Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Thioflavin T (ThT) Assay
2.2. Cell Culture
2.3. Cell Cytotoxicity
2.4. Neuroprotective Activity Assay
2.5. Measurement of Reactive Oxygen Species (ROS)
2.6. Mitochondrial Membrane Potential (ΔΨm) Assay
2.7. Molecular Docking
2.8. Statistical Analysis
3. Results
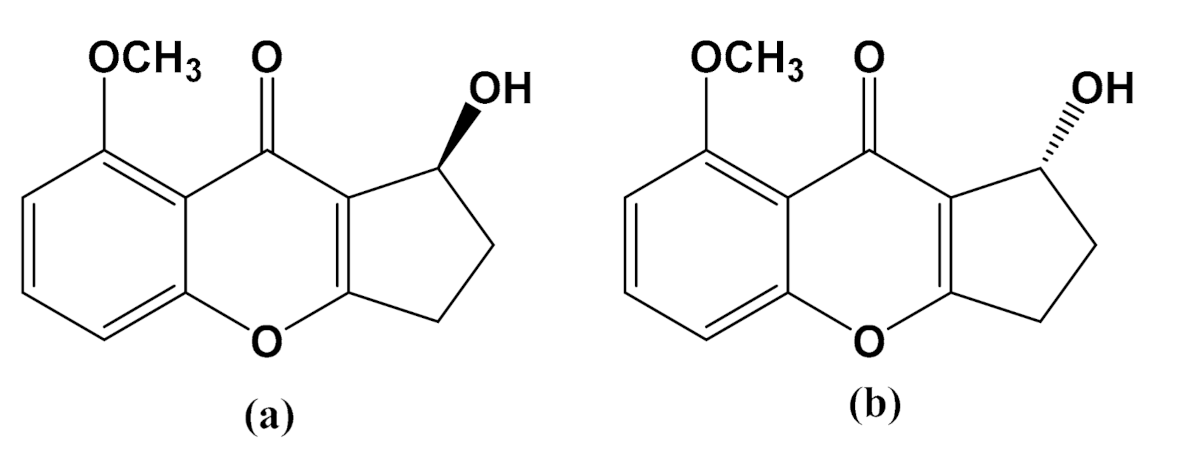
3.1. Effects of Diaportheone A1 and Diaportheone A2 on Thioflavin T (ThT) Assay
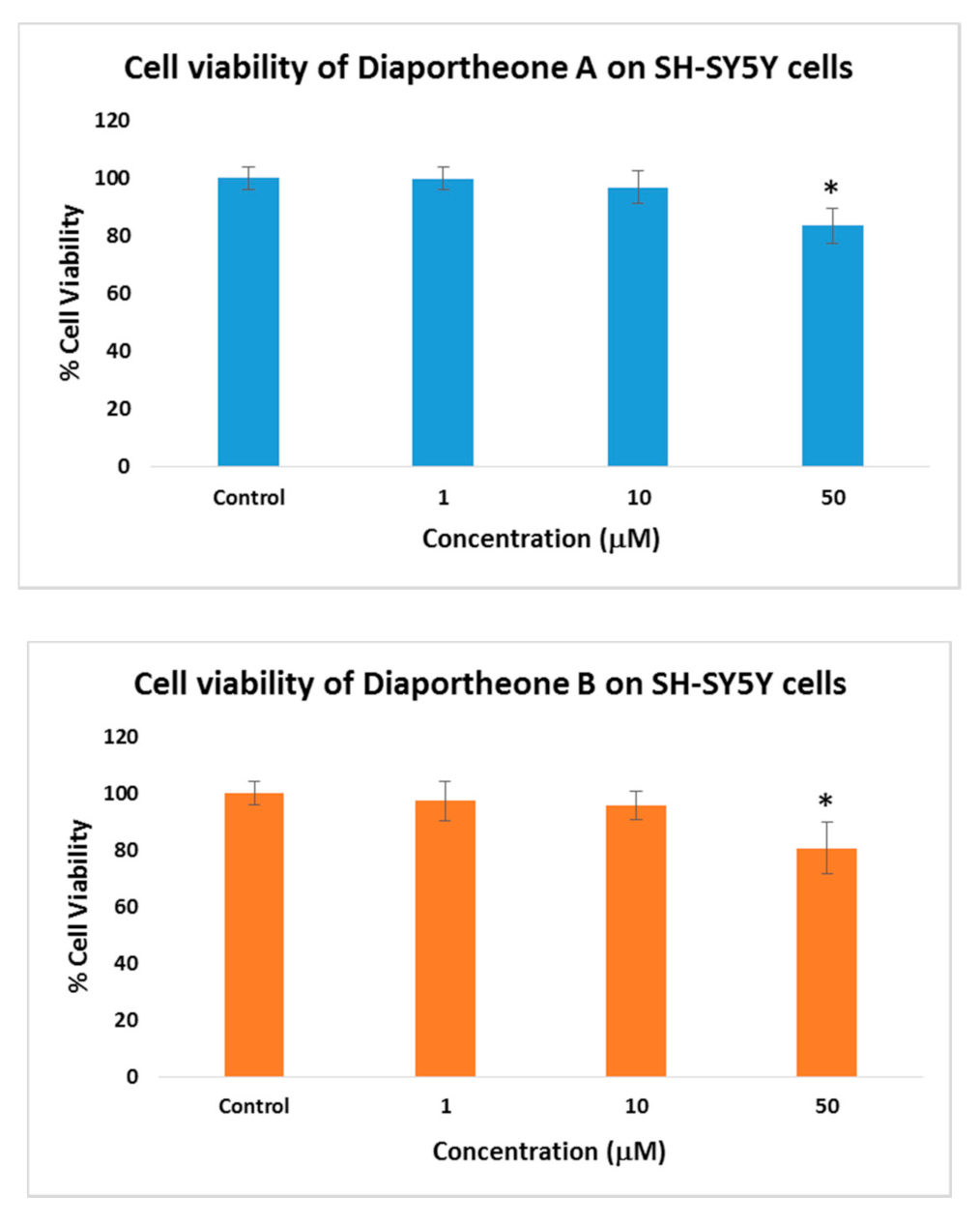
3.2. Cytotoxicity of Diaportheone A1 and Diaportheone A2
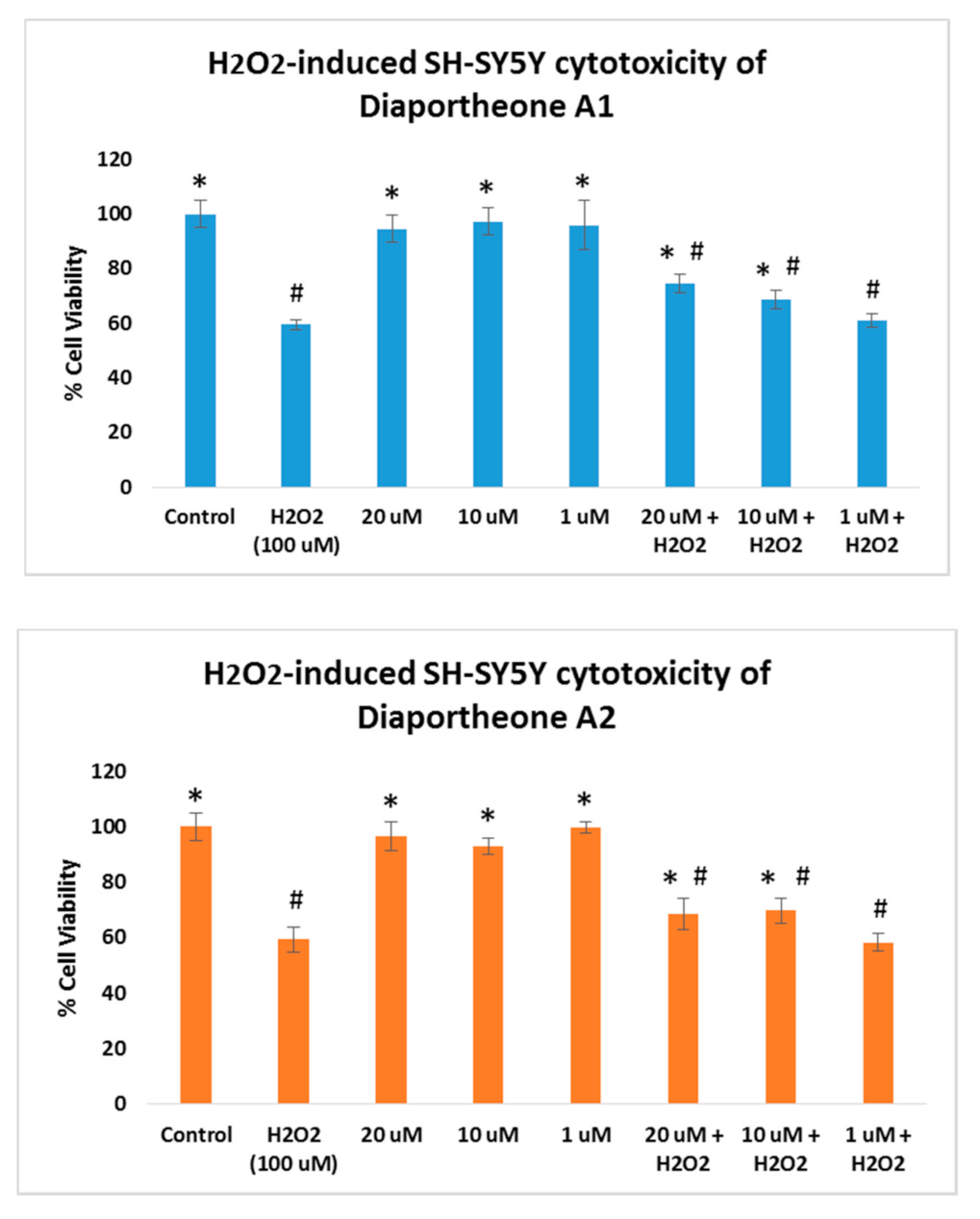
3.3. Neuroprotective Effects of Diaportheone A1 and Diaportheone A2
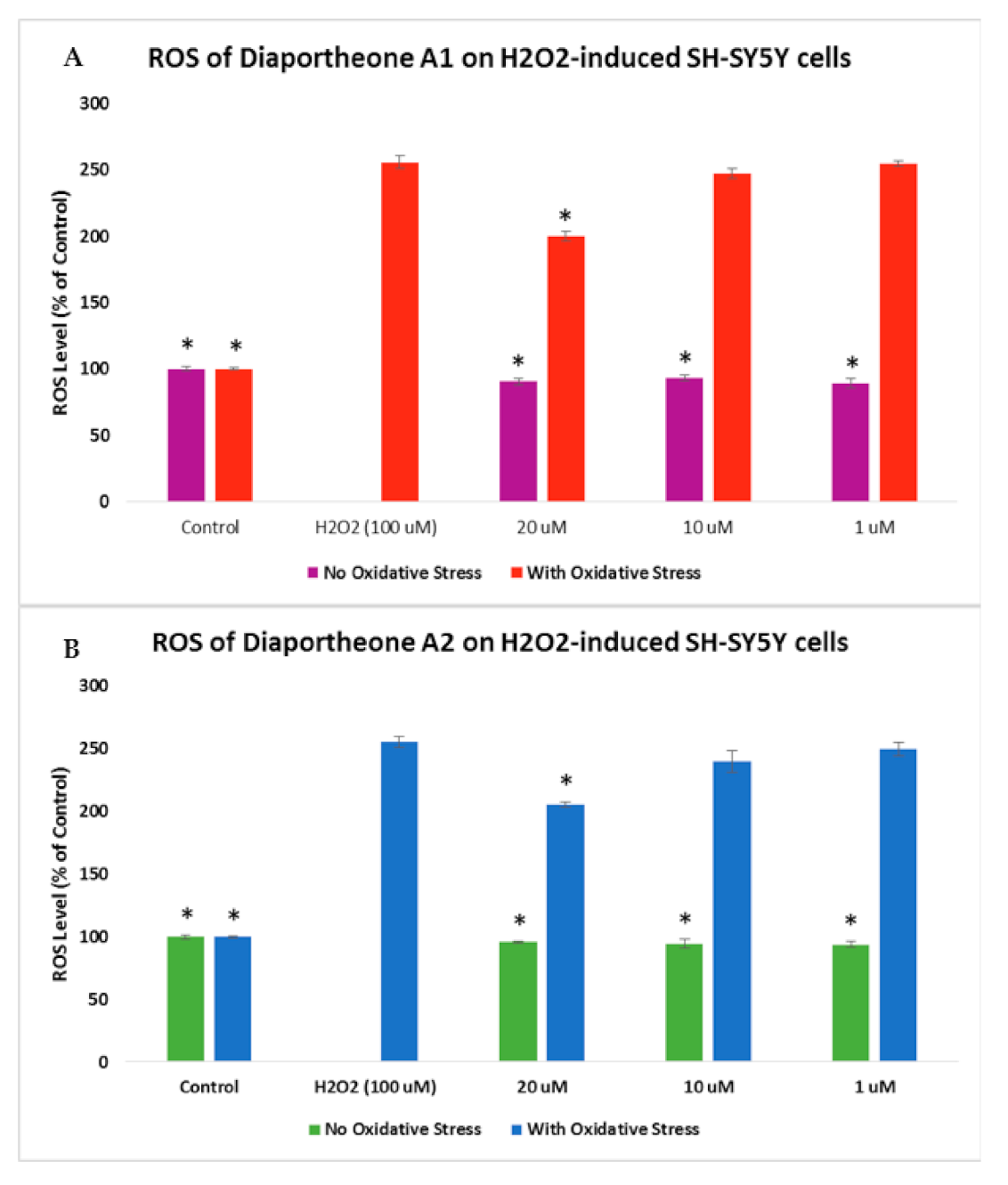
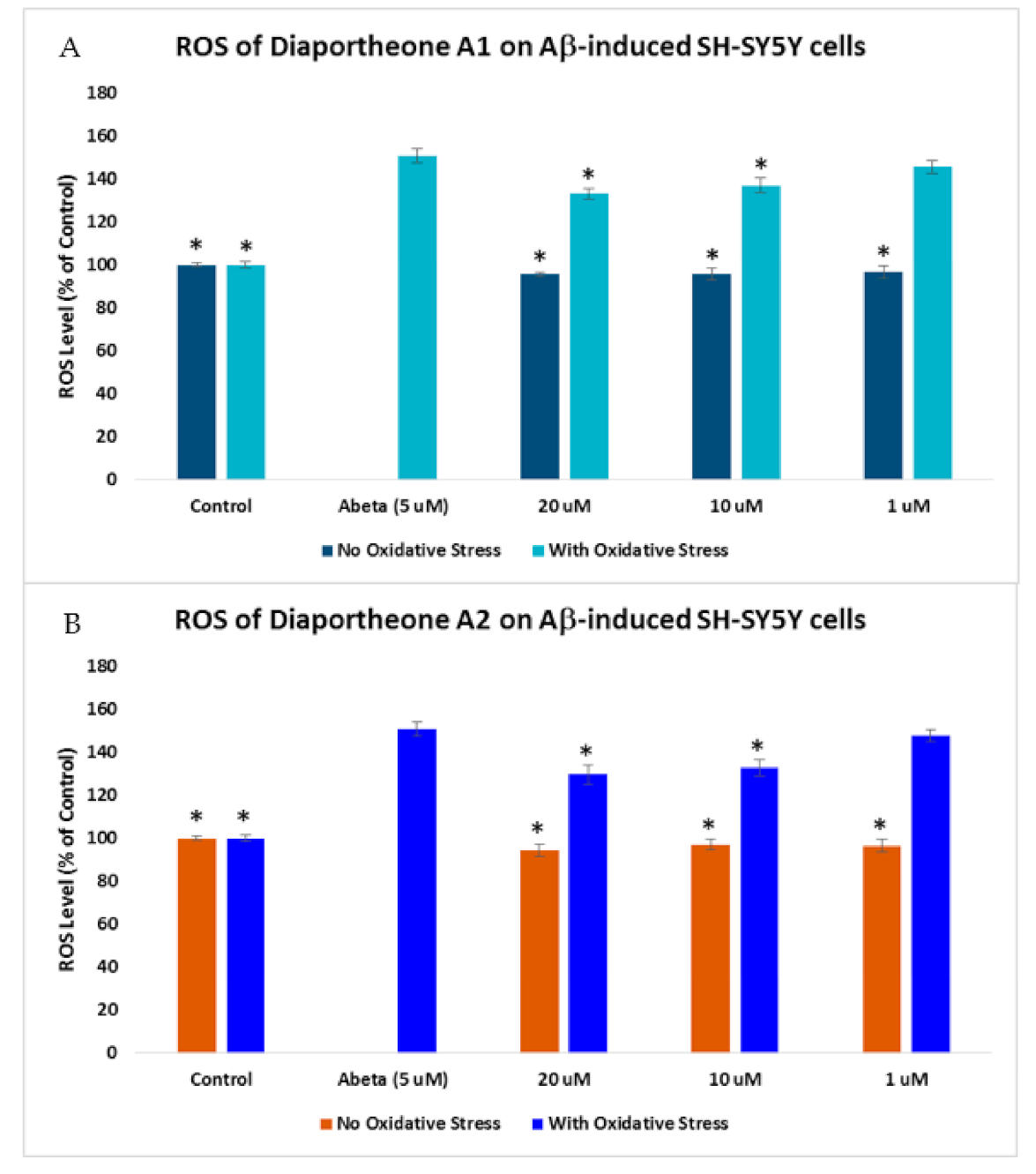
3.4. Effects on Intracellular Reactive Oxygen Species (ROS) Production
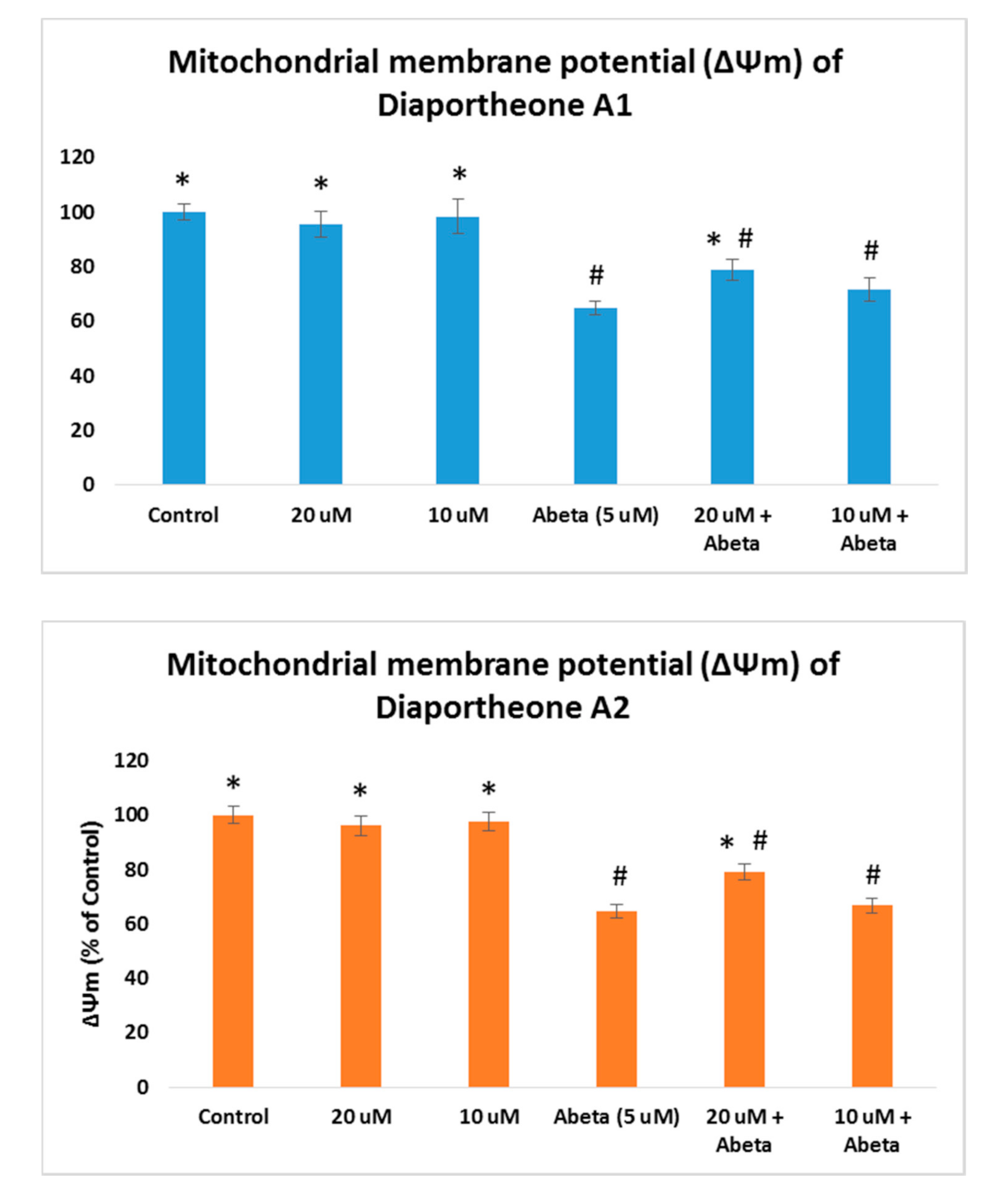
3.5. Effects on Mitochondrial Membrane Potential
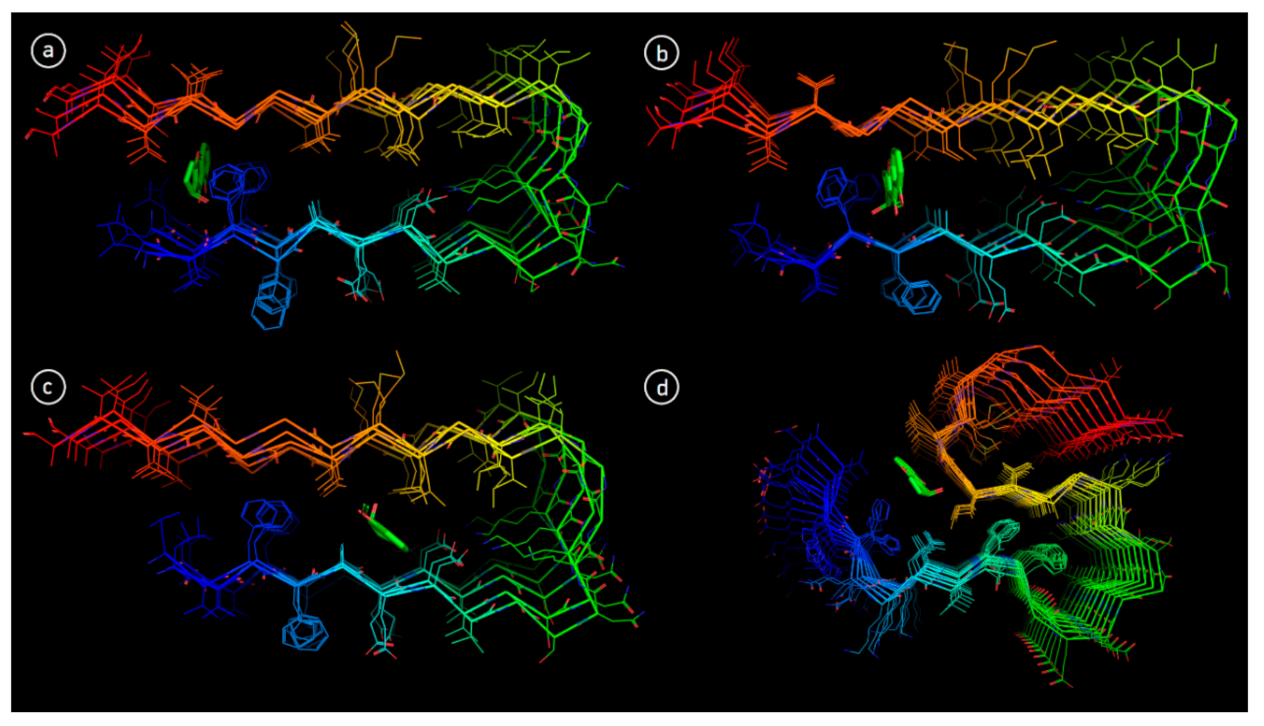
