Husk of Agarwood Fruit-Based Hydrogel Beads for Adsorption of Cationic and Anionic Dyes in Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
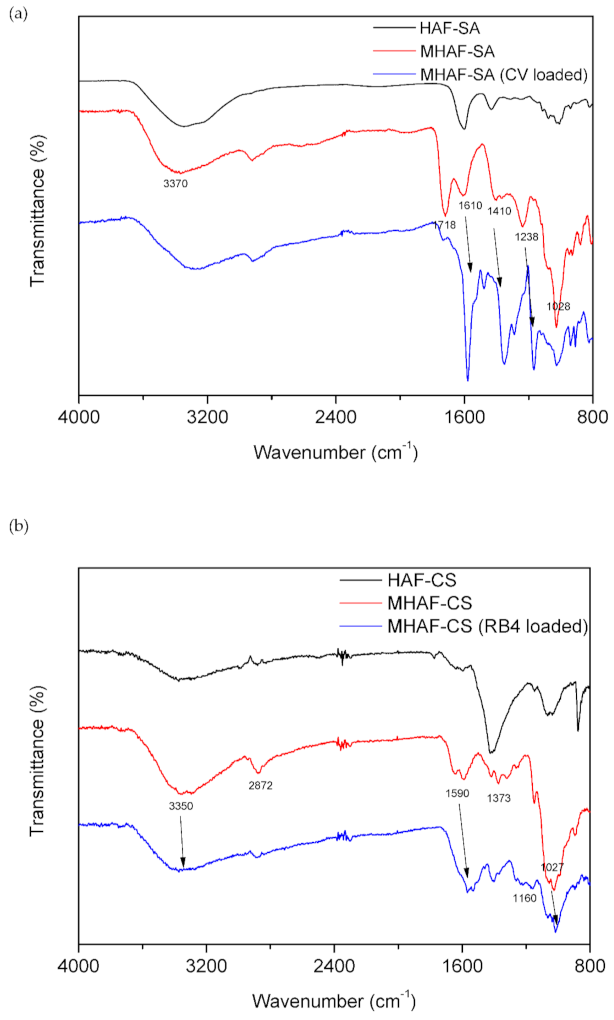
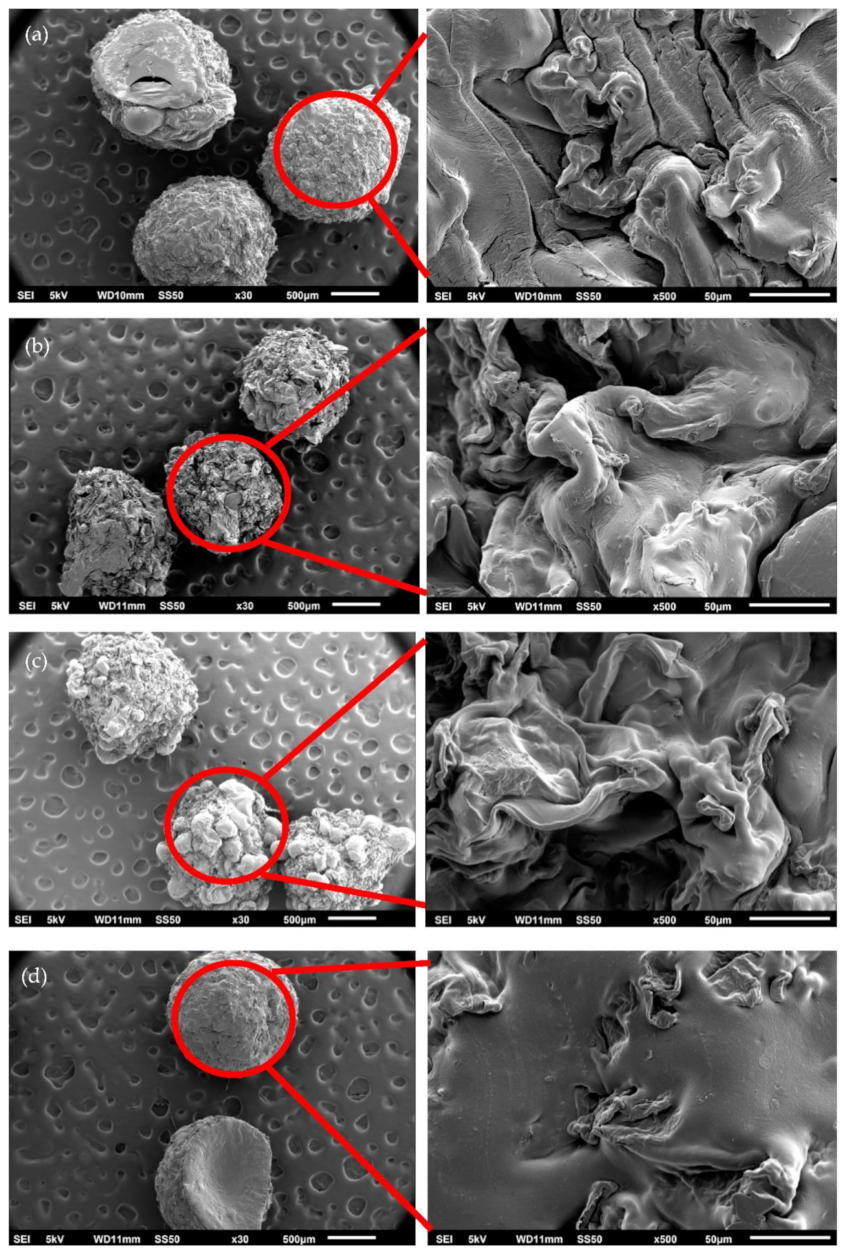
2.1. Characterization
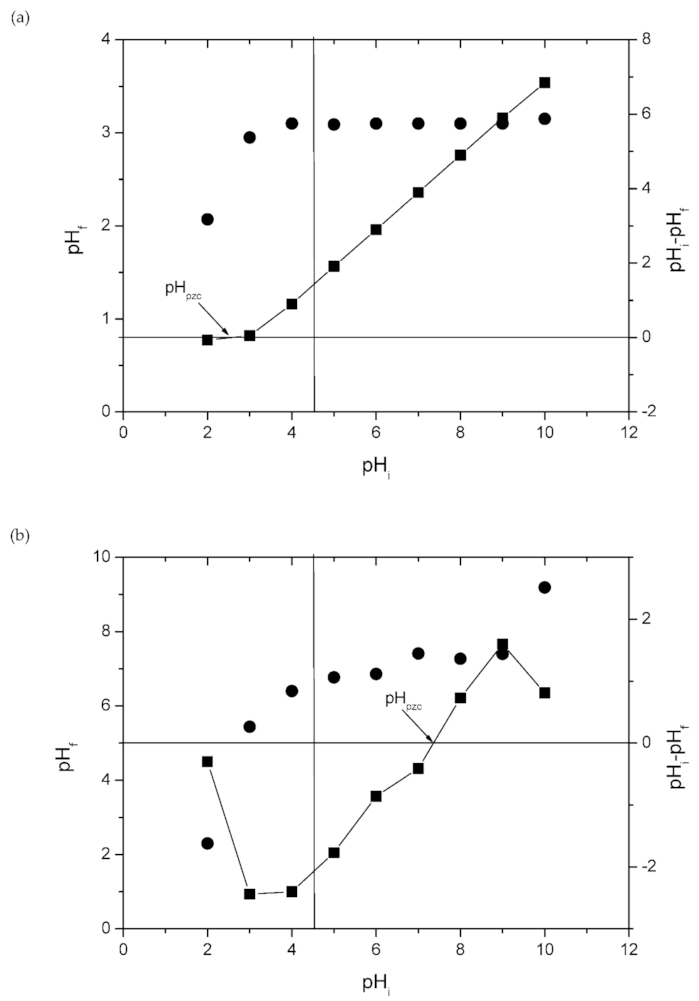
2.2. Effect of pH
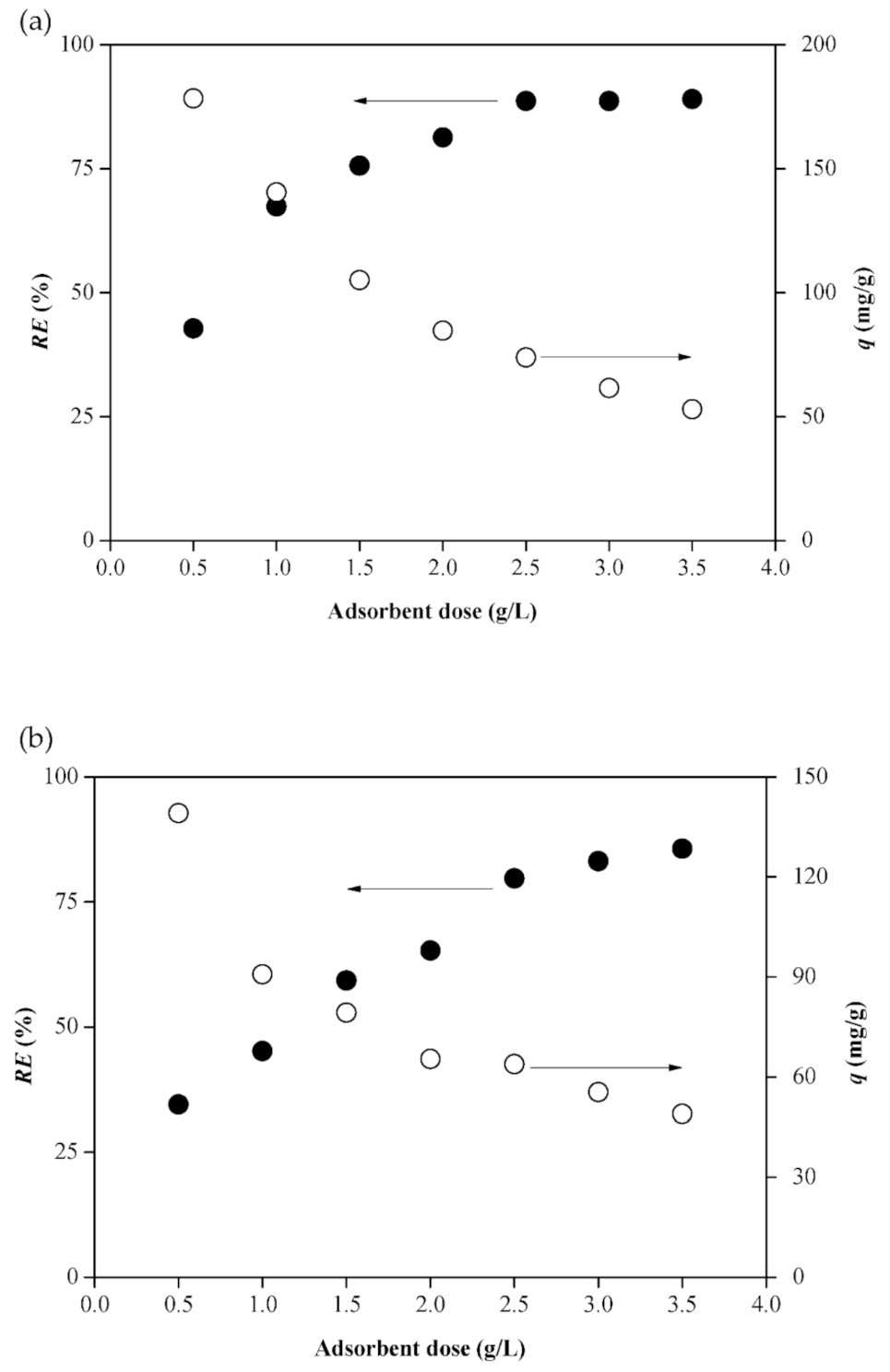
2.3. Effect of Dosage
2.4. Effect of Contact Time
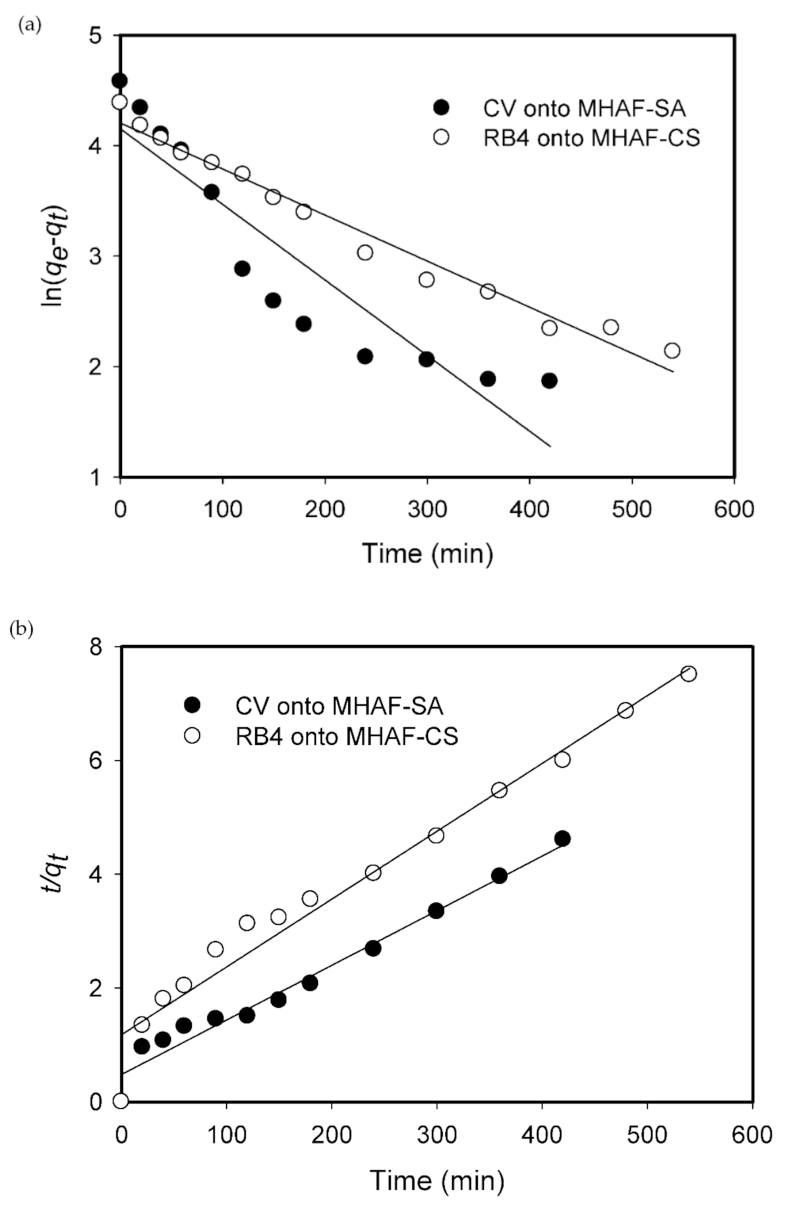
2.5. Adsorption Kinetics
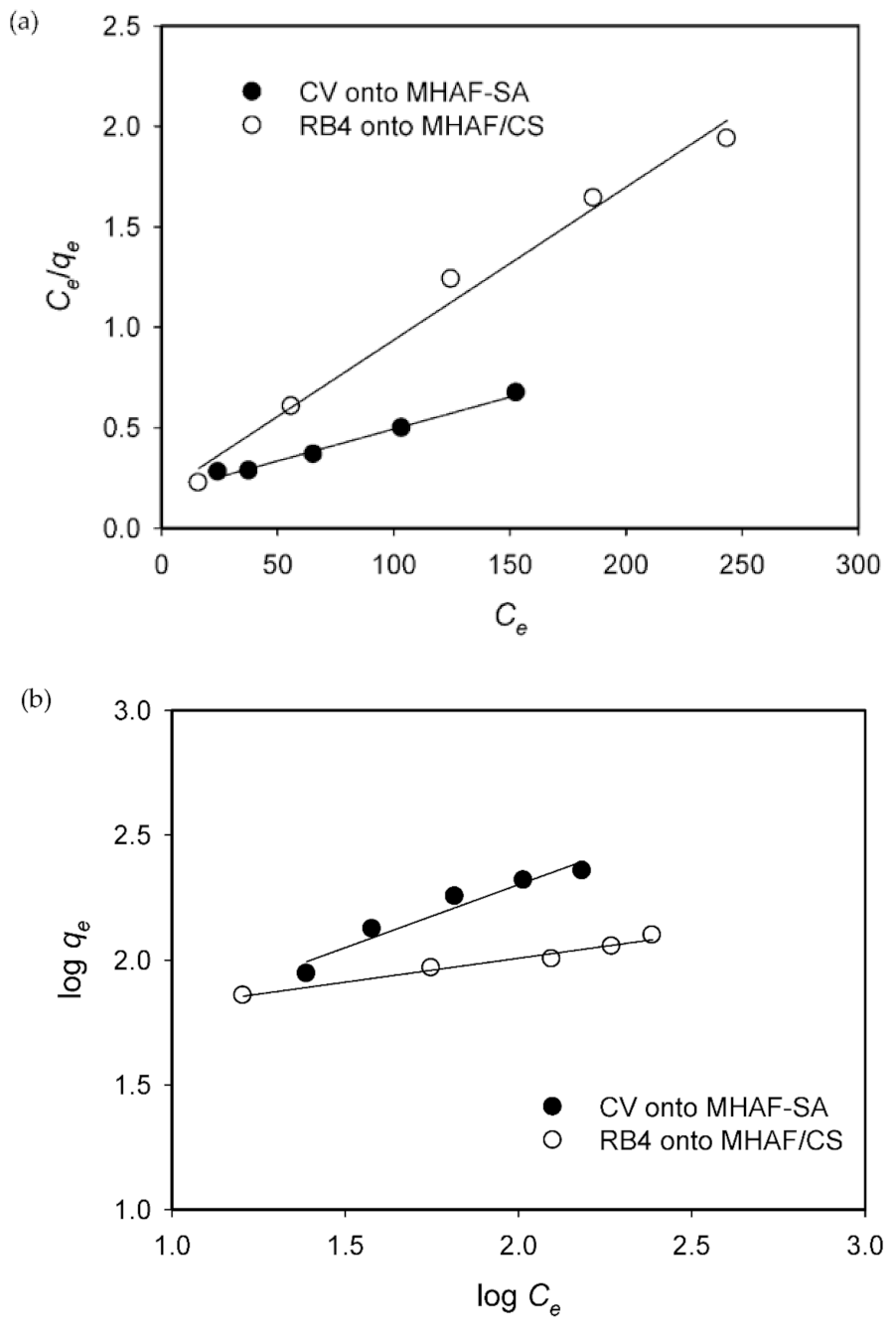
2.6. Adsorption Isotherms
2.7. Selective Adsorption of Hydrogel Beads
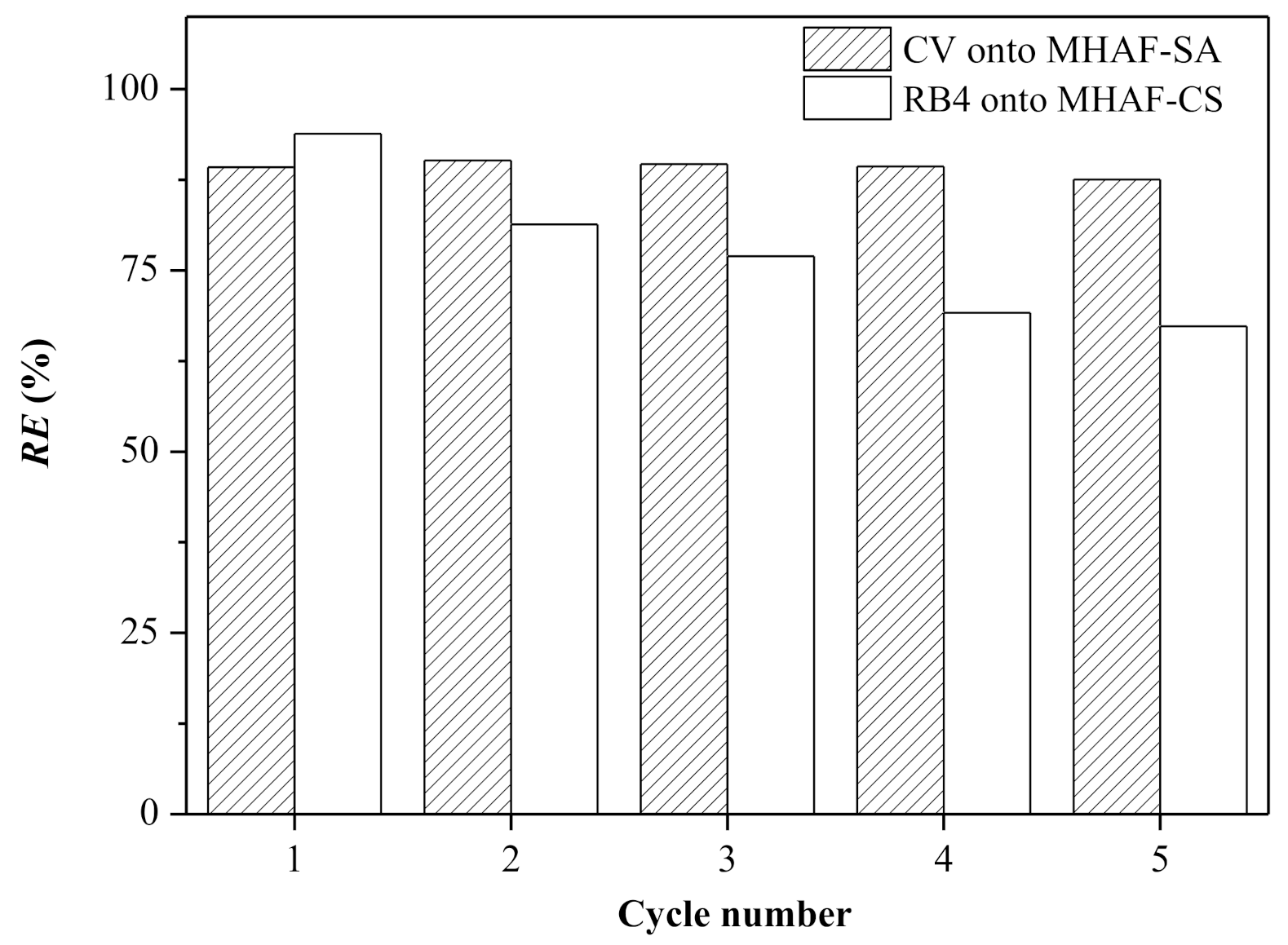
2.8. Regeneration of Hydrogel Beads
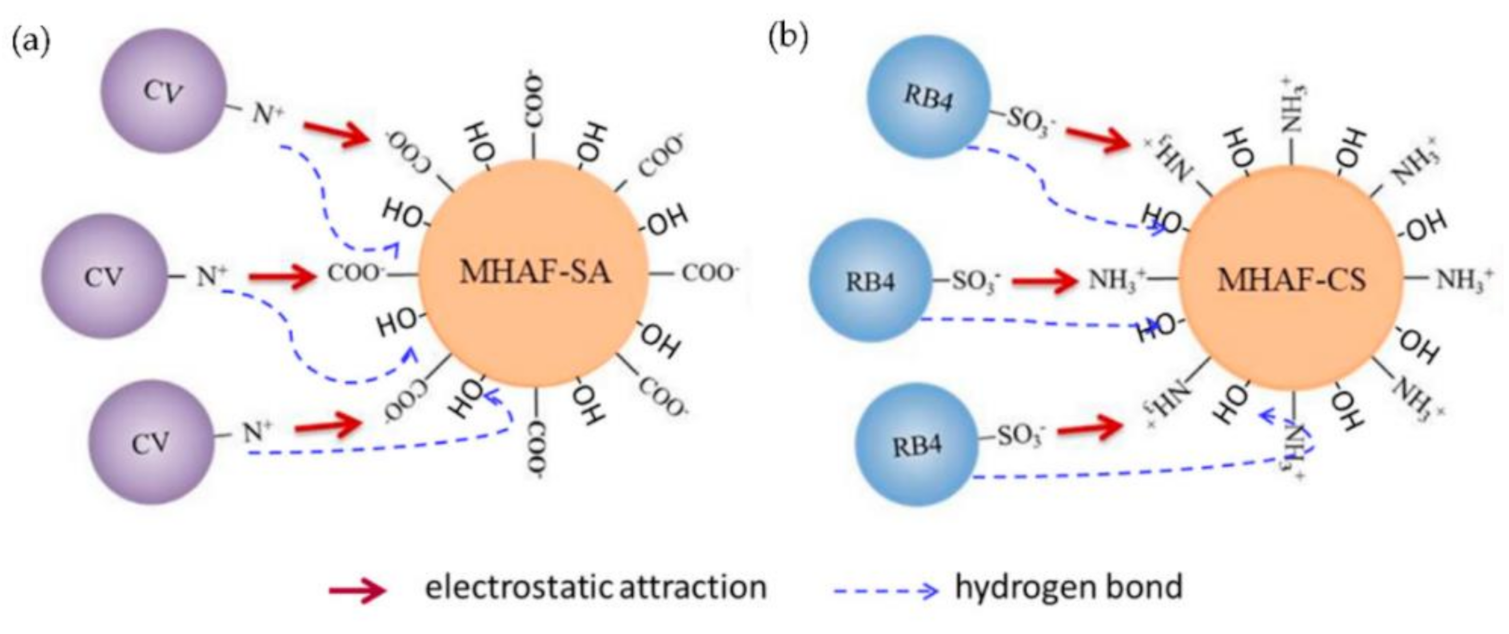
2.9. Adsorption Mechanism
3. Materials and Methods
3.1. Materials
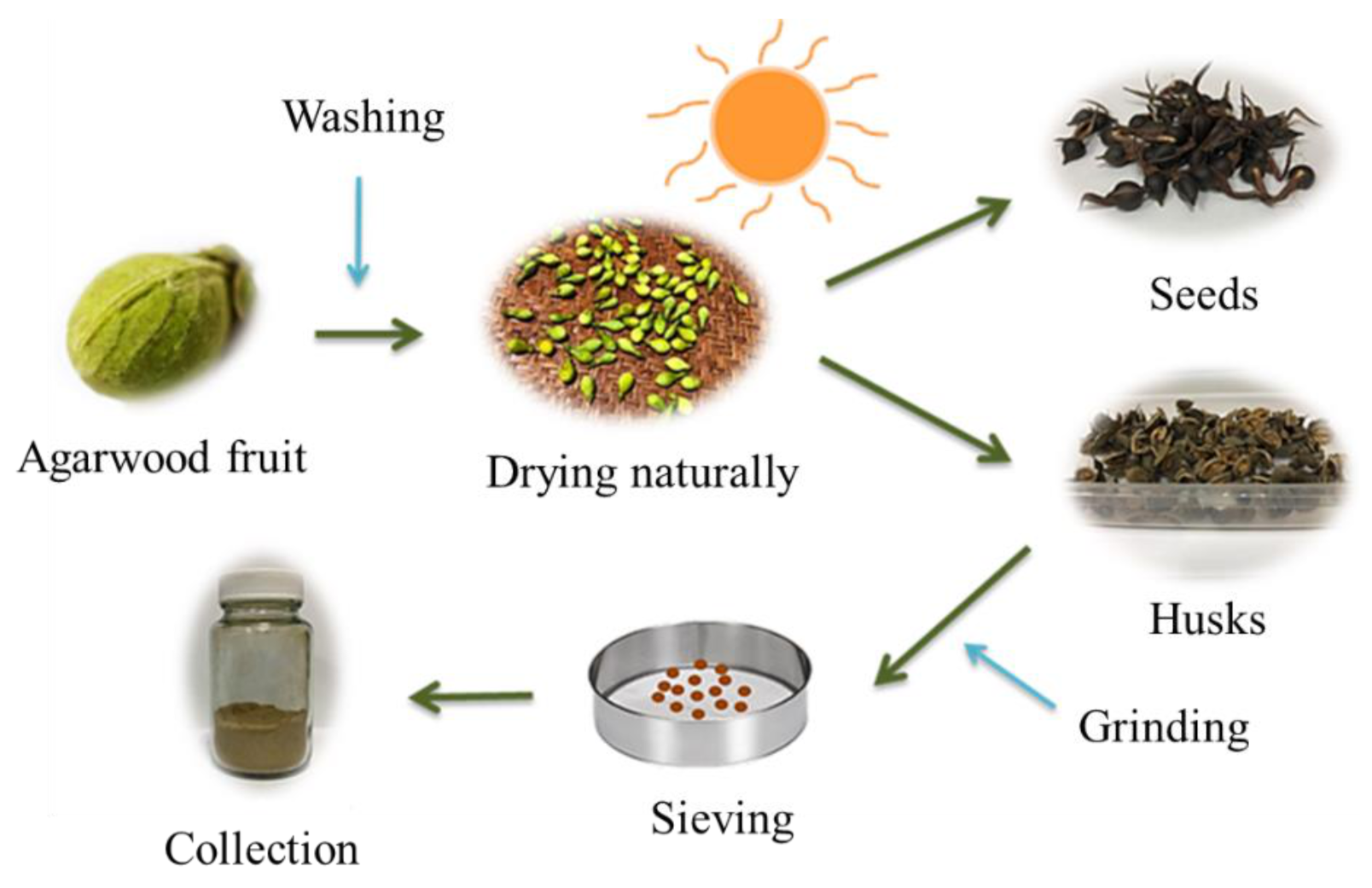
3.2. Agarwood Fruit Treatment
3.3. Preparation of HAF-SA Hydrogel Beads
3.4. Preparation of HAF-CS Hydrogel Beads
3.5. Characterization
3.6. Batch Adsorption Experiments
3.7. Selective Adsorption Study
3.8. Regeneration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sahraei, R.; Pour, Z.S.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Kurczewska, J.; Ceglowski, M.; Schroeder, G. Alginate/PAMAM dendrimer- Halloysite beads for removal of cationic and anionic dyes. Int. J. Biol. Macromol. 2019, 123, 398–408. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.V.; Quyen, T.T.H.; Trung, T.S. One-step facile synthesis of mesoporous graphene/Fe3O4/chitosan nanocomposite and its adsorption capacity for a textile dye. J. Water Process Eng. 2016, 9, 170–178. [Google Scholar]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent cellulose-clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Mondal, S.; Bobde, K.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: Equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manag. 2016, 182, 581–594. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, B.; Cao, B.; Wang, J.; Zhang, J. Fabrication of the novel hydrogel based on waste corn stalk for removal of methylene blue dye from aqueous solution. Appl. Surf. Sci. 2017, 422, 944–952. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, Y.K. Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl. Surf. Sci. 2017, 423, 800–809. [Google Scholar] [CrossRef]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Enhancing adsorption of malachite green dye using base-modified Artocarpus odoratissimus leaves as adsorbents. Environ. Technol. Inno. 2019, 13, 211–223. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Schwartz, F.W.; Jeong, Y.; Song, H. Magnetic chitosan composite for adsorption of cationic and anionic dyes in aqueous solution. J. Ind. Eng. Chem. 2015, 28, 60–66. [Google Scholar] [CrossRef]
- Cinar, S.; Kaynar, U.H.; Aydemir, T.; Kaynar, S.C.; Ayvacikli, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/Chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luan, Q.; Tang, H.; Huang, F.; Zheng, M.; Deng, Q.; Xiang, X.; Yang, C.; Shi, J.; Zheng, C.; et al. Removal of methyl orange from aqueous solutions by adsorption on cellulose hydrogel assisted with Fe2O3 nanoparticles. Cellulose 2017, 24, 903–914. [Google Scholar] [CrossRef]
