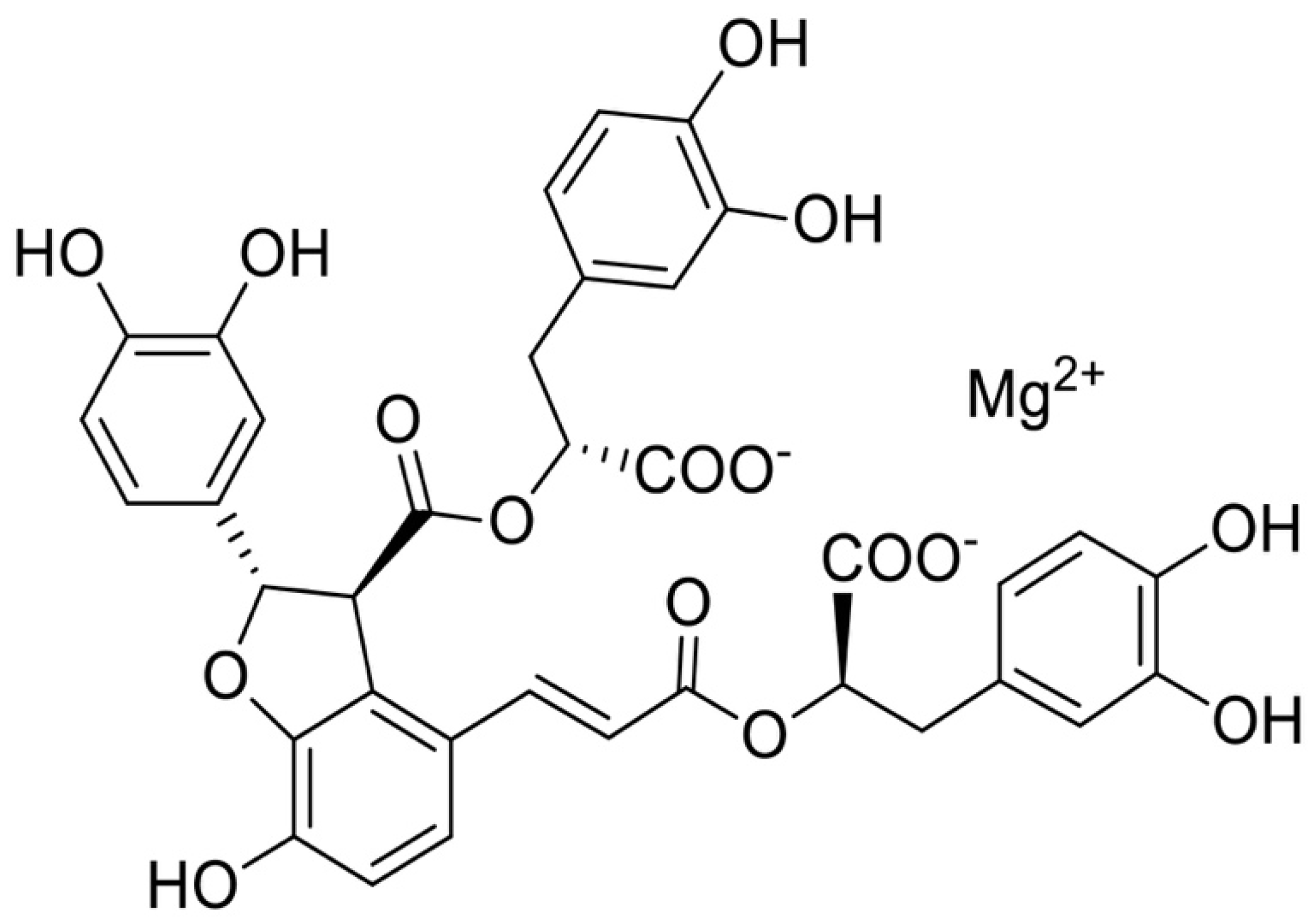
Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling
Abstract
1. Introduction
2. Results
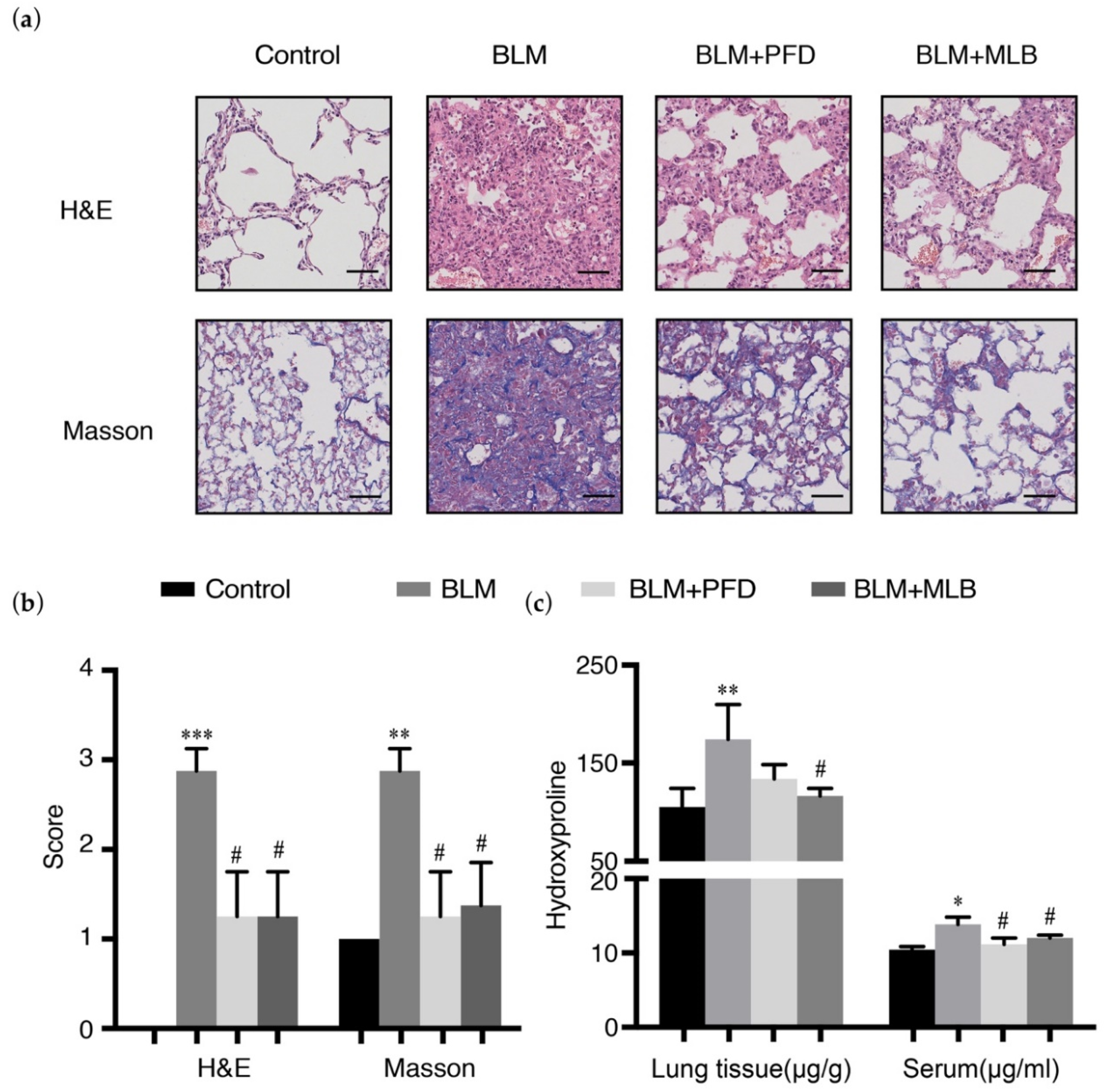
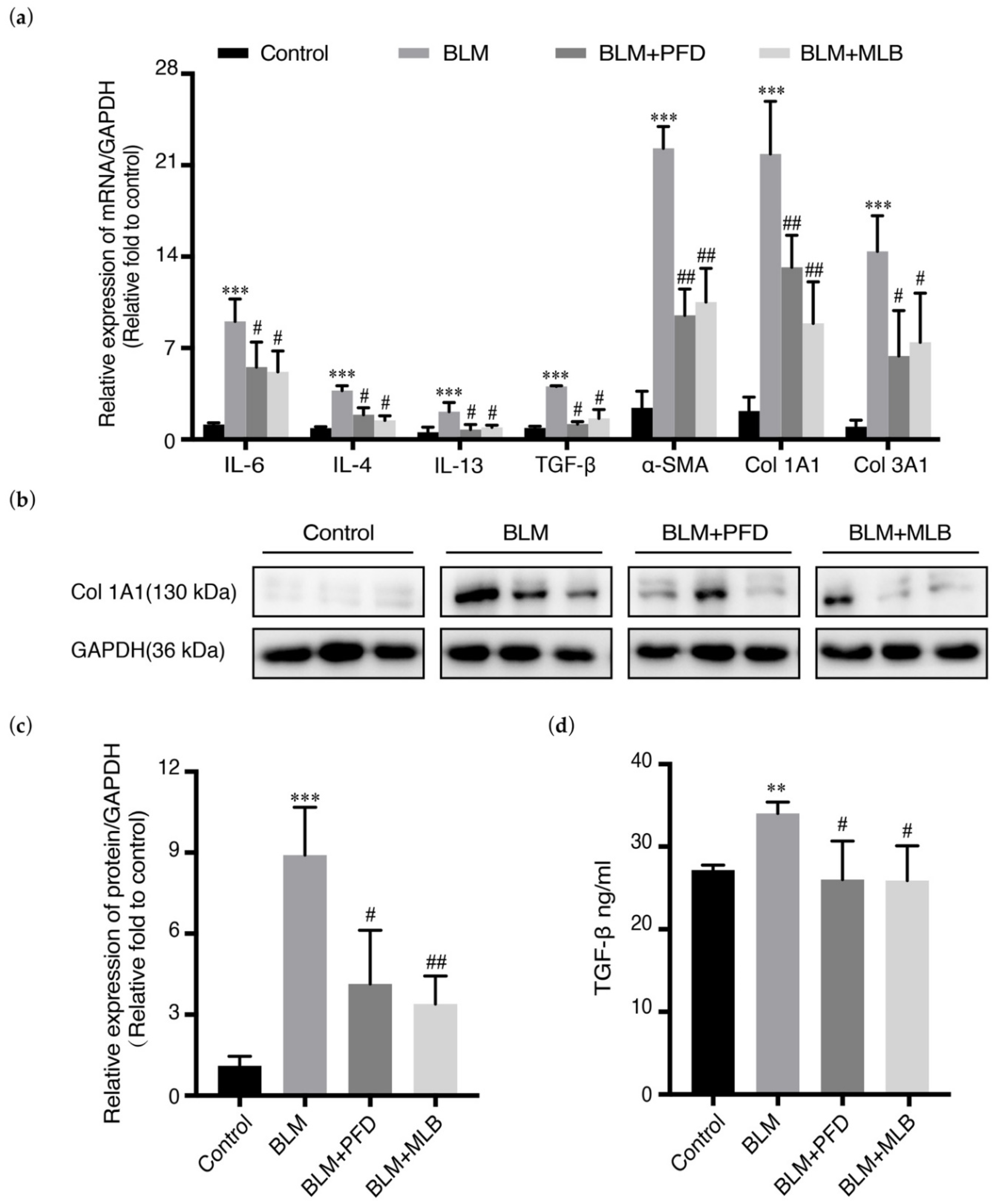
2.1. MLB Could Alleviate Bleomycin-Induced Pulmonary Fibrosis in Mice
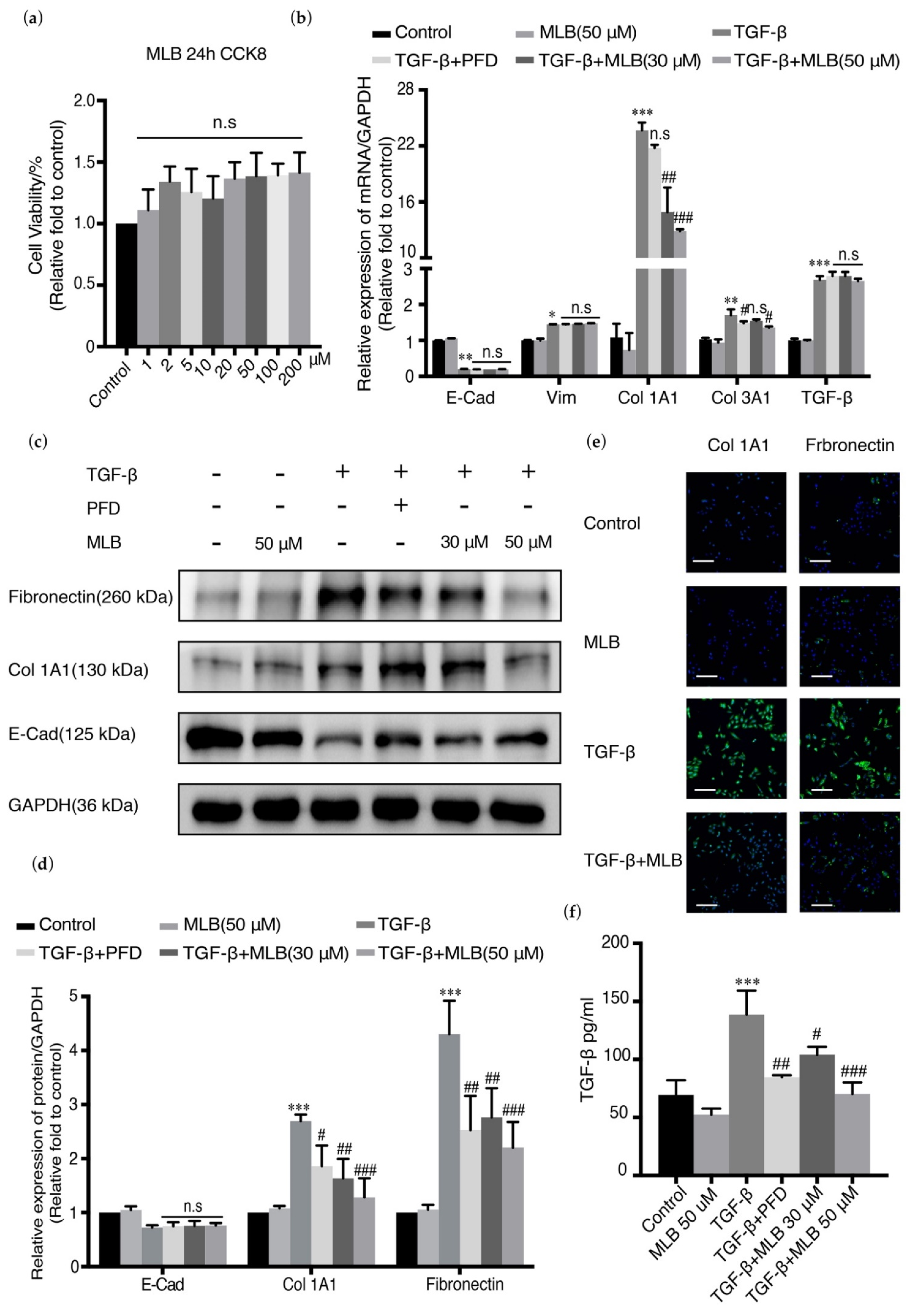
2.2. MLB Could Inhibit the Expression of Col 1A1 and TGF-β Release in A549 Cells
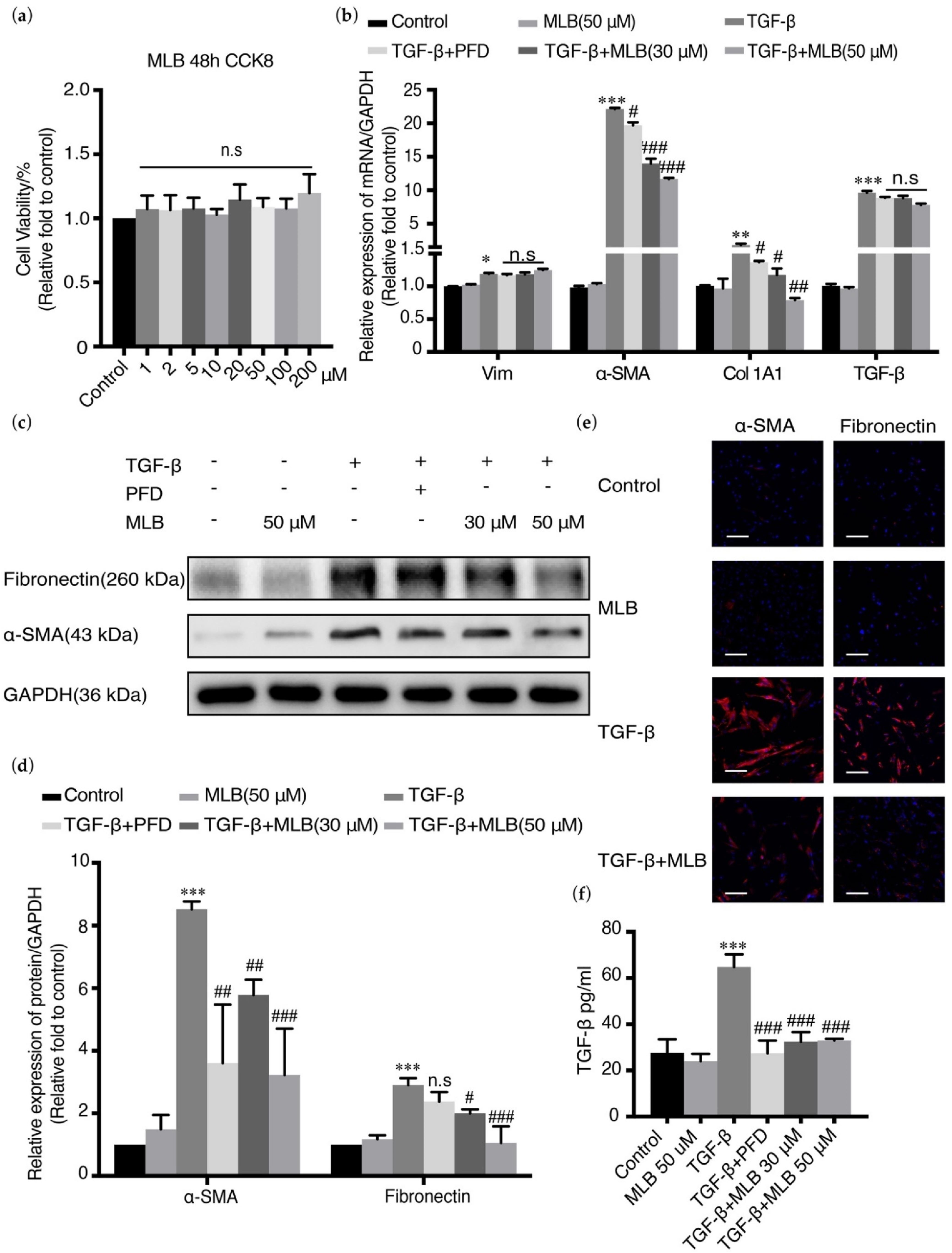
2.3. MLB Could Inhibit TGF-β-Induced Myofibroblast Transdifferentiation in MRC-5 Cells
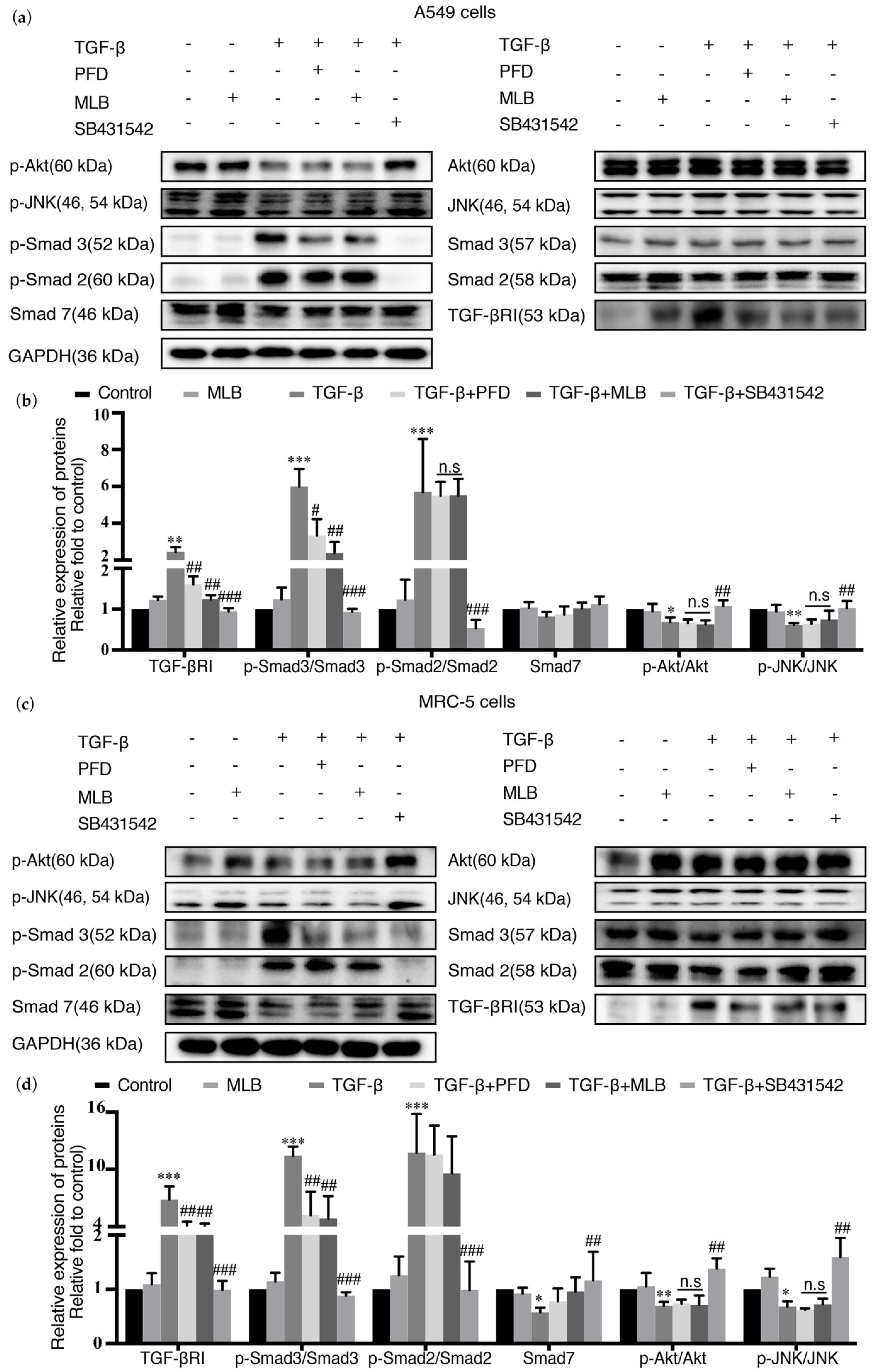
2.4. MLB Could Inhibit TGF-βRI/Smad Signaling in TGF-β-Stimulated A549 and MRC-5 Cells
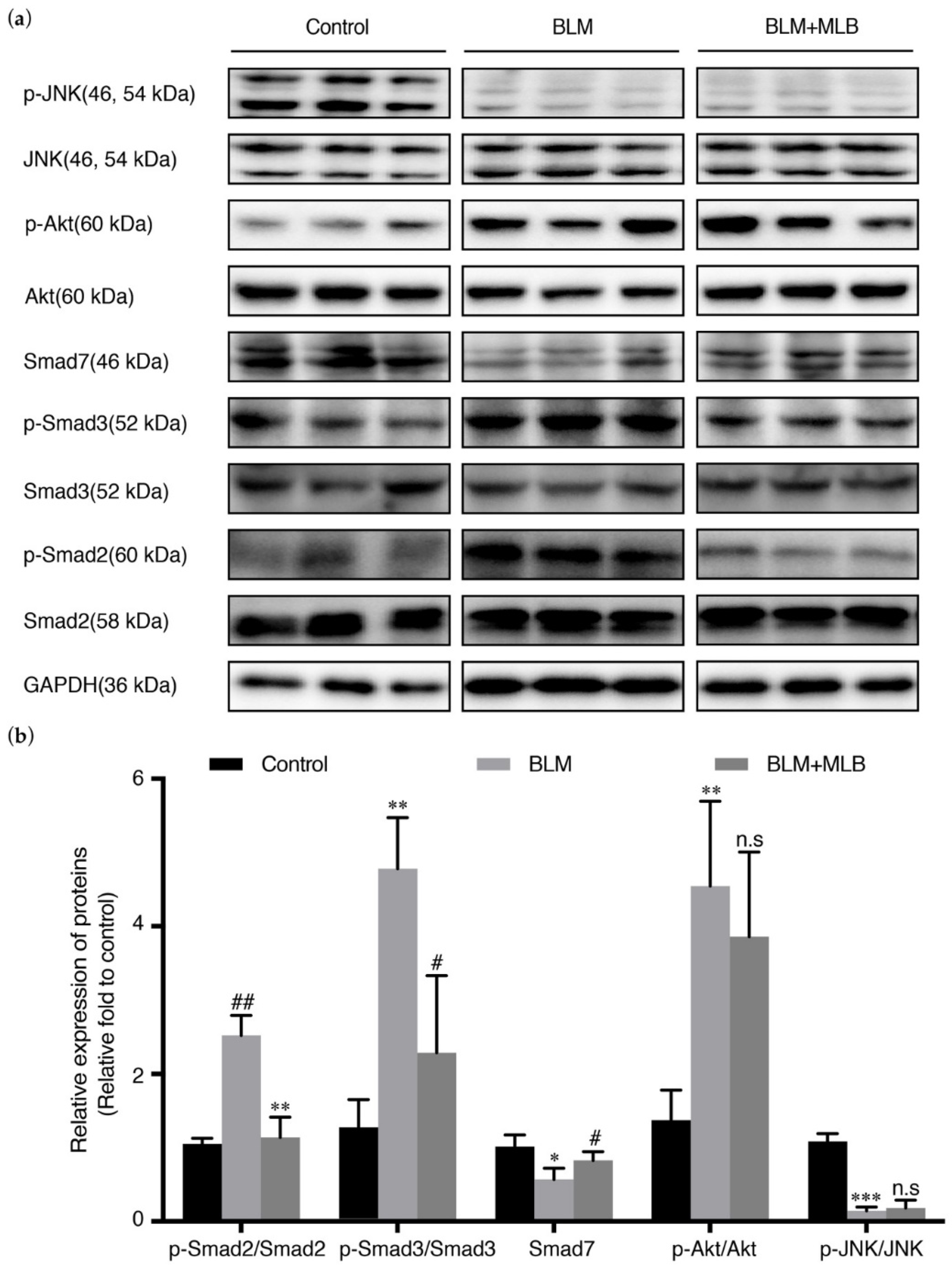
2.5. MLB Could Inhibit TGF-β/Smad Signaling in BLM-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Bleomycin-Induced Pulmonary Fibrosis Mouse Model
4.3. Histology Analysis and Scoring
4.4. Hydroxyproline
4.5. Cell Culture and Treatment
4.6. Cell Viability Assay
4.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.8. Western Blot Analysis
4.9. Cell Immunofluorescence Staining
4.10. Enzyme Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nicholson, A.G.; Colby, T.V.; Dubois, R.M.; Hansell, D.M.; Wells, A.U. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 2000, 162, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Pulmonary fibrosis, part i: Epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Staitieh, B.S.; Renzoni, E.A.; Veeraraghavan, S. Pharmacologic therapies for idiopathic pulmonary fibrosis, past and future. Ann. Med. 2015, 47, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Bonella, F.; Spagnolo, P. Update on therapeutic management of idiopathic pulmonary fibrosis. Ther. Clin. Risk Manag. 2015, 11, 359–370. [Google Scholar]
- Liu, Y.; Lu, F.; Kang, L.; Kang, Z.; Wang, Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating nrf2/bach1 equilibrium. BMC Pulm. Med. 2017, 17, 63. [Google Scholar] [CrossRef]
- Azuma, A.; Nukiwa, T.; Tsuboi, E.; Suga, M.; Abe, S.; Nakata, K.; Taguchi, Y.; Nagai, S.; Itoh, H.; Ohi, M.; et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171, 1040–1047. [Google Scholar] [CrossRef]
