BivCaE in B. ignitus was carried out by Northern blot analysis (NB; lower panel) using total RNA from the epidermis (lane 1), the fat body (lane 2), the gut (lane 3), muscle (lane 4), and the venom gland (lane 5) of B. ignitus worker bees. The ethidium bromide-stained RNA gel (EtBr; upper panel) is shown. BivCaE transcripts are indicated. (B) Expression of BivCaE in B. ignitus was analyzed using 12% SDS-PAGE (left) and Western blotting technique by employing anti-BivCaE antibody (right). Protein samples were prepared from the epidermis (lane 1), the fat body (lane 2), the gut (lane 3), muscle (lane 4), the venom gland (lane 5), and the secreted venom (lane 6) of B. ignitus worker bees. The molecular weight standard (M) and BivCaE proteins (arrow) are shown.
2.2. BivCaE Functions as a Carboxylesterase
To assess BivCaE as a carboxylesterase, we assayed the enzymatic property of recombinant BivCaE protein. The enzyme activity of recombinant BivCaE protein was determined at varying pH levels, temperatures, and incubation times. When assayed under the condition of pH 8.5, the optimum temperature for the activity of recombinant BivCaE protein was 40 °C (Figure 4A). When assayed under conditions of 40 °C for 1 h, recombinant BivCaE protein showed the optimum activity at pH 8.5 (Figure 4B). The optimum incubation time for recombinant BivCaE activity was 120 min at 40 °C and pH 8.5 (Figure 4C). These results indicate that BivCaE is a carboxylesterase.
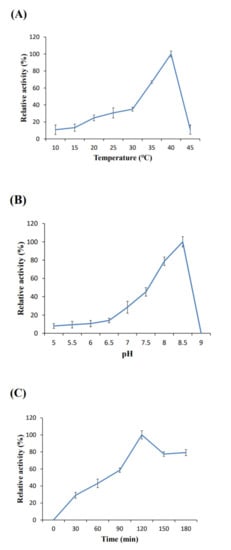
Figure 4.
Enzymatic properties of recombinant BivCaE protein. Temperature (A), pH (B), and incubation time (C) for the optimum activity of recombinant BivCaE protein (n = 3). Error bars represent SD.
2.3. BivCaE Acts as a Lipolytic Agent
Because in this study, recombinant BivCaE protein revealed carboxylesterase activity, we first determined whether BivCaE exhibited lipolytic activity against triglycerides. The triglyceride degradation assay showed that recombinant BivCaE protein degraded triglycerides in a BivCaE concentration-dependent manner (Figure 5A). Next, the substrate specificity assay of BivCaE using tributyrin (C4), tricaprylin (C8), and triolein (C18:1) indicated that BivCaE exhibited high lipolytic activity against longer chains, such as tricaprylin and triolein (Figure 5B). These results support the fact that BivCaE in bumblebee venom acts as a lipolytic agent that degrades triglycerides.
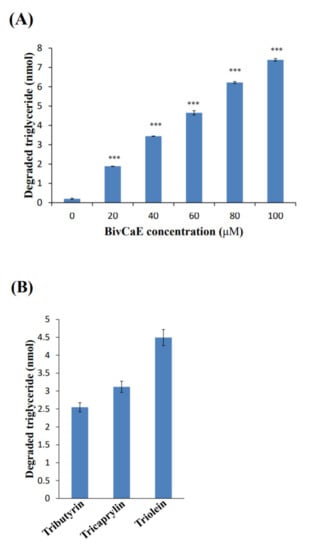
Figure 5.
Lipolytic activity of recombinant BivCaE protein. (A) Degradation of triglyceride by recombinant BivCaE protein. Triglyceride was treated with recombinant BivCaE protein (0, 20, 40, 60, 80, or 100 μM) for 20 min (n = 3). Data are represented as the mean ± SD. *** p < 0.001. (B) Substrate specificity of recombinant BivCaE protein against triglycerides. Tributyrin (C4), tricaprylin (C8), and triolein (C18:1) were treated with recombinant BivCaE protein (60 μM) for 20 min (n = 3). Data are represented as the mean ± SD.