3.6. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-L.; Tang, G.-H.; Guo, Y.-Q.; Xu, Y.-K.; Huang, Z.-S.; Yin, S. Mulberry Diels-Alder-type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochem 2019, 157, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, K.; Xu, H.; Qiao, Z.; Cai, S.; Wang, Y.; Huang, L. Predicting the progression of mild cognitive impairment to Alzheimer’s disease by longitudinal magnetic resonance imaging-based dictionary learning. Clin. Neurophysiol. 2020, 131, 2429–2439. [Google Scholar] [CrossRef]
- Poloni, K.M.; Duarte de Oliveira, I.A.; Tam, R.; Ferrari, R.J. Brain MR image classification for Alzheimer’s disease diagnosis using structural hippocampal asymmetrical attributes from directional 3-D log-Gabor filter responses. Neurocomputing 2021, 419, 126–135. [Google Scholar] [CrossRef]
- Reus, L.M.; Stringer, S.; Posthuma, D.; Teunissen, C.E.; Scheltens, P.; Pijenburg, Y.; Visser, P.J.; Tijms, B.M. Degree of genetic liability for Alzheimer’s disease associated with specific proteomic profiles in cerebrospinal fluid. Neurobiol. Aging 2020, 93, e1–e15. [Google Scholar] [CrossRef]
