Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats
Abstract
1. Introduction
2. Results
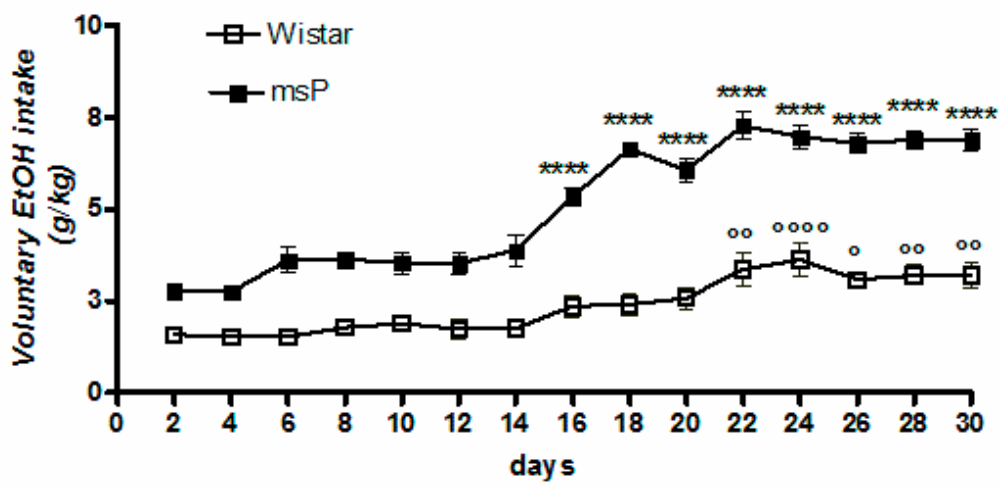
2.1. Voluntary 10% EtOH Intake
2.2. Gene Expression Analysis in the AMY of Water Controls and 10% EtOH Exposed Wistar and msP Rats
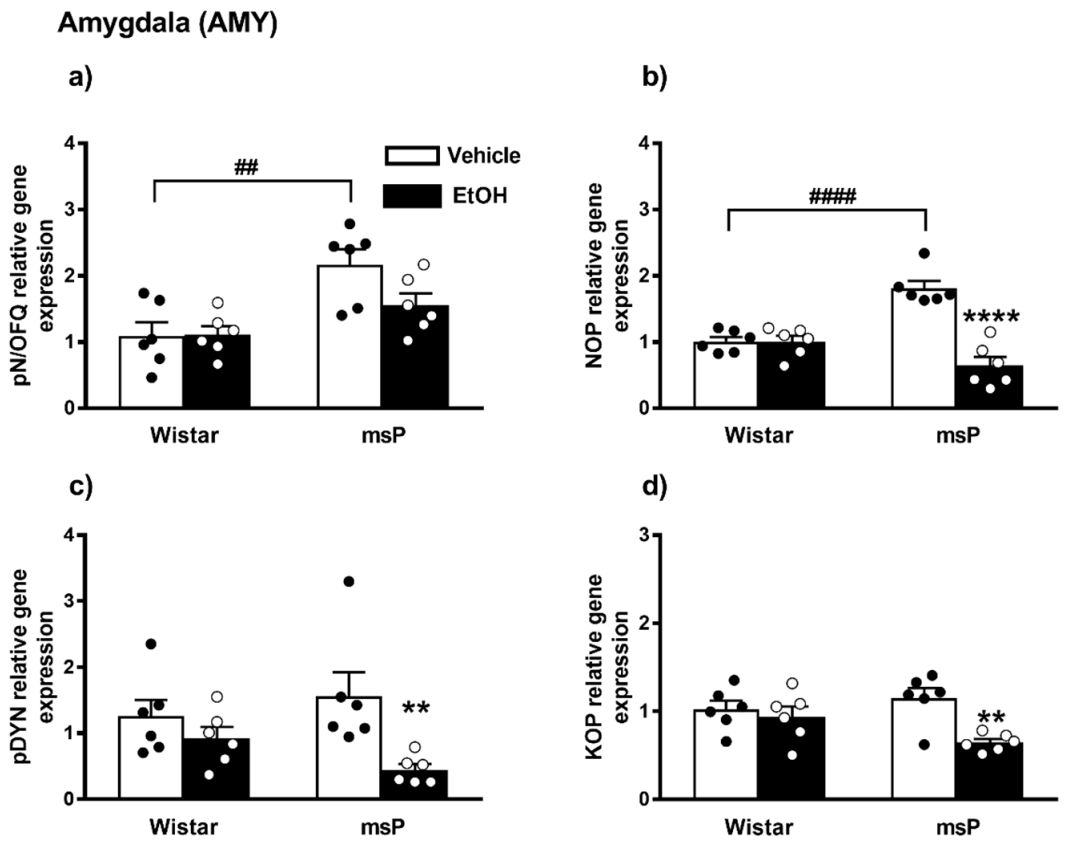
2.2.1. pN/OFQ Expression
2.2.2. NOP Expression
2.2.3. pDYN Expression
2.2.4. KOP Expression
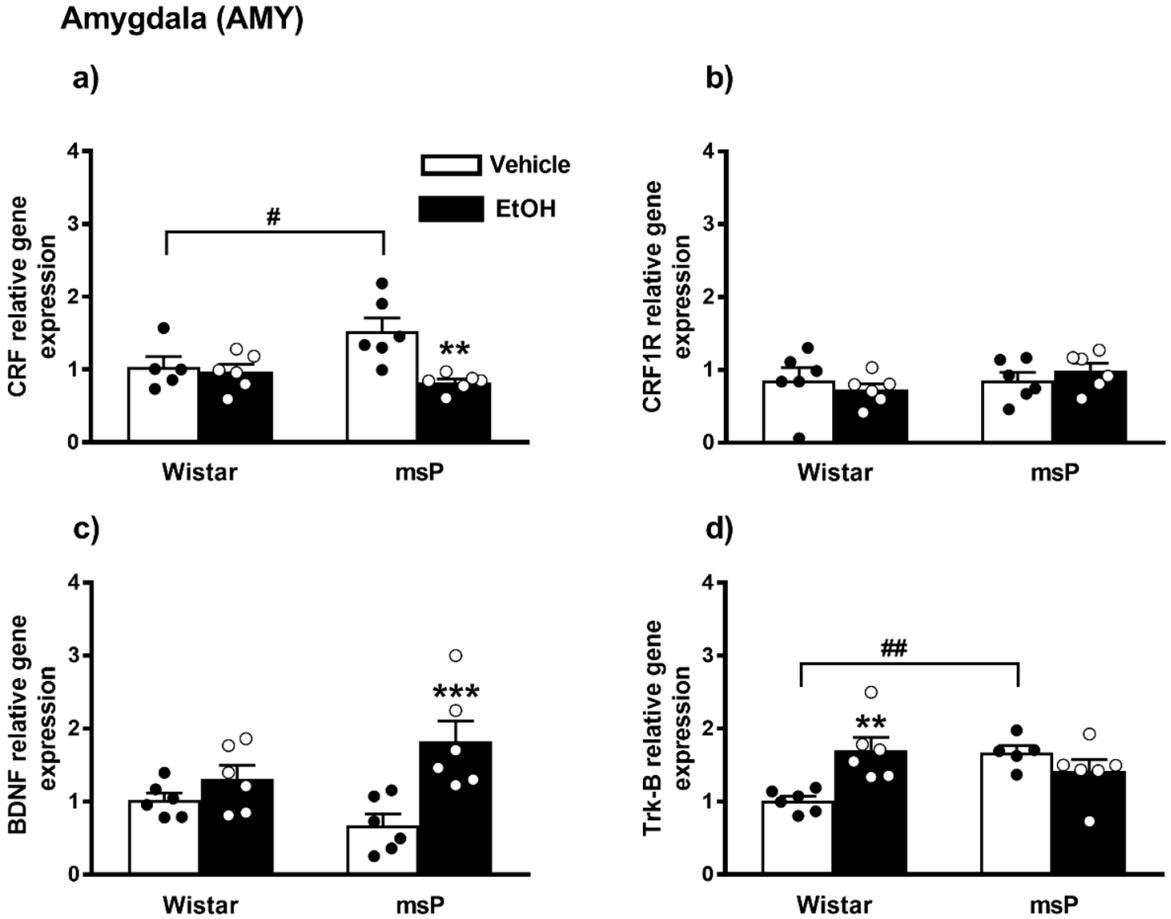
2.2.5. CRF Expression
2.2.6. CRF1R Expression
2.2.7. BDNF Expression
2.2.8. Trk-B Expression
2.3. Gene Expression Analysis in the BNST of Water Controls and 10% EtOH Exposed Wistar and msP Rats
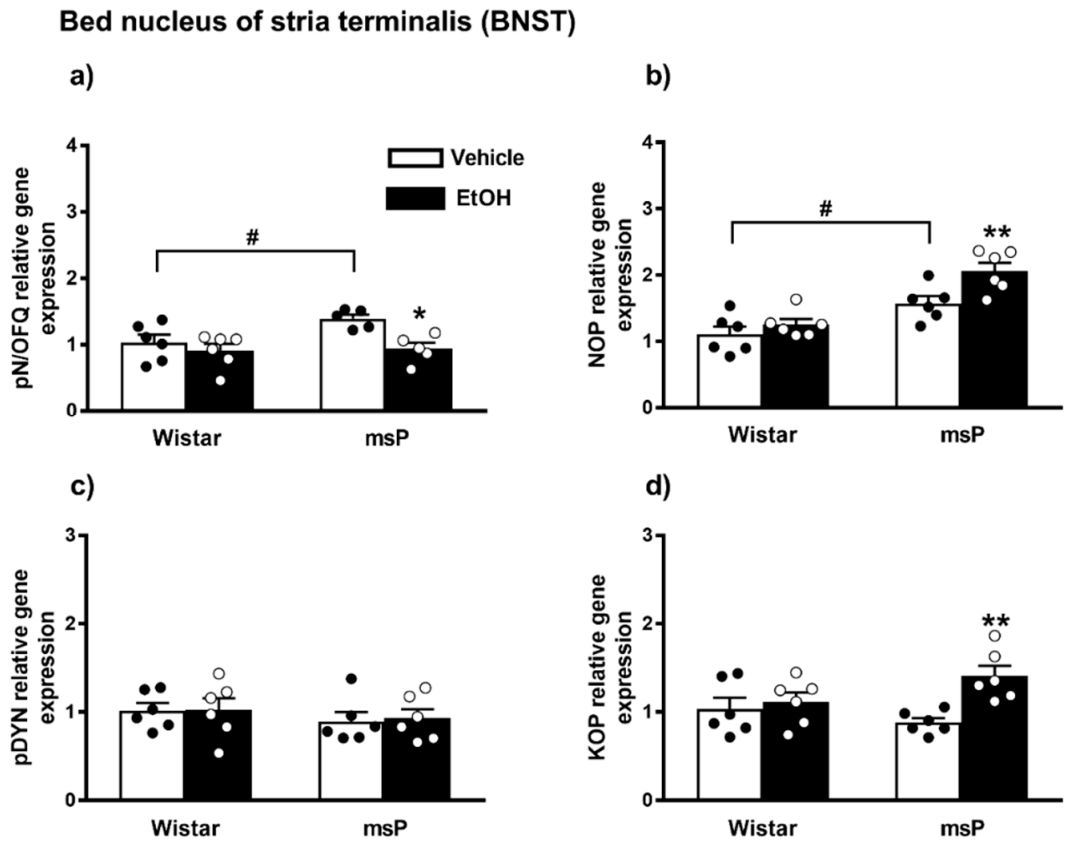
2.3.1. pN/OFQ Expression
2.3.2. NOP Expression
2.3.3. pDYN Expression
2.3.4. KOP Expression
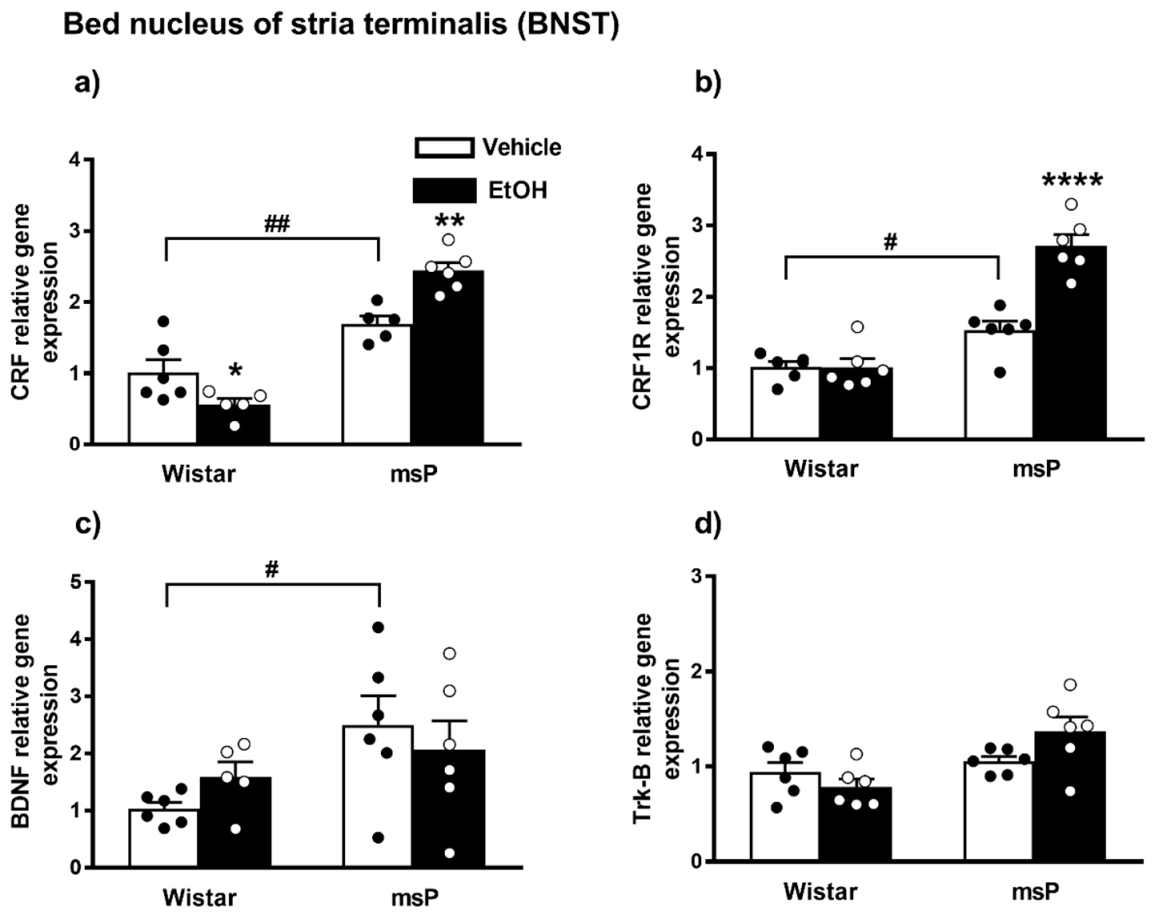
2.3.5. CRF Expression
2.3.6. CRF1R Expression
2.3.7. BDNF Expression
2.3.8. Trk-B Expression
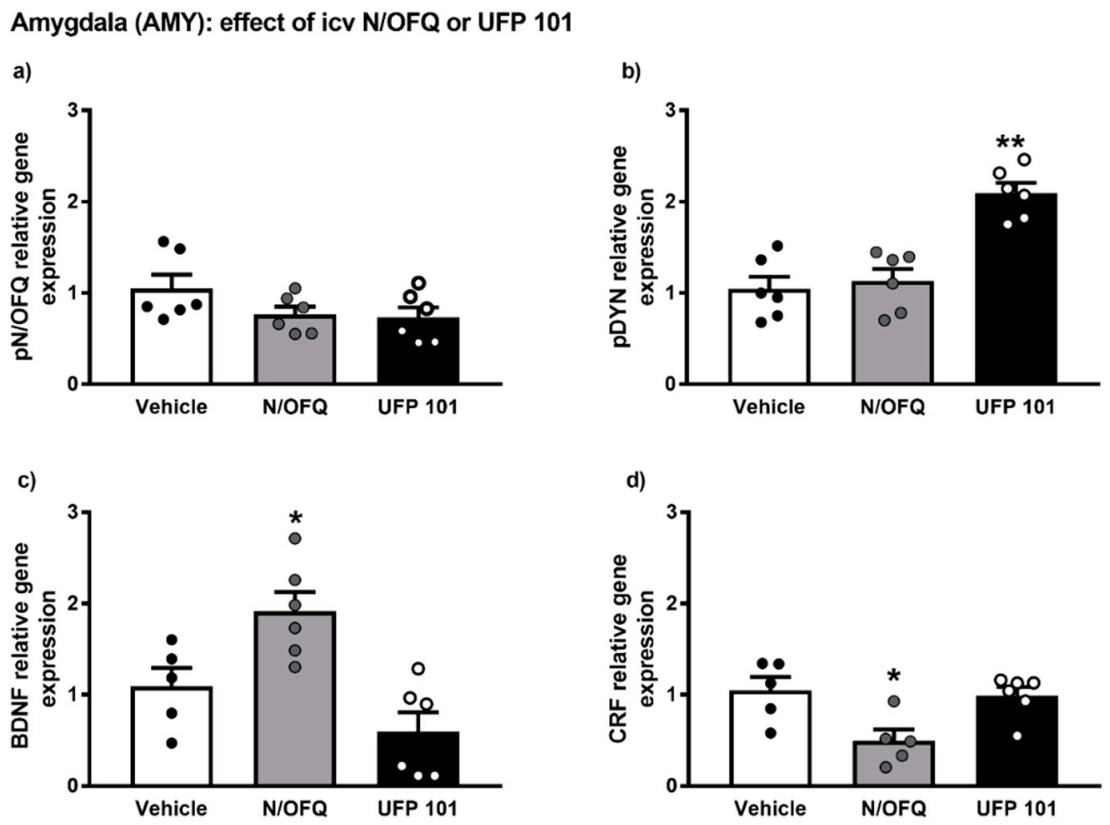
2.4. Effect of Intracerebroventricular Injection of N/OFQ or UFP101 on Neuropeptide Gene Expression in the Amygdala of msP Rats
3. Discussion
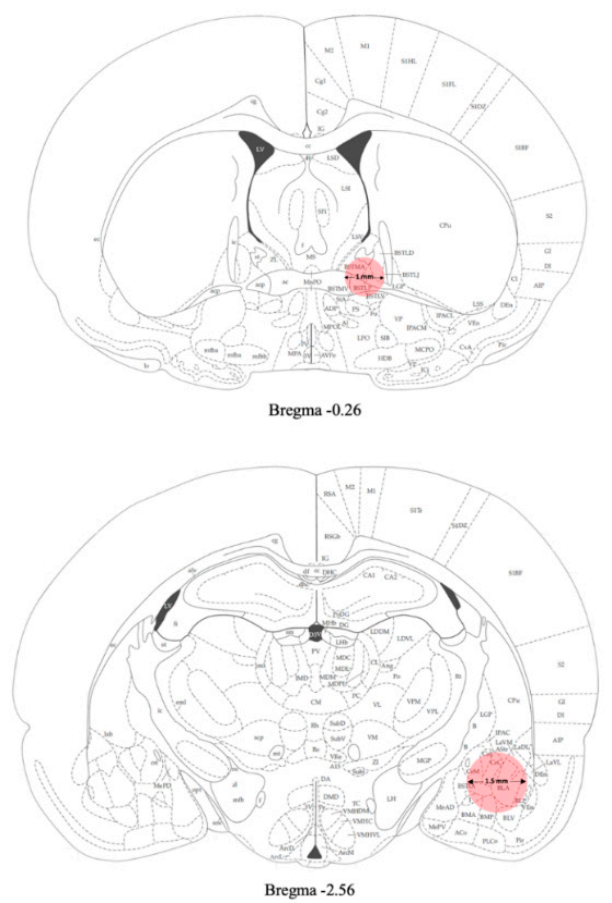
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Surgical Procedures