- Gan, L.; Li, H.; Chen, L.; Xu, L.; Liu, J.; Geng, A.; Mei, C.; Shang, S. Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym. Sci. 2018, 296, 607–615. [Google Scholar] [CrossRef]
- Perez-Calderon, J.; Santos, M.V.; Zaritzky, N. Reactive RED 195 dye removal using chitosan coacervated particles as bio-sorbent: Analysis of kinetics, equilibrium and adsorption mechanisms. J. Environ. Chem. Eng. 2018, 6, 6749–6760. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, L. Unique gelation of chitosan in an alkali/urea aqueous solution. Polymers 2018, 141, 124–131. [Google Scholar] [CrossRef]
- Kahya, S.; Solak, E.K.; Sanh, O. Sodium alginate/poly(vinyl alcohol) alloy membranes for the pervaporation, vapour permeation and vapour permeation with temperature difference separation of dimethylformamide/water mixtures: A comparative study. Vacuum 2010, 84, 1092–1102. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Hu, S.; Zhang, X.; Luo, X. Core-shell zeolite@Alg-Ca particles for removal of strontium from aqueous solutions. RSC Adv. 2016, 6, 73959–73973. [Google Scholar] [CrossRef]
- Shajahan, A.; Shankar, S.; Sathiyaseelan, A.; Narayan, K.S.; Narayanan, V.; Kaviyarasan, V.; Ignacimuthu, S. Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int. J. Biol. Macromol. 