As previously reported, the lipolytic enzyme PLA2 binds to mammalian cells and induces apoptosis [24]. Thus, to assess whether BivCaE affects the mammalian cell membrane, we investigated the recombinant BivCaE protein’s cell binding activity. A cell binding assay was performed using the Western blot technique, which indicated that recombinant BivCaE protein did not bind to mammalian cells (Figure 6A). We further investigated the binding of recombinant BivCaE to mammalian cells using immunofluorescence staining. We confirmed that recombinant BivCaE did not affect mammalian cells, whereas B. terrestris PLA2 bound to mammalian cells and induced apoptosis (Figure 6B), consistent with the findings of B. ignitus PLA2 [25]. Collectively, these results indicate that BivCaE in bumblebee venom can act as a lipolytic agent by degrading blood triglycerides but not whole mammalian cells.
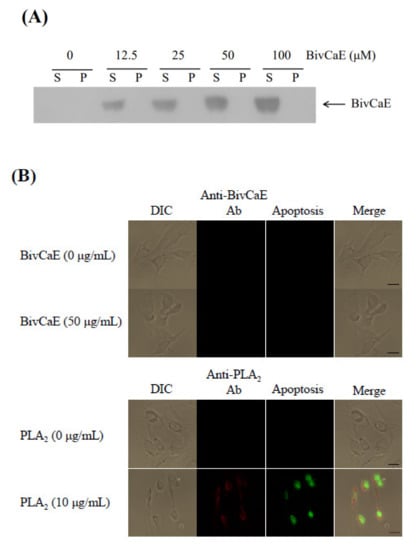
Figure 6.
Cell binding assay of recombinant BivCaE protein. (A) Western blot analysis of the binding of recombinant BivCaE protein to mammalian cells. NIH 3T3 cells were incubated with recombinant BivCaE protein (0, 12.5, 25, 50, or 100 μg/mL of the medium) for 24 h. Recombinant BivCaE protein bound to the pellet (P) and free recombinant BivCaE protein in the supernatant (S) were analyzed using Western blotting by employing anti-His-tag antibody. (B) Representative immunofluorescence images of the recombinant BivCaE protein or B. terrestris PLA2 bound to mammalian cells. NIH 3T3 cells were incubated with recombinant BivCaE protein (0 or 50 μg/mL of the medium) or B. terrestris PLA2 (0 or 10 μg/mL of the medium) for 24 h. Apoptosis (green) and the binding of recombinant BivCaE protein (red) or B. terrestris PLA2 (red) to the cells were observed. Merged confocal images are shown. The scale bar is 20 µm.
3. Discussion
Bee venom is a complex mixture of bioactive substances, including various enzymes [9,10,13,14,26]. Considering that bee venom components may both facilitate venom distribution and cause tissue damage [9], the biological role of bee venom components is associated with toxicological outcomes but can also have pharmacological applications [13]. Bee venom’s pharmacotherapeutic effects have already been reported in treating several diseases, such as arthritic rheumatism, pain, and neoplastic diseases [13,14,15]. Moreover, bee venom proteomic studies have unraveled the venom composition and extended knowledge of potential venom allergens [9,10,18,19]. Although the bee venom carboxylesterase was identified as an allergen in honeybees and bumblebees [9,10], the enzyme’s functions as a bee venom component are not yet fully understood. Here, we reported the functional role of BivCaE as a bumblebee venom carboxylesterase.