4.4. Experimental Procedures
4.4.1. Chronic Intermittent EtOH Consumption in a Two Bottle Free-Choice Paradigm
4.4.2. Intracerebroventricular Injection of N/OFQ and UFP-101
4.5. Tissue Collection
4.6. Quantitative Real-Time RT-PCR
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koob, G.F.; Roberts, A.J.; Kieffer, B.L.; Heyser, C.J.; Katner, S.N.; Ciccocioppo, R.; Weiss, F. Animal Models of Motivation for Drinking in Rodents with a Focus on Opioid Receptor Neuropharmacology. Recent Dev. Alcohol. 2002, 16, 263–281. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Panocka, I.; Froldi, R.; Colombo, G.; Gessa, G.L.; Massi, M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology 1999, 144, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Economidou, D.; Cippitelli, A.; Cucculelli, M.; Ubaldi, M.; Soverchia, L.; Lourdusamy, A.; Massi, M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: An animal model to study the neurobiology of alcoholism. Addict. Biol. 2006, 11, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Fox, H.C.; Hong, K.A.; Bergquist, K.; Bhagwagar, Z.; Siedlarz, K.M. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology 2008, 34, 1198–1208. [Google Scholar] [CrossRef]
- Hansson, A.C.; Cippitelli, A.; Sommer, W.H.; Fedeli, A.; Bjork, K.; Soverchia, L.; Terasmaa, A.; Massi, M.; Heilig, M.; Ciccocioppo, R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. USA 2006, 103, 15236–15241. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular mechanisms of drug addiction. J. Neurosci. 1992, 12, 2439–2450. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Koob, G.F. Theoretical frameworks and mechanistic aspects of alcohol addiction: Alcohol addiction as a reward deficit disorder. Curr. Top. Behav. Neurosci. 2013, 13, 3–30. [Google Scholar]
- Spanagel, R.; Noori, H.R.; Heilig, M. Stress and alcohol interactions: Animal studies and clinical significance. Trends Neurosci. 2014, 37, 219–227. [Google Scholar] [CrossRef]
- McCaul, M.E.; Hutton, H.E.; Stephens, M.A.C.; Xu, X.; Wand, G.S. Anxiety, Anxiety Sensitivity, and Perceived Stress as Predictors of Recent Drinking, Alcohol Craving, and Social Stress Response in Heavy Drinkers. Alcohol. Clin. Exp. Res. 2017, 41, 836–845. [Google Scholar] [CrossRef]