2017, 104, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene blue removal from water using the hydrogel beads of poly(vinyl alcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cui, T.; Yang, C.; Dai, X.; Ma, J. κ-Carrageenan/Sodium alginate double-network hydrogel with enhanced mechanical properties, anti-swelling, and adsorption capacity. Chemosphere 2019, 237, 124417–124423. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Islam, M.T.; Zusoh, Z.M.; Khan, S.I. Agarwood production-a multidisciplinary field to be explored in Bangladesh. Int. J. Pharm. Life Sci. 2013, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Quansah, J.O.; Hlaing, T.; Lyonga, F.N.; Kyi, P.P.; Hong, S.H.; Lee, C.G.; Park, S.J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020, 10, 3437. [Google Scholar] [CrossRef]
- Mohanty, S.; Moulick, S.; Maji, S.K. Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J. Water Process. Eng. 2020, 37, 101428. [Google Scholar] [CrossRef]
- Carneiro, P.A.; Fugivara, C.S.; Nogueira, R.F.P.; Boralle, N.; Zanoni, M.V.B. A comparative study on chemical and electrochemical degradation of Reactive Blue 4 dye. Port. Electrochimica Acta 2003, 21, 49–67. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, L.; Wang, K.; Yao, J.; Li, D.; Simon, G.P.; Wang, R.; Wang, H. Significantly enhanced water flux in forward osmosis desalination with polymer-graphene composite hydrogels as a draw agent. RSC Adv. 2013, 3, 887–894. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef]