- Emmerzaal, T.L.; Kiliaan, A.J.; Gustafson, D.R. 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimers Dis. 2015, 43, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Cashman, N.R. Passive immunotherapies targeting Aβ and tau in Alzheimer’s disease. Neurobiol. Dis. 2020, 144, 105010. [Google Scholar] [CrossRef]
- Tang, K.S. The potential role of nanoyttria in alleviating oxidative stress biomarkers: Implications for Alzheimer’s disease therapy. Life Sci. 2020, 259, 118287. [Google Scholar] [CrossRef]
- Sieteiglesias, V.; Gonzalez-Burgos, E.; Bermejo-Bescos, P.; Divakar, P.K.; Gomez-Serranillos, M.P. Lichens of parmelioid clade as promising multitarget neuroprotective agents. Chem. Res. Toxicol. 2019, 32, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Montine, T.J.; Montine, K.S.; McMahan, W.; Markesbery, W.R.; Quinn, J.F.; Morrow, J.D. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid. Redox Signal 2005, 7, 269–275. [Google Scholar] [CrossRef]
- Reddy, P.H. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer disease. J. Neurochem. 2006, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimers Dis. 2018, 62, 1335–1399. [Google Scholar] [CrossRef]
- Dey, A.; Bhattacharya, R.; Mukherjee, A.; Pandey, D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017, 35, 178–216. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Ko, H.; Yang, H.; Wang, X. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology 2010, 59, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Doig, A.J.; Derreumaux, P. Inhibition of protein aggregation and amyloid formation by small molecules. Curr. Opin. Struct. Biol. 2015, 30, 50–56. [Google Scholar] [CrossRef]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Lagamayo, M.W.D.; Alejandro, G.J.D.; An, S.S.A. Anti-amyloidogenic and cyclooxygenase inhibitory activity of Guettarda speciosa. Molecules 2019, 24, 4112. [Google Scholar] [CrossRef]
- Tan, M.A.; Gonzalez, S.J.B.; Alejandro, G.J.D.; An, S.S.A. Neuroprotective effects of vomifoliol, isolated from Tarenna obtusifolia Merr. (Rubiaceae), against amyloid-beta(1-42)-treated neuroblastoma SH-SY5Y cells. 3 Biotech 2020, 10, 424. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Nunez-Sanchez, M.A.; Tomas-Barberan, F.A.; Espin, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolited against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Chen, Z.-Y.; Sun, B.; Liu, J.; Meng, F.-Y.; Liu, Y.; Tian, T.; Jin, A.; Ruan, H.-L. Lignans from the fruit of Schisandra glaucescens with antioxidant and neuroprotective properties. J. Nat. Prod. 2014, 77, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, P.; Zodio, S.; Lucas, R.; de-Paz, M.V.; Morales, J.C. Neuroprotective and anti-inflammatory effects of pterostilbene metabolites in human neuroblastoma SH-SY5Y and RAW 264.7 macrophage cells. J. Agric. Food Chem. 2020, 68, 1609–1620. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Lacret, R.; Oves-Costales, D.; Genilloud, O.; Reyes, F.; Alfonso, A.; Botana, L.M.; Caniferolide, A. A macrolide from Streptomyces caniferus, attenuates neuroinflammation, oxidative stress, amyloid-beta, and tau pathology in vitro. Mol. Pharm. 2019, 16, 1456–1466. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comp. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Döbeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Aβ(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef]
- Wu, C.; Lei, H.; Wang, Z.; Zhang, W.; Duan, Y. Phenol red interacts with the protofibril-like oligomers of an amyloidogenic hexapeptide NFGAIL through both hydrophobic and aromatic contacts. Biophys. J. 2006, 91, 3664–3672. [Google Scholar] [CrossRef] [PubMed]
- Sereia, A.L.; de Oliveira, M.T.; Baranoski, A.; Marques, L.L.; Ribeiro, F.; Isolani, R.; de Medeiros, D.; Chierrito, D.; Bidoia, D.; Zielinski, A.; et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef]
- Kuang, G.; Murugan, N.A.; Tu, Y.; Nordberg, A.; Agren, H. Investigation of the binding profiles of AZD2184 and Thioflavin T with amyloid-β(1–42) fibril by molecular docking and molecular dynamics methods. J. Phys. Chem. B 2015, 119, 11560–11567. [Google Scholar] [CrossRef]
- Espargaró, A.; Ginex, T.; Vadell, M.D.; Busquets, M.A.; Estelrich, J.; Muñoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a privileged scaffold in drug discovery: Recent advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef]