- D’Addario, C.; Caputi, F.F.; Ekström, T.J.; Di Benedetto, M.; Maccarrone, M.; Romualdi, P.; Candeletti, S. Ethanol Induces Epigenetic Modulation of Prodynorphin and Pronociceptin Gene Expression in the Rat Amygdala Complex. J. Mol. Neurosci. 2012, 49, 312–319. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, C.; Caputi, F.F.; Rimondini, R.; Gandolfi, O.; Del Borrello, E.; Candeletti, S.; Romualdi, P. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict. Biol. 2011, 18, 425–433. [Google Scholar] [CrossRef]
- Koob, G.F. The dark side of emotion: The addiction perspective. Eur. J. Pharmacol. 2015, 753, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Logrip, M.L.; Barak, S.; Warnault, V.; Ron, D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015, 1628, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; Sánchez-Catalán, M.J.; Hipólito, L.; Rosas, M.; Porru, S.; Bennardini, F.; Romualdi, P.; Caputi, F.F.; Candeletti, S.; Polache, A.; et al. Mystic Acetaldehyde: The Never-Ending Story on Alcoholism. Front. Behav. Neurosci. 2017, 11, 81. [Google Scholar] [CrossRef]
- Ryabinin, A.E.; Giardino, W.J. Contribution of Urocortin to the Development of Excessive Drinking. Int. Rev. Neurobiol. 