- La Mora, D.A.L.-D.; Sanchez-Roque, C.; Montoya-Buelna, M.; Sanchez-Enriquez, S.; Lucano-Landeros, S.; Macias-Barragan, J.; Armendariz-Borunda, J. Role and new insights of pirfenidone in fibrotic diseases. Int. J. Med. Sci. 2015, 12, 840–847. [Google Scholar] [CrossRef]
- Gulati, S.; Luckhardt, T.R. Updated Evaluation of the Safety, Efficacy and Tolerability of Pirfenidone in the Treatment of Idiopathic Pulmonary Fibrosis. Drug Health Patient Saf. 2020, 12, 85–94. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; Kasselman, L.J.; Glass, A.D.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Molecular mechanisms and potential treatment approaches. Respir. Investig. 2020, 58, 320–335. [Google Scholar] [CrossRef]
- May, R.D.; Fung, M. Strategies targeting the il-4/il-13 axes in disease. Cytokine 2015, 75, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Fernandez, I.E.; Eickelberg, O. The impact of tgf-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. Tgf-β1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S417–S421. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.C.; Rogers, D.S.; Sharma, V.; Vittal, R.; White, E.S.; Cui, Z.; Thannickal, V.J. Combinatorial activation of fak and akt by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell. Signal. 2007, 19, 761–771. [Google Scholar] [CrossRef]
- Goldmann, T.; Zissel, G.; Watz, H.; Drömann, D.; Reck, M.; Kugler, C.; Rabe, K.F.; Marwitz, S. Human alveolar epithelial cells type ii are capable of tgfβ-dependent epithelial-mesenchymal-transition and collagen-synthesis. Respir. Res. 2018, 19, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (emt): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 136–137. [Google Scholar] [CrossRef]
- Upagupta, C.; Shimbori, C.; Alsilmi, R.; Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 180033. [Google Scholar] [CrossRef]
- Degryse, A.L.; Tanjore, H.; Xu, X.C.; Polosukhin, V.V.; Jones, B.R.; McMahon, F.B.; Gleaves, L.A.; Blackwell, T.S.; Lawson, W.E. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L442–L452. [Google Scholar] [CrossRef]
- Shimbori, C.; Bellaye, P.S.; Xia, J.; Gauldie, J.; Ask, K.; Ramos, C.; Becerril, C.; Pardo, A.; Selman, M.; Kolb, M. Fibroblast growth factor-1 attenuates tgf-beta1-induced lung fibrosis. J. Pathol. 2016, 240, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-A.; Kim, M.-J.; Park, S.-Y.; Kim, J.S.; Lee, S.J.; Woo, H.A.; Kim, D.-K.; Nam, J.-S.; Sheen, Y.Y. Ew-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking tgf-β/smad and ros signaling. Cell. Mol. Life Sci. 2015, 72, 2023–2039. [Google Scholar] [CrossRef]