- Tan, M.A.; Zueger, P.P.; Roggo, S. Total synthesis of diaportheone A. Tet. Lett. 2019, 60, 52–54. [Google Scholar] [CrossRef]
- Brown, D.G.; Wobst, H.K. Opportunities and challenges in phenotypic screening for neurodegenerative disease research. J. Med. Chem. 2020, 63, 1823–1840. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hu, X.; Tan, R.; Feng, Y.; Sun, M.; Ma, N.; Li, X.; Huang, L.; An, J.; Ge, Q.; et al. Neuroprotective effect of green tea extractives against oxidative stress by enhancing the survival and proliferation of PC12 cells. Mol. Cell. Toxicol. 2019, 15, 391–397. [Google Scholar] [CrossRef]

| % Inhibition of Aβ1-42 Aggregation a | |
|---|---|
| Diaportheone A1 (5 μM) | 34.75% ± 2.5 * |
| Diaportheone A1 (50 μM) | 80.41% ± 1.40 * |
| Diaportheone A2 (5 μM) | 35.21% ± 2.80 * |
| Diapotheone A2 (50 μM) | 73.68% ± 1.70 * |
| Phenol Red (50 μM) (Positive control) | 65.78% ± 2.97 |
| Model | 2MXU | 2BEG | ||
|---|---|---|---|---|
| Diaportheone A1 | Diaportheone A2 | Diaportheone A1 | Diaportheone A2 | |