2017, 136, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Ciccocioppo, R.; Parsons, L.H.; Katner, S.N.; Liu, X.; Zorrilla, E.P.; Valdez, G.R.; Ben-Shahar, O.; Angeletti, S.; Richter, R.R. Compulsive Drug-Seeking Behavior and Relapse. Ann. N. Y. Acad. Sci. 2006, 937, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Caputi, F.F.; Rullo, L.; Stamatakos, S.; Candeletti, S.; Romualdi, P. Modulation of the Negative Affective Dimension of Pain: Focus on Selected Neuropeptidergic System Contributions. Int. J. Mol. Sci. 2019, 20, 4010. [Google Scholar] [CrossRef]
- Caputi, F.F.; Caffino, L.; Candeletti, S.; Fumagalli, F.; Romualdi, P. Short-term withdrawal from repeated exposure to cocaine during adolescence modulates dynorphin mRNA levels and BDNF signaling in the rat nucleus accumbens. Drug Alcohol. Depend. 2019, 197, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.; Grisel, J.; Reinscheid, R.; Civelli, O.; Belknap, J.; Grandy, D. Orphanin FQ is a functional anti-opioid peptide. Neuroscience 1996, 75, 333–337. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Angeletti, S.; Sanna, P.P.; Weiss, F.; Massi, M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000, 404, 153–159. [Google Scholar] [CrossRef]
