Development of Antibacterial, Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper betle Linn Oil as Active Biodegradable Packaging Material
Abstract
:Highlights
1. Introduction
2. Experimental
2.1. Materials
2.2. Analyses of PBLO
2.3. Preparation of pCS-PBLO films
2.4. Film Characterization
2.5. Bacterial Inhibition Test
2.6. Antioxidant Test
2.7. Surface Tension, Work of Adhension, and Spreading Coefficient
2.8. Coating of King Oranges
3. Results and Discussion
3.1. Chemical Composition of Piper betle Linn oil
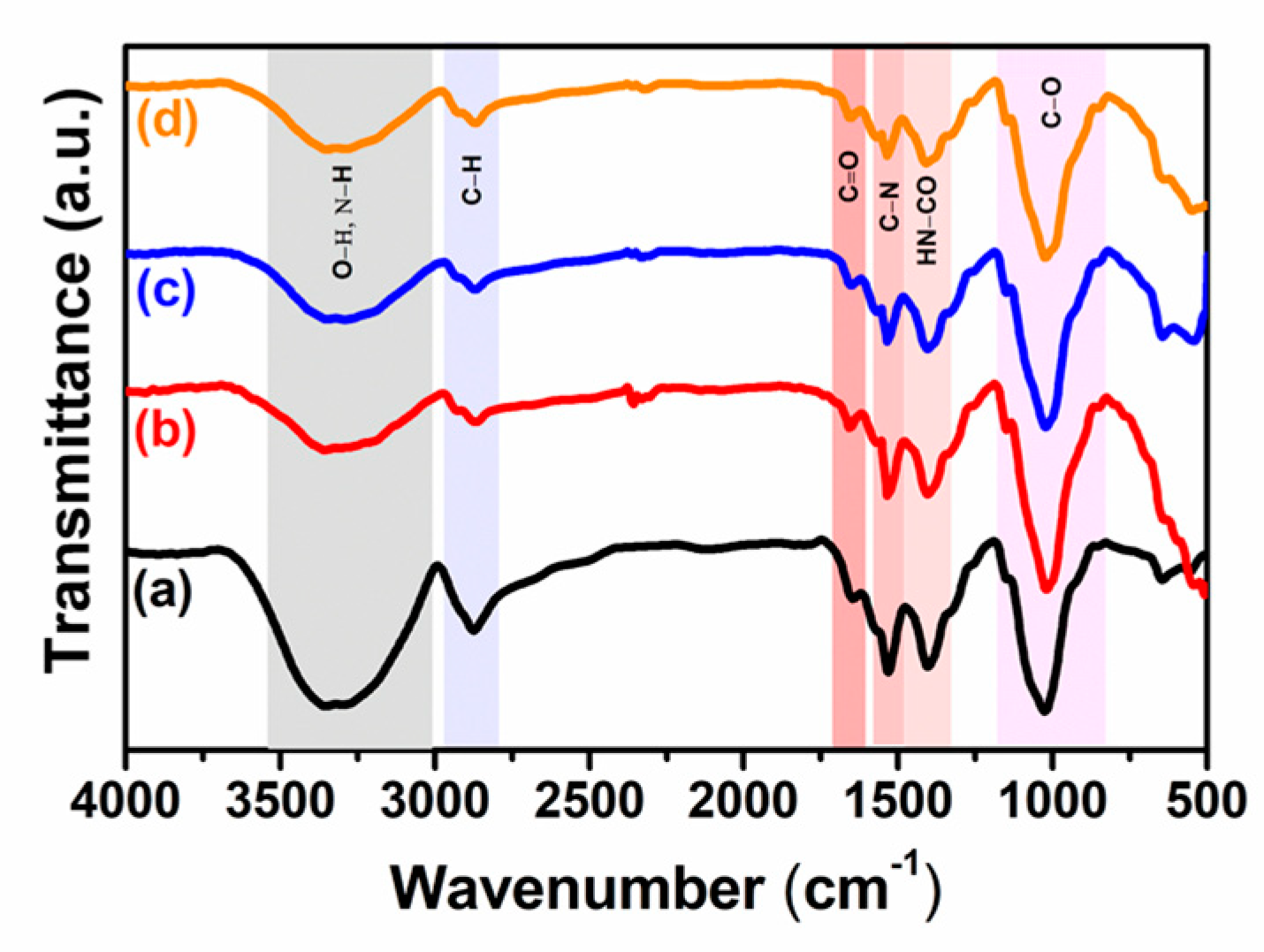
3.2. ATR/FTIR Analyses
3.3. XRD Analyses
3.4. Thermo-Stability
3.5. The Surface Morphology
3.6. UV-Protective Property
3.7. Mechanical Behavior
3.8. Swelling Degree
3.9. Antibacterial Activity
3.10. Antioxidant Activity
3.11. Preservative Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, S. Plastic Pollution Affects Sea Life Throughout the Ocean. In Proceedings of the Preventing Ocean Plastics. The Pew Charitable Trusts, New Zealand, September 2018; Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2018/09/24/plastic-pollution-affects-sea-life-throughout-the-ocean (accessed on 5 February 2021).