- Keenan, C.R.; Langenbach, S.Y.; Jativa, F.; Harris, T.; Li, M.; Chen, Q.; Xia, Y.; Gao, B.; Schuliga, M.J.; Jaffar, J.; et al. Casein kinase 1δ/ε inhibitor, pf670462 attenuates the fibrogenic effects of transforming growth factor-β in pulmonary fibrosis. Front. Pharmacol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Lv, Z.; Liu, S.; Zhang, L.; Huang, K.; Gao, S.; Gan, W.; Liu, X.; Zhang, S.; Helian, K.; et al. Anlotinib attenuated bleomycin-induced pulmonary fibrosis via the tgf-β1 signalling pathway. J. Pharm. Pharmacol. 2020, 72, 44–55. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.-M.; Xi, C.; Li, H.-H.; Liu, Y.-L.; Wang, Y.-P.; Xuan, L.-J. Magnesium lithospermate b protects the endothelium from inflammation-induced dysfunction through activation of nrf2 pathway. Acta Pharmacol. Sin. 2019, 40, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Han, L.; Xue, Y.; Deng, Q.; Wu, Z.; Peng, H.; Zhang, Y.; Xuan, L.; Pan, G.; Fu, Q. The protective effect of magnesium lithospermate b on hepatic ischemia/reperfusion via inhibiting the jak2/stat3 signaling pathway. Front. Pharmacol. 2019, 10, 620. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Ding, H.; Li, D.; Wen, G.; Zhang, C.; Lua, W.; Chen, M.; Yang, Y. Salvianolic acid b exerts anti-liver fibrosis effects via inhibition of mapk-mediated phospho-smad2/3 at linker regions in vivo and in vitro. Life Sci. 2019, 239, 116881. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wu, M.; Qin, J.; Yang, J.; Ye, C.; Wang, C. Magnesium lithospermate b improves renal hemodynamics and reduces renal oxygen consumption in 5/6th renal ablation/infarction rats. BMC Nephrol. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Tao, Y.-Y.; Yuan, J.-L.; Shen, L.; Liu, C.-H. Salvianolic acid b prevents epithelial-to-mesenchymal transition through the tgf-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Wells, A.U. Acute exacerbations of idiopathic pulmonary fibrosis. Respiration 2013, 86, 265–274. [Google Scholar] [CrossRef]