We hypothesized that BivCaE is a B. ignitus venom carboxylesterase because it possesses a catalytic triad of Ser, Glu, and His residues and the classical GXSXG pentapeptide motif contains Ser residue, which are typical features of carboxylesterases [2,3,6]. Notably, the catalytic triad of Ser–Glu–His, a typical α/β hydrolase structure, and the consensus active site motif GXSXG were observed to be identical in bee venom carboxylesterases. Considering the protein sequence identity of BivCaE with putative venom carboxylesterases in other bee species, BivCaE is highly likely to be a B. ignitus bee venom carboxylesterase. Additionally, the expression profile obtained using Northern and Western blot analyses indicated that besides being a component of B. ignitus worker bee venom, BivCaE is present in many tissues, including the epidermis, the fat body, the gut, muscle, and the venom gland. The enzyme activity of BivCaE was confirmed using the recombinant BivCaE produced in insect cells. Together, these results indicate that BivCaE is a B. ignitus venom carboxylesterase.
Carboxylesterase, which belongs to the lipolytic enzyme family, exhibits lipolytic activity against triglycerides [2,3,6]. Because BivCaE is a bee venom carboxylesterase, we determined whether BivCaE also exhibits lipolytic activity and found that recombinant BivCaE degrades triglycerides and exhibits strong lipolytic activity against longer-chain triglycerides. Our data were similar to the results showing that carboxylesterases have a broad substrate specificity [1] and exhibit lipolytic activity against various triglycerides [2,3]. Collectively, our results show that BivCaE functions as a lipolytic agent, defining the role of BivCaE in B. ignitus venom.
Bee venom PLA2, known as a lipolytic enzyme, cleaves the glycerol backbone of phospholipids and induces cytotoxicity in mammalian cells [24,27]. Our present study revealed that purified B. terrestris PLA2 bound to mammalian cells and induced apoptosis, consistent with the findings of B. ignitus PLA2 in our previous study [25]; in contrast, recombinant BivCaE did not affect mammalian cells. Because bee venom is a mixture of toxic components that mediate the dissemination of venom and may also damage tissues [9], we also hypothesized that BivCaE might serve as a toxic component. Considering that bumblebee venom serine protease facilitates the distribution of venom throughout the bloodstream in mammals via fibrin(ogen)olytic activity [20,22], our results suggest that the lipolytic activity of BivCaE in bumblebee venom contributes toward the distribution of the venom components via degradation of blood triglycerides.
In conclusion, our data show evidence of the lipolytic activity of BivCaE against various triglycerides and of the role of BivCaE as a lipolytic agent in bumblebee venom. Together, our findings not only establish the biological and toxicological basis of bee venom carboxylesterase but may also offer insight into potential biomedical applications, including VT and VIT treatments.
4. Materials and Methods
4.1. Bumblebees and Venom Preparation
Bumblebee (Bombus ignitus and B. terrestris) worker bees were obtained from the National Academy of Agricultural Science, Republic of Korea. Venom from B. ignitus and B. terrestris worker bees was collected by stimulating their stings on the inside walls of collection tubes.
4.2. Complementary DNA (cDNA) Cloning and Sequence Analysis of BivCaE
The cDNA cloning of BivCaE cDNA was carried out using the B. ignitus cDNA library as previously described [20]. Plasmid DNA extraction was performed using the Wizard Mini-Prep Kit (Promega, Madison, WI, USA). The cDNA sequence was analyzed using the Basic Local Alignment Search Tool (BLAST) programs from National Center for Biotechnology Information (NCBI) [28]. The deduced protein sequences of BivCaE were aligned using MacVector (ver. 6.5, Oxford Molecular Ltd., Oxford, UK). The prediction of the signal sequence of BivCaE was carried out by the SignalP 4.1 Server [29]. The protein sequence of BivCaE was registered in GenBank (accession number MW699017).
4.3. Tissue Collection
Tissue samples of B. ignitus worker bees were collected using a stereo microscope (Zeiss, Jena, Germany) and washed with phosphate-buffered saline (PBS; 140 mM NaCl, 27 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4; pH 7.4). The fresh tissues were used for total RNA and protein sample preparation.