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Umar, R.A.; Zahary, M.N.; Ismail, S.; Rohin, M.A.K. Chemical Composition and The Potential Biological Activities Of Piper Betel—A Review. Malaysian J. Appl. Sci. 2018, 3, 1–8. [Google Scholar]
- Kanjwani, D.G.; Marathe, T.P.; Chiplunkar, S.V.; Sathaye, S.S. Evaluation of immunomodulatory activity of methanolic extract of Piper betel. Scand. J. Immunol. 2008, 67, 589–593. [Google Scholar] [CrossRef]
- Muruganandam, L.; Krishna, A.; Reddy, J.; Nirmala, G.S. Optimization studies on extraction of phytocomponents from betel leaves. Resour. Technol. 2017, 3, 385–393. [Google Scholar] [CrossRef]
- Das, S.; Parida, R.; Sriram Sandeep, I.; Nayak, S.; Mohanty, S. Biotechnological intervention in betelvine (Piper betle L.): A review on recent advances and future prospects. Asian Pac. J. Trop. Med. 2016, 9, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for Eriochrome black T removal from aqueous solutions. Appl. Water Sci. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. Coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Hemalatha, T.; UmaMaheswari, T.; Senthil, R.; Krithiga, G.; Anbukkarasi, K. Efficacy of chitosan films with basil essential oil: Perspectives in food packaging. J. Food Meas. Charact. 2017, 11, 2160–2170. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, N.; Li, H.Z.; Li, X.J.; Cao, J.M.; Zhang, G.P.; He, D.L. Preparation and characterization of biocomposite chitosan film containing Perilla frutescens (L.) Britt. essential oil. Ind. Crops Prod. 2018, 112, 660–667. [Google Scholar] [CrossRef]
- Hafsa, J.; ali Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hosseini, S.E.; Sharifan, A. Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Sci. Nutr. 2018, 6, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Thuong, N.T.; Ngoc Bich, H.T.; Thuc, C.N.H.; Quynh, B.T.P.; Minh, L.V. Preparation and characterization of Piper betle Linn. leaf extract incorporated chitosan films as potential active food packaging materials. ChemistrySelect 2019, 4, 8150–8157. [Google Scholar] [CrossRef]
- ASTM G40-15 Standard Terminology Relating to Wear and Erosion; ASTM: West Conshohocken, PA, USA, 2015.
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact Angle Measurements. In Membrane Characterization; Hilal, N., Matsuura, T., Oatley-Radcliffe, D., Fauzi Ismail, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 122, pp. 219–255. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A. Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity. Ind. Crops Prod. 2019, 138, 111578. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An investigation on the effect of polyphenolic extracts of Nigella sativa seedcake on physicochemical properties of chitosan-based films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2015, 3, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Go, E.J.; Song, K. Bin Effect of java citronella essential oil addition on the physicochemical properties of Gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, T.T.; Nguyen, H.N.; Bui, Q.T.P. Simultaneous determination of active compounds in Piper betle Linn. leaf extract and effect of extracting solvents on bioactivity. Eng. Rep. 2020, 2, 2–9. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Intawiwat, N.; Pettersen, M.K.; Rukke, E.O.; Meier, M.A.; Vogt, G.; Dahl, A.V.; Skaret, J.; Keller, D.; Wold, J.P. Effect of different colored filters on photooxidation in pasteurized milk. J. Dairy Sci. 2010, 93, 1372–1382. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, B.; Almasi, H. Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef]