- Oussalah, A.; Boukerroui, A.; Aichour, A.; Djellouli, B. Cationic and anionic dyes removal by low-cost hybrid alginate/natural bentonite composite beads: Adsorption and reusability studies. Int. J. Biol. Macromol. 2019, 124, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tan, J.; Wang, G.; Zhou, L. Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohydr. Polym. 2018, 199, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, W.; Mao, Q.; Bai, Y.; Ma, H. Facile synthesis of chitosan/gelatin filled with graphene bead adsorbent for orange II removal. Chem. Eng. Res. Des. 2019, 144, 35–46. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloester stoffe, Kungliga Svenska Vetenskapsakad. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption process. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Li, T.; Zhou, C. Physicochemical studies toward the removal of Zn(II) and Pb(II) ions through adsorption on montmorillonite-supported zero-valent iron nanoparticles. RSC Adv. 2015, 5, 29859–29871. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über die absorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Yang, H.; Li, A.; Cheng, R. Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem. Eng. J. 2013, 223, 402–411. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Guo, W.; Tian, Y. Removal of malachite green and crystal violet cationic dyes from aqueous solution using activated sintering process red mud. Appl. Clay Sci. 2014, 93–94, 85–93. [Google Scholar] [CrossRef]
- Tahir, N.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Biopolymers composites with peanut hull waste biomass and application for Crystal Violet adsorption. Int. J. Biol. Macromol. 2017, 94, 210–220. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Wang, X.; Huang, Q.; Min, Y.; Ma, T.; Niu, J. Removal of crystal violet by a novel cellulose-based adsorbent: Comparison with native cellulose. J. Ind. Eng. Chem. 2014, 53, 5498–5506. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Celik, G.; Arica, M.Y. Biosorption of reactive blue 4 dye by native and treated fungus phanerocheate chrysosporium: Batch and continuous flow system studies. J. Hazard. Mater. 2006, 137, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Binupriya, A.R.; Sathishkumar, M.; Jung, S.H.; Song, S.H.; Yun, S.I. A novel method in utilization of bokbunja seed wastes from wineries in liquid-phase sequestration of reactive blue 4. Int. J. Environ. Res. 2009, 3, 1–12. [Google Scholar]
- Sun, D.; Zhang, Z.; Wang, M.; Wu, Y. Adsorption of reactive dyes on activated carbon developed from enteromorpha prolifera. Am. J. Analyt. Chem. 2013, 4, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.M.; Hong, G.B.; Wang, Y.K. Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent. Materials 2020, 13, 2764. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Li, J.; Wang, L.; Jin, W.; Liu, J.; Pei, Y.; Tang, K. Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution. Int. J. Biol. Macromol. 2018, 107, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Romanov, A.; Rooney, J.; Chen, W. Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran. Biomaterials 2008, 29, 3905–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwakeel, K.Z.; El-Kousy, S.; El-Shorbagy, H.G.; El-Ghaffar, M.A.A. Comparison between the removal of reactive black 5 from aqueous solutions by 3-amino-1,2,4 triazole,5-thiol and melamine grafted chitosan prepared through four different routes. J. Environ. Chem. Eng. 2016, 4, 733–745. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P. Water absorption, retention and the swelling characteristics of cassava starch grafted with polyacrylic acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwakeel, K.Z. Removal of reactive black 5 from aqueous solutions using magnetic chitosan resins. J. Hazard. Mater. 2009, 167, 383–392. [Google Scholar] [CrossRef] [PubMed]




| Dye | CV | RB4 |
|---|---|---|
| qexp (mg/g) | 97.56 | 80.41 |
| PFOM | ||
| k1 (1/min) | 0.0068 | 0.0042 |
| qe (mg/g) | 63.7 | 83.0 |
| R2 | 0.8353 | 0.9739 |
| PSOM | ||
| k2 (g/mg·g) | 1.92 × 10−4 | 1.20 × 10−4 |
| qe (mg/g) | 104.17 | 84.03 |
| R2 | 0.9715 | 0.9635 |
| Dye | CV | RB4 | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | 30 | 40 | 50 | 30 | 40 | 50 |
| Langmuir | ||||||
| qm (mg/g) | 232.56 | 312.50 | 370.37 | 156.25 | 222.22 | 270.27 |
| KL (L/mg) | 0.022 | 0.018 | 0.016 | 0.027 | 0.014 | 0.013 |
| R2 | 0.975 | 0.989 | 0.982 | 0.998 | 0.995 | 0.998 |
| Freundlich | ||||||
| KF (mg/g)(L/mg)1/n | 22.43 | 19.60 | 16.39 | 37.13 | 42.09 | 56.87 |
| n | 2.41 | 1.98 | 1.74 | 6.84 | 5.21 | 4.90 |