4.4. RNA Extraction and Northern Blot Analysis
Total RNA was extracted with a Total RNA Extraction Kit (Promega) and was separated in a 1.0% formaldehyde agarose gel (5 µg/lane). After electrophoresis, total RNA was transferred onto a nylon blotting membrane (Schleicher & Schuell, Dassel, Germany) and hybridized with BivCaE cDNA labeled with [α-32P]dCTP (Amersham Biosciences, Piscataway, NJ, USA). The cDNA labeling was performed using the Prime-It II Random Primer Labeling Kit (Stratagene, La Jolla, CA, USA). Hybridization and exposure were carried out using the method described by Choo et al. [20] and Yang et al. [30].
4.5. Production of Recombinant BivCaE Protein
The production of recombinant BivCaE proteins was performed using a baculovirus expression system [31]. The BivCaE cDNA, including His-tag sequence, was PCR-amplified using the following primer set: forward (1–18) 5′-ATGGAACTATCAGTTATC-3′ and reverse (1651–1668) 5′-TTACTCCTGCCCACTTAT-3′. The BivCaE cDNA was introduced into the baculovirus vector pBacPAK8 (Clontech, Palo Alto, CA, USA) to construct the pBacPAK8-BivCaE. For generating BivCaE protein-expressing recombinant baculoviruses, pBacPAK8-BivCaE (500 ng) was co-transfected with baculoviral DNA (100 ng) into insect Sf9 cells (1.0–1.5 × 106 cells/well of a 6-well plate) using Lipofectin transfection reagent (Gibco BRL, Gaithersburg, MD, USA). Recombinant BivCaE proteins in insect Sf9 cells were produced by infection of recombinant baculoviruses [30]. Recombinant BivCaE proteins were purified using the MagneHisTM Protein Purification System (Promega). Concentrations of recombinant BivCaE proteins were analyzed using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA).
4.6. PLA2 Purification
B. terrestris PLA2 from B. terrestris venom was purified using size-exclusion column chromatography [25]. The conditions for chromatography were as follows: Elution was performed at a flow rate of 0.5 mL/min using a HiPrep 16/60 SephacrylTM S-100 column (Amersham Biosciences, Baie d’Urfé, PQ, Canada). The column was equilibrated with 0.05 M sodium phosphate buffer (pH 7.2) containing 0.15 M NaCl.
4.7. Polyclonal Antibody Production
A polyclonal antibody was produced in mice [32]. Purified recombinant BivCaE (5 μg) or purified B. terrestris PLA2 (5 μg) mixed with Freund’s complete adjuvant (Sigma, St. Louis, MO, USA) was injected into Institute of Cancer Research (ICR) mice (Samtako Bio Korea Co., Osan, Korea). At 1-week intervals, the mice were given two successive injections of recombinant BivCaE or B. terrestris PLA2 mixed with Freund’s incomplete adjuvant (Sigma). The last injection was administered using recombinant BivCaE protein (5 μg) or B. terrestris PLA2 (5 μg) without an adjuvant. Three days after the last injection, the collected blood was centrifuged at 15,000× g for 10 min at 4 °C. The serum was used for Western blot analysis and immunofluorescence staining.
4.8. Protein Analysis
Protein analysis was carried out using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Protein samples were subjected to 10% or 12% SDS-PAGE and were stained with Coomassie Brilliant Blue R-250 [33]. Western blot analysis was carried out using the enhanced chemiluminescence (ECL) Western blot system (Amersham Biosciences) with anti-BivCaE antibodies. Glycoprotein was stained using Gel Code Glycoprotein Staining Kit (Pierce, Rockford, IL, USA).