| 1 | −8.0 | −7.9 | −10.3 | −11.0 |
| 2 | −5.8 | - | −8.7 | −8.6 |
| 3 | −6.8 | −6.6 | −9.7 | −10.1 |
| 4 | −8.3 | −8.5 | −6.8 | −6.6 |
| 5 | −6.6 | −6.2 | −8.8 | −8.9 |
| 6 | −7.3 | −6.9 | −10.4 | −9.7 |
| 7 | - | - | −8.3 −8.1 | −8.4 −7.4 |
| 8 | −7.1 | −7.1 | −7.8 −10.7 | −7.6 −10.8 |
| 9 | −7.5 | −7.2 | −9.1 | −8.8 |
| 10 | −7.4 | −7.4 | −8.8 | −8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.A.; Zakharova, E.; An, S.S.A. Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biology 2021, 10, 199. https://doi.org/10.3390/biology10030199
Tan MA, Zakharova E, An SSA. Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biology. 2021; 10(3):199. https://doi.org/10.3390/biology10030199
Chicago/Turabian StyleTan, Mario A., Elena Zakharova, and Seong Soo A. An. 2021. "Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress" Biology 10, no. 3: 199. https://doi.org/10.3390/biology10030199
APA StyleTan, M. A., Zakharova, E., & An, S. S. A. (2021). Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biology, 10(3), 199. https://doi.org/10.3390/biology10030199