- Li, L.C.; Kan, L.D. Traditional chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J. Ethnopharmacol. 2017, 198, 45–63. [Google Scholar] [CrossRef]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial-mesenchymal transition in the pathogenesis of idiopathic pulmonary fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef]
- Chen, K.-J.; Li, Q.; Wen, C.-M.; Duan, Z.-X.; Zhang, J.Y.; Xu, C.; Wang, J.-M. Bleomycin (blm) induces epithelial-to-mesenchymal transition in cultured a549 cells via the tgf-β/smad signaling pathway. J. Cancer 2016, 7, 1557–1564. [Google Scholar] [CrossRef]
- Baek, A.R.; Lee, J.M.; Seo, H.J.; Park, J.S.; Lee, J.H.; Park, S.W.; Jang, A.S.; Kim, D.J.; Koh, E.S.; Uh, S.T.; et al. Apolipoprotein a1 inhibits tgf-β1-induced epithelial-to-mesenchymal transition of alveolar epithelial cells. Tuberc. Respir. Dis. 2016, 79, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gan, C.; Liu, H.; Wang, L.; Li, Y.; Tan, Z.; You, J.; Yao, Y.; Xie, Y.; Yin, W.; et al. Cryptotanshinone reverses the epithelial-mesenchymal transformation process and attenuates bleomycin-induced pulmonary fibrosis. Phytother. Res. 2020, 34, 2685–2696. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Li, Y.-N.; Wang, X.-L.; Ye, C.-L.; Zhu, X.-Y.; Li, H.-P.; Yang, T.; Liu, Y.-J. Probucol ameliorates emt and lung fibrosis through restoration of sirt3 expression. Pulm. Pharmacol. Ther. 2019, 57, 101803. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. Tgf-β signaling in lung health and disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Muhammad, S.; Shi, J.; Meng, Y.; Wang, J. Computational investigation of tgf-β receptor inhibitors for treatment of idiopathic pulmonary fibrosis: Field-based qsar model and molecular dynamics simulation. Comput. Biol. Chem. 2018, 76, 139–150. [Google Scholar] [CrossRef]
- Paik, Y.-H.; Yoon, Y.J.; Lee, H.C.; Kil Jung, M.; Kang, S.H.; Chung, S.I.; Kim, J.K.; Cho, J.Y.; Lee, K.S.; Han, K.-H. Antifibrotic effects of magnesium lithospermate b on hepatic stellate cells and thioacetamide-induced cirrhotic rats. Exp. Mol. Med. 2011, 43, 341–349. [Google Scholar] [CrossRef]
- Sun, X.; Nkennor, B.; Mastikhina, O.; Soon, K.; Nunes, S.S. Endothelium-mediated contributions to fibrosis. Semin. Cell Dev. Biol. 2020, 101, 78–86. [Google Scholar] [CrossRef]