4.9. Enzymatic Assay
Esterase activity of recombinant BivCaE protein was assayed using the method described by Rejón et al. [34]. The effect of pH (5–9), temperature (10–45 °C), and time of incubation (30–180 min) was measured by the amount of p-nitrophenol (p-NP) formed from p-nitrophenyl butyrate (p-NPB) ester. Recombinant BivCaE protein (0.05 mol/L) was added to a solution of 880 μL of 50 mM Tris-HCl (pH 8.0), 100 μL of 0.4% (v/v) Triton X-100, and 10 μL of 1.76% p-NPB (v/v in acetonitrile), and the reaction mixture was incubated for 30 min at 30 °C. The amount of p-NP released was analyzed at 405 nm. The enzyme activity of recombinant BivCaE protein was represented as the % of control activity. The triglyceride degradation assay was performed using a Chemical Chemistry Analyzer (Bio-Rad) according to the manufacturer’s instructions. Recombinant BivCaE (0–100 μM) was incubated with 10 nmol triglyceride for 20 min, and the degraded triglycerides were measured. The enzyme substrate specificity was assayed using tributyrin (C4), tricaprylin (C8), and triolein (C18:1) as substrates [3]. Recombinant BivCaE (60 μM) was incubated with 10 nmol substrates for 20 min, and the degraded triglycerides were measured.
4.10. Cell Binding Assay
The cell binding assay for recombinant BivCaE proteins was carried out using the murine fibroblast cell line NIH 3T3 [32]. NIH 3T3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma) supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 atmosphere. The cells cultured onto 6-well plates (1 × 106 cells/well) were incubated with serially diluted recombinant BivCaE protein (0, 12.5, 25, 50, or 100 μg per mL of the medium) for 24 h. After incubation, the media were collected by centrifugation at 1000× g for 5 min, whereas the cell pellets were resuspended in PBS after washing two times with PBS. Western blot analysis of cell binding of recombinant BivCaE protein was performed using the cell pellets and supernatant samples with the anti-BivCaE antibody.
4.11. Immunofluorescence Staining
NIH 3T3 cells were treated with recombinant BivCaE protein (50 μg per mL of the medium) or B. terrestris PLA2 (10 μg per mL of the medium) for 24 h. Subsequently, the NIH 3T3 cells were double-labeled with mouse anti-BivCaE or anti-PLA2 antibody using an in situ cell death detection kit (Roche Applied Science, Mannheim, Germany). The cell binding assays of the recombinant BivCaE or PLA2 on the NIH 3T3 cells were carried out by incubation with mouse anti-BivCaE or anti-PLA2 antibody (diluted 1:200 (v/v)). Subsequently, the NIH 3T3 cells were incubated with tetramethylrhodamine isothiocyanate-conjugated goat anti-mouse Immunoglobulin G (IgG; diluted 1:300 (v/v); Santa Cruz Biotech, Inc., Santa Cruz, CA, USA). Apoptosis assay was performed in NIH 3T3 cells by incubation with the Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) reaction mixture, including terminal deoxynucleotidyl transferase (TdT) and fluorescein-conjugated dUTP. After incubation at 37 °C for 1 h, binding of the recombinant BivCaE or PLA2 on NIH 3T3 cell surfaces and apoptosis of NIH 3T3 cells were observed using laser-scanning confocal microscopy (Carl Zeiss LSM 510, Zeiss, Jena, Germany).
4.12. Statistical Analysis
Data were represented as the mean ± standard deviation (SD). All data were analyzed through independent unpaired two-tailed Student’s t-test using statistical software (SPSS 22.0, IBM, Chicago, IL, USA). Statistical significance was represented as p values (* p < 0.05, ** p < 0.01, and *** p < 0.001).