- Baskar, D.; Sampath Kumar, T.S. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohydr. Polym. 2009, 78, 767–772. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Alavi Rafiee, S.; Farhoosh, R.; Sharif, A. Antioxidant Activity of Gallic Acid as Affected by an Extra Carboxyl Group than Pyrogallol in Various Oxidative Environments. Eur. J. Lipid Sci. Technol. 2018, 120, 1–8. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]






| No. | Retention Time (min) | Compound Name | Area Percent (%) |
|---|---|---|---|
| 1 | 7.261 | α-Pinene | 1.051 |
| 2 | 7.837 | Camphene | 0.314 |
| 3 | 9.081 | β-Pinene | 0.171 |
| 4 | 11.862 | Eucalyptol | 1.376 |
| 5 | 16.15 | β-Linalool | 0.36 |
| 6 | 26.481 | δ-Elemene | 0.364 |
| 7 | 26.847 | Chavicol acetate | 3.004 |
| 8 | 27.088 | 4-Allylphenyl acetate | 0.422 |
| 9 | 27.642 | Eugenol | 14.209 |
| 10 | 27.673 | Copaene | 3.098 |
| 11 | 27.746 | 3-Allyl-6-methoxyphenol | 1.824 |
| 12 | 27.977 | Geraniol acetate | 1.19 |
| 13 | 28.227 | β-Elemene | 2.343 |
| 14 | 28.928 | α-Bergamotene | 0.952 |
| 15 | 29.043 | Caryophyllene | 9.107 |
| 16 | 29.315 | β-copaene | 0.486 |
| 17 | 29.514 | α-Bergamotene | 0.711 |
| 18 | 29.597 | Aromadendrene | 0.794 |
| 19 | 30.016 | α-Caryophyllene | 1.732 |
| 20 | 30.152 | β-Farnesene | 0.383 |
| 21 | 30.695 | γ-Muurolene | 6.015 |
| 22 | 30.81 | Germacrene D | 3.945 |
| 23 | 30.904 | 2-Isopropenyl-4a,8-dimethyl 1,2,3,4,4a,5,6,7-octahydronaphthalene | 0.998 |
| 24 | 31.208 | β-Cyclogermacrane | 3.964 |
| 25 | 31.333 | α-Muurolene | 1.053 |
| 26 | 31.542 | β-Bisabolene | 1.34 |
| 27 | 31.678 | γ-Cadinene | 0.767 |
| 28 | 31.908 | β-Cadinene | 3.361 |
| 29 | 32.086 | Acetyleugenol | 17.511 |
| 30 | 32.327 | α-Calacorene | 0.777 |
| 31 | 32.902 | Palustrol | 0.24 |
| 32 | 33.247 | Globulol | 0.589 |
| 33 | 33.404 | Viridiflorol | 0.446 |
| 34 | 33.602 | cis-Eudesm-6-en-11-ol | 0.228 |
| 35 | 33.916 | Junenol | 0.151 |
| 36 | 34.073 | 1,10-Diepicubenol | 0.33 |
| 37 | 34.366 | 4-Allyl-1,2-diacetoxybenzene | 10.922 |
| 38 | 34.543 | α-Cadinol | 0.948 |
| Sample Codes | Tensile Strength (MPa) | Elongation at Break (%) | Elastic Modulus (MPa) | Swelling Degree (%) |
|---|---|---|---|---|
| pCS | 15.26 ± 0.38 | 17.49 ± 1.09 | 111.96 ± 13.31 | 50.10 ± 2.56 |
| pCS-0.4PBLO | 11.98 ± 0.41 | 24.97 ± 0.88 | 51.00 ± 1.48 | 52.57 ± 3.01 |
| pCS-1PBLO | 7.71 ± 0.36 | 20.62 ± 0.88 | 32.16 ± 2.85 | 62.05 ± 4.50 |
| pCS-1.2PBLO | 5.42 ± 0.40 | 17.06 ± 1.06 | 25.95 ± 1.58 | 70.54 ± 2.66 |
| Sample Codes | Total Colony Forming Units (CFU/mL) | ||||
|---|---|---|---|---|---|
| 0 h | 4 h | 8 h | 11 h | 24 h | |
| pCS | 7.50 × 109 | 8.30 × 109 | GO | GO | GO |
| pCS-0.4PBLO | 7.80 × 109 | 7.40 × 109 | 5.70 × 109 | 6.20 × 109 | 12.30 × 109 |
| pCS-1PBLO | 7.60 × 109 | 5.45 × 109 | 4.65 × 109 | 2.35 × 109 | 2.00 × 108 |
| pCS-1.2PBLO | 7.50 × 109 | 4.65 × 109 | 1.45 × 109 | 4.00 × 108 | ++++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Nguyen, T.-T.T.; Tran, T.V.; Tan, L.V.; Danh, L.T.; Than, V.T. Development of Antibacterial, Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper betle Linn Oil as Active Biodegradable Packaging Material. Coatings 2021, 11, 351. https://doi.org/10.3390/coatings11030351
Nguyen TT, Nguyen T-TT, Tran TV, Tan LV, Danh LT, Than VT. Development of Antibacterial, Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper betle Linn Oil as Active Biodegradable Packaging Material. Coatings. 2021; 11(3):351. https://doi.org/10.3390/coatings11030351
Chicago/Turabian StyleNguyen, Thuong Thi, Thu-Thao Thi Nguyen, Thuan Van Tran, Lam Van Tan, Luu Thai Danh, and Van Thai Than. 2021. "Development of Antibacterial, Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper betle Linn Oil as Active Biodegradable Packaging Material" Coatings 11, no. 3: 351. https://doi.org/10.3390/coatings11030351