- Sun, X.; Nkennor, B.; Mastikhina, O.; Soon, K.; Nunes, S.S. Src family kinases and pulmonary fibrosis: A review. Biomed. Pharmacother. 2020, 127, 110183. [Google Scholar]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef]
- Hill, C.; Jones, M.; Davies, D.; Wang, Y. Epithelial-mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. J. Lung Health Dis. 2019, 3, 31–35. [Google Scholar] [CrossRef]
- Hill, C.; Jones, M.; Davies, D.; Wang, Y. Epithelial injury and dysfunction in the pathogenesis of idiopathic pulmonaryfibrosis. Am. J. Med. Sci. 2019, 357, 374–378. [Google Scholar]
- Parimon, T.; Yao, C.; Stripp, B.R.; Noble, P.W.; Chen, P. Alveolar epithelial type ii cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 2020, 21, 2269. [Google Scholar] [CrossRef]
- Feng, F.; Cheng, P.; Xu, S.; Li, N.; Wang, H.; Zhang, Y.; Wang, W. Tanshinone iia attenuates silica-induced pulmonary fibrosis via nrf2-mediated inhibition of emt and tgf-β1/smad signaling. Chem. Biol. Interact. 2020, 319, 109024. [Google Scholar] [CrossRef]
- Gong, L.; Wu, X.; Li, X.; Ni, X.; Gu, W.; Wang, X.; Ji, H.; Hu, L.; Zhu, L. S1pr3 deficiency alleviates radiation-induced pulmonary fibrosis through the regulation of epithelial-mesenchymal transition by targeting mir-495-3p. J. Cell. Physiol. 2020, 235, 2310–2324. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 macrophages induce emt through the tgf-beta/smad2 signaling pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef]
- Murray, L.A.; Chen, Q.; Kramer, M.S.; Hesson, D.P.; Argentieri, R.L.; Peng, X.; Gulati, M.; Homer, R.J.; Russell, T.; Van Rooijen, N.; et al. Tgf-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid p. Int. J. Biochem. Cell Biol. 2011, 43, 154–162. [Google Scholar] [CrossRef]
- Xu, Y.D.; Hua, J.; Mui, A.; O’Connor, R.; Grotendorst, G.; Khalil, N. Release of biologically active tgf-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L527–L539. [Google Scholar] [CrossRef]
- Broekelmann, T.J.; Limper, A.H.; Colby, T.V.; McDonald, J.A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 1991, 88, 6642–6646. [Google Scholar] [CrossRef]