Author Contributions
Conceptualization, B.R.J.; Data curation, Y.D.; Formal analysis, Y.D., B.Y.K., H.W., J.L., K.S.L. and B.R.J.; Investigation, Y.D. and B.Y.K.; Resources, K.Y.L. and H.J.Y.; Writing—original draft, B.R.J.; Writing—review & editing, B.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Dong-A University Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Dong-A University Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lian, J.; Nelson, R.; Lehner, R. Carboxylesterases in lipid metabolism: From mouse to human. Protein Cell 2018, 9, 178–195. [Google Scholar] [CrossRef]
- Kang, C.H.; Oh, K.H.; Lee, M.H.; Oh, T.K.; Kim, B.H.; Yoon, J.H. A novel family VII esterase with industrial potential from compost metagenomic library. Microb. Cell Fact. 2011, 10, 41. [Google Scholar] [CrossRef]
- Privé, F.; Newbold, J.C.; Kaderbhai, N.N.; Girdwood, S.G.; Golyshina, O.V.; Golyshin, P.N.; Scollan, N.D.; Huws, S.A. Isolation and characterization of novel lipases/sterases from a bovine rumen metagenome. Appl. Microbiol. Biotechnol. 2015, 99, 5475–5485. [Google Scholar] [CrossRef]
- Badiou-Bénéteau, A.; Carvalho, S.M.; Brunet, J.L.; Carvalho, G.A.; Buleté, A.; Giroud, B.; Belzunces, L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxan. Ecotoxicol. Environ. Saf. 2012, 82, 22–31. [Google Scholar] [CrossRef]
- Du Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S.W. Detoxification mecanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jia, H.; Cui, X.; Zhai, N.; Wang, H.; Guo, X.; Xu, B. Isolation of carboxylesterase (esterase FE4) from Apis cerana cerana and its role in oxidative resistance during adverse environmental stress. Biochimie 2018, 144, 85–97. [Google Scholar] [CrossRef]
- Mao, K.; Ren, Z.; Li, W.; Cai, T.; Qin, X.; Wan, H.; Jin, B.R.; He, S.; Li, J. Carboxylesterase genes in nitenpyram-resistant brown planthopper, Nilapavata lugens. Insect Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.; Ge, P.; Yang, M.; Guo, Y.; Zhu, K.Y.; Ma, E.; Zhang, J. RNA interference revealed the roles of two carboxylesterase genes in insecticide detoxification in Locusta migratoria. Chemosphere 2013, 93, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Van Vaerenbergh, M.; Debyser, G.; Devreese, B.; de Graaf, D.C. Exploring the hidden honeybee (Apis mellifera) venom proteome by integrating a combinatorial peptide ligand library approach with FTMS. J. Proteom. 2014, 99, 169–178. [Google Scholar] [CrossRef]
- Van Vaerenbergh, M.; Debyser, G.; Smagghe, G.; Devreese, B.; de Graaf, D.C. Unraveling the venom proteome of the bumblebee (Bombus terrestris) by integrating a combinatorial peptide ligand library approach with FT-ICR MS. Toxicon 2015, 102, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Yang, X.; Liu, M.; Liu, C.; Han, L.; Guo, H.; Li, C.; Sun, Y.; Li, X.; Xiao, M.; et al. Biopanning of allergens from wasp sting patients. Biosci. Rep. 2018, 38, BSR20181113. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Aerts, M.; Danneels, E.; Devreese, B. Bee, wasp and ant venomics pave the way for a component-resolved diagnosis of sting allergy. J. Proteom. 2009, 72, 145–154. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Chen, J.; Lariviere, W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef]
- Hossen, S.; Shapla, U.M.; Gan, S.H.; Khalil, I. Impact of bee venom enzymes on diseases and immune responses. Molecules 2017, 22, 25. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Kelly, D.; Hamilton, R.G.; Craig, T.J. Venom immunotherapy reduces large local reactions to insect stings. J. Allergy Clin. Immunol. 2009, 123, 1371–1375. [Google Scholar] [CrossRef]
- Ozdemir, C.; Kucuksezer, U.C.; Akdis, M.; Akdis, C.A. Mechanisms of immunotherapy to wasp and bee venom. Clin. Exp. Allergy 2011, 41, 1226–1234. [Google Scholar] [CrossRef]