| R2 | 0.875 | 0.936 | 0.957 | 0.983 | 0.974 | 0.858 |
| Dye | Adsorbent | Adsorption Capacity (mg/g) | Reusability (Five Cycle) | Reference |
|---|---|---|---|---|
| CV | NaOH-modified rice husk | 44.87 | - | [40] |
| Chitosan magnetic composite microspheres | 86.6 | −1.6% | [41] | |
| Acid-activated sintering process red mud | 336.3 | - | [42] | |
| Chitosan pyrrole | 150.16 | - | [43] | |
| Cellulose-based adsorbent | 182.15 | >−25% | [44] | |
| MHAF-SA | 232.56–370.37 | −1.69% | This study | |
| RB4 | Heat-treated fungal biomass | 156.9 | [45] | |
| Bokbunja seed wastes | 26.1 | [46] | ||
| rice bran/ Fe3O4 | 185.19 | −4.6% | [9] | |
| Activated carbon | 131.9 | [47] | ||
| rice bran/SnO2/Fe3O4 | 218.82 | −12% | [48] | |
| MHAF-CS | 156.25–270.27 | ~−20% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.M.; Yang, B.-Y.; Hong, G.-B. Husk of Agarwood Fruit-Based Hydrogel Beads for Adsorption of Cationic and Anionic Dyes in Aqueous Solutions. Molecules 2021, 26, 1437. https://doi.org/10.3390/molecules26051437
Ma CM, Yang B-Y, Hong G-B. Husk of Agarwood Fruit-Based Hydrogel Beads for Adsorption of Cationic and Anionic Dyes in Aqueous Solutions. Molecules. 2021; 26(5):1437. https://doi.org/10.3390/molecules26051437
Chicago/Turabian StyleMa, Chih Ming, Bo-Yuan Yang, and Gui-Bing Hong. 2021. "Husk of Agarwood Fruit-Based Hydrogel Beads for Adsorption of Cationic and Anionic Dyes in Aqueous Solutions" Molecules 26, no. 5: 1437. https://doi.org/10.3390/molecules26051437