- Coker, R.K.; Laurent, G.J.; Jeffery, P.K.; Du Bois, R.M.; Black, C.M.; McAnulty, R.J. Localisation of transforming growth factor beta1 and beta3 mrna transcripts in normal and fibrotic human lung. Thorax 2001, 56, 549–556. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into tgf-β/smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. Tgf-β—An excellent servant but a bad master. J. Transl. Med. 2012, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-smad pathways in tgf-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Luo, K. Signaling cross talk between tgf-β/smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Lu, C.-Y.; Li, S.-W.; Lai, C.-C.; Hua, C.-H.; Huang, S.-H.; Lin, Y.-J.; Hour, M.-J.; Lin, C.-W. Sars coronavirus papain-like protease up-regulates the collagen expression through non-samd tgf-β1 signaling. Virus Res. 2017, 235, 58–66. [Google Scholar] [CrossRef]
- Wang, R.; Song, F.; Li, S.; Wu, B.; Gu, Y.; Yuan, Y. Salvianolic acid a attenuates ccl(4)-induced liver fibrosis by regulating the pi3k/akt/mtor, bcl-2/bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des. Dev. Ther. 2019, 13, 1889–1900. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. Tgf-β/smad pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, W.; Zhang, X.; Lu, J.; Xu, S.; Chen, S.; Zhong, Z.; Zhou, T.; Wang, Q.; Chen, J.; et al. Cryptotanshinone protects against pulmonary fibrosis through inhibiting smad and stat3 signaling pathways. Pharmacol. Res. 2019, 147, 104307. [Google Scholar] [CrossRef]
- Hwangbo, C.; Tae, N.; Lee, S.; Kim, O.; Park, O.K.; Kim, J.; Kwon, X.-H.; Lee, J.H. Syntenin regulates tgf-beta1-induced smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated tgf-beta type i receptor internalization. Oncogene 2016, 35, 389–401. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.-F. Signaling cross-talk between tgf-beta/bmp and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Liu, M.-H.; Lin, A.-H.; Ko, H.-K.; Perng, D.-W.; Lee, T.-S.; Kou, Y.R. Prevention of bleomycin-induced pulmonary inflammation and fibrosis in mice by paeonol. Front. Physiol. 2017, 8, 193. [Google Scholar] [CrossRef]
- Gamad, N.; Malik, S.; Suchal, K.; Vasisht, S.; Tomar, A.; Arava, S.; Arya, D.S.; Bhatia, J. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: Pharmacological effects and molecular mechanisms. Biomed. Pharmacother. 2018, 97, 1544–1553. [Google Scholar] [CrossRef]