- Martin-Fardon, R.; Zorrilla, E.P.; Ciccocioppo, R.; Weiss, F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010, 1314, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Statnick, M.A.; Rorick-Kehn, L.M.; Pintar, J.E.; Ansonoff, M.; Chen, Y.; Tucker, R.C.; Ciccocioppo, R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Ther. 2014, 141, 283–299. [Google Scholar] [CrossRef]
- Logrip, M.L.; Janak, P.H.; Ron, D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008, 22, 2393–2404. [Google Scholar] [CrossRef]
- Matsushita, S.; Kimura, M.; Miyakawa, T.; Yoshino, A.; Murayama, M.; Masaki, T.; Higuchi, S. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol. Clin. Exp. Res. 2004, 28, 1609–1612. [Google Scholar] [CrossRef]
- You, C.; Zhang, H.; Sakharkar, A.J.; Teppen, T.; Pandey, S.C. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int. J. Neuropsychopharmacol. 2014, 17, 313–322. [Google Scholar] [CrossRef]
- Caputi, F.F.; Palmisano, M.; D’Addario, C.; Candeletti, S.; Romualdi, P. Effects of acute ethanol exposure on class I HDACs family enzymes in wild-type and BDNF(+/−) mice. Drug Alcohol. Depend. 2015, 155, 68–75. [Google Scholar] [CrossRef]
- Pandey, S.C.; Roy, A.; Zhang, H.; Xu, T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J. Neurosci. 2004, 24, 5022–5030. [Google Scholar] [CrossRef]