- Matysiak, J.; Hajduk, J.; Mayer, F.; Hebeler, R.; Kokot, Z.J. Hyphenated LC-MALDI-ToF/ToF and LC-ESI-QToF approach in proteomic characterization of honeybee venom. J. Pharm. Biomed. Anal. 2016, 121, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Barkan, N.P.; Bayazit, M.B.; Demiralp, D.O. Proteomic characterization of the venom of five Bombus (Thoracobombus) species. Toxins 2017, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.M.; Lee, K.S.; Yoon, H.J.; Kim, B.Y.; Sohn, M.R.; Roh, J.Y.; Je, Y.H.; Kim, N.J.; Kim, I.; Woo, S.D.; et al. Dual function of a bee venom serine protease: Prophenoloxidase-activating factor in arthropods and fibrin(ogen)olytic enzyme in mammals. PLoS ONE 2010, 5, e10393. [Google Scholar] [CrossRef]
- Choo, Y.M.; Lee, K.S.; Yoon, H.J.; Qiu, Y.; Wan, H.; Sohn, M.R.; Sohn, H.D.; Jin, B.R. Antifibrinolytic role of a bee venom serine protease inhibitor that acts as a plasmin inhibitor. PLoS ONE 2012, 7, e32269. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Choo, Y.M.; Yoon, H.J.; Jia, J.; Cui, Z.; Wang, D.; Kim, D.H.; Sohn, H.D.; Jin, B.R. Fibrin(ogen)olytic activity of bumblebee venom serine protease. Toxicol. Appl. Pharmacol. 2011, 255, 207–213. [Google Scholar] [CrossRef]
- Qiu, Y.; Lee, K.S.; Choo, Y.M.; Kong, D.; Yoon, H.J.; Jin, B.R. Molecular cloning and antifibrinolytic activity of a serine protease inhibitor from bumblebee (Bombus terrestris) venom. Toxicon 2013, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mingarro, I.; Pérez-Payá, E.; Pinilla, C.; Appel, J.R.; Houghten, R.A.; Blondelle, S.E. Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett. 1995, 372, 131–134. [Google Scholar] [CrossRef]
- Xin, Y.; Choo, Y.M.; Hu, Z.; Lee, K.S.; Yoon, H.J.; Cui, Z.; Sohn, H.D.; Jin, B.R. Molecular cloning and characterization of a venom phospholipase A2 from the bumblebee Bombus ignitus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Danneels, E.L.; Van Vaerenbergh, M.; Debyser, G.; Devreese, B.; de Graaf, D.C. Honeybee venom proteome profile of queens and winter bees as determined by a mass spectrometric approach. Toxins 2015, 7, 4468. [Google Scholar] [CrossRef]
- Guillaume, C.; Calzada, C.; Lagarde, M.; Schrével, J.; Deregnaucourt, C. Interplay between lipoproteins and bee venom phospholipase A2 in relation to their anti-plasmodium toxicity. J. Lipid Res. 2006, 47, 1493–1506. [Google Scholar] [CrossRef]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/BLAST (accessed on 18 February 2021).
- SignalP 4.1 Server. Available online: http://www.cbs.dtu.dk/services/SignalP (accessed on 18 February 2021).
- Yang, J.; Lee, K.S.; Kim, B.Y.; Choi, Y.S.; Yoon, H.J.; Jia, J.; Jin, B.R. Anti-fibrinolytic and anti-microbial activities of a serine protease inhibitor from honeybee (Apis cerana) venom. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2017, 201, 11–18. [Google Scholar] [CrossRef]
- Je, Y.H.; Chang, J.H.; Kim, M.H.; Roh, J.Y.; Jin, B.R.; O’Reilly, D.R.; Kang, S.K. A defective viral genome maintained in Escherichia coli for the generation of baculovirus expression vectors. Biotechnol. Lett. 2001, 23, 575–582. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.Y.; Wan, H.; Li, J.; Jin, B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018, 85, 51–60. [Google Scholar] [CrossRef]
- Wasko, A.; Kieliszek, M.; Targonski, Z. Purification and characterization of a proteinase from the probiotic Lactobacillus rhamnosus OXY. Prep. Biochem. Biotechnol. 2012, 42, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Rejón, J.D.; Zienkiewicz, A.; Rodríguez-García, M.I.; Castro, A.J. Profiling and finctional classification of esterases in olive (Olea europaea) pollen during germination. Ann. Bot. 2012, 110, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).