- Van Der Velden, J.L.; Alcorn, J.F.; Chapman, D.G.; Lundblad, L.K.A.; Irvin, C.G.; Davis, R.J.; Butnor, K.; Janssen-Heininger, Y.M.W. Airway epithelial specific deletion of jun-n-terminal kinase 1 attenuates pulmonary fibrosis in two independent mouse models. PLoS ONE 2020, 15, e0226904. [Google Scholar] [CrossRef]
- Liu, Q.; Chu, H.; Ma, Y.; Wu, T.; Qian, F.; Ren, X.; Tu, W.; Zhou, X.; Jin, L.; Wu, W.; et al. Salvianolic acid b attenuates experimental pulmonary fibrosis through inhibition of the tgf-beta signaling pathway. Sci. Rep. 2016, 6, 27610. [Google Scholar] [CrossRef]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.-H.; Landström, M. Tgf-β promotes pi3k-akt signaling and prostate cancer cell migration through the traf6-mediated ubiquitylation of p85α. Sci. Signal. 2017, 10, eaal4186. [Google Scholar] [CrossRef]
- Hamidi, A.; Song, J.; Thakur, N.; Itoh, S.; Marcusson, A.; Bergh, A.; Heldin, C.-H.; Landström, M. Tgf-β-mediated lefty/akt/gsk-3β/snail axis modulates epithelial-mesenchymal transition and cancer stem cell properties in ovarian clear cell carcinomas. Mol. Carcinog. 2018, 57, 957–967. [Google Scholar]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. Tgf-β induces m2-like macrophage polarization via snail-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Suwanabol, P.A.; Seedial, S.M.; Zhang, F.; Shi, X.; Si, Y.; Liu, B.; Kent, K.C. Tgf-β and smad3 modulate pi3k/akt signaling pathway in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2211–H2219. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lakhia, R.; Thomas, S.S.; Dong, Y.; Wang, X.H.; Silva, K.A.S.; Zhang, L. Interactions between p-akt and smad3 in injured muscles initiate myogenesis or fibrogenesis. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E367–E375. [Google Scholar] [CrossRef] [PubMed]
- Shihata, W.A.; Putra, M.R.A.; Chin-Dusting, J.P.F. Is there a potential therapeutic role for caveolin-1 in fibrosis? Front. Pharmacol. 2017, 8, 567. [Google Scholar] [CrossRef]
- Sanders, Y.Y.; Cui, Z.; Le Saux, C.J.; Horowitz, J.C.; Rangarajan, S.; Kurundkar, A.; Antony, V.B.; Thannickal, V.J. Smad-independent down-regulation of caveolin-1 by tgf-beta: Effects on proliferation and survival of myofibroblasts. PLoS ONE 2015, 10, e0116995. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, H.; Xiang, F.; Song, L.-J.; Zhou, L.-L.; Cai, P.-C.; Zhang, J.-C.; Yu, F.; Shi, H.-Z.; Su, Y.; et al. Mir-18a-5p inhibits sub-pleural pulmonary fibrosis by targeting tgf-β receptor ii. Mol. Ther. 2017, 25, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Wang, C.-Y.; Jou, Y.-J.; Yang, T.-C.; Huang, S.-H.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Sars coronavirus papain-like protease induces egr-1-dependent up-regulation of tgf-β1 via ros/p38 mapk/stat3 pathway. Sci. Rep. 2016, 6, 25754. [Google Scholar] [CrossRef]
- Kato, S.; Inui, N.; Hakamata, A.; Suzuki, Y.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; Watanabe, H.; Suda, T. Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir. Res. 2018, 19, 127. [Google Scholar] [CrossRef]
- Liu, T.; De Los Santos, F.G.; Phan, S.H. The bleomycin model of pulmonary fibrosis. Methods Mol. Biol. 2017, 1627, 27–42. [Google Scholar] [PubMed]
- Chaudhary, N.I.; Schnapp, A.; Park, J.E. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am. J. Respir. Crit. Care Med. 2006, 173, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef]
- Oku, H.; Shimizu, T.; Kawabata, T.; Nagira, M.; Hikita, I.; Ueyama, A.; Matsushima, S.; Torii, M.; Arimura, A. Antifibrotic action of pirfenidone and prednisolone: Different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur. J. Pharmacol. 2008, 590, 400–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Deng, Q.; Xue, Y.; Zhang, T.; Wu, Z.; Peng, H.; Xuan, L.; Pan, G. Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling. Molecules 2021, 26, 1715. https://doi.org/10.3390/molecules26061715
Luo X, Deng Q, Xue Y, Zhang T, Wu Z, Peng H, Xuan L, Pan G. Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling. Molecules. 2021; 26(6):1715. https://doi.org/10.3390/molecules26061715
Chicago/Turabian StyleLuo, Xin, Qiangqiang Deng, Yaru Xue, Tianwei Zhang, Zhitao Wu, Huige Peng, Lijiang Xuan, and Guoyu Pan. 2021. "Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling" Molecules 26, no. 6: 1715. https://doi.org/10.3390/molecules26061715
APA StyleLuo, X., Deng, Q., Xue, Y., Zhang, T., Wu, Z., Peng, H., Xuan, L., & Pan, G. (2021). Anti-Fibrosis Effects of Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-βRI/Smad Signaling. Molecules, 26(6), 1715. https://doi.org/10.3390/molecules26061715