- Schoenbaum, G.; Stalnaker, T.A.; Shaham, Y. A role for BDNF in cocaine reward and relapse. Nat. Neurosci. 2007, 10, 935–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Rubio, F.J.; Zeric, T.; Bossert, J.M.; Kambhampati, S.; Cates, H.M.; Kennedy, P.J.; Liu, Q.-R.; Cimbro, R.; Hope, B.T.; et al. Incubation of Methamphetamine Craving Is Associated with Selective Increases in Expression of Bdnf and Trkb, Glutamate Receptors, and Epigenetic Enzymes in Cue-Activated Fos-Expressing Dorsal Striatal Neurons. J. Neurosci. 2015, 35, 8232–8244. [Google Scholar] [CrossRef]
- Barker, J.M.; Taylor, J.R.; De Vries, T.J.; Peters, J. Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res. 2015, 1628, 68–81. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef]
- Carnicella, S.; Ron, D.; Barak, S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 2014, 48, 243–252. [Google Scholar] [CrossRef]
- Delker, E.; Brown, Q.; Hasin, D.S. Alcohol Consumption in Demographic Subpopulations: An Epidemiologic Overview. Alcohol Res. 2016, 38, 7–15. [Google Scholar]
- Kranzler, H.R.; Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R.; Emson, P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods 1980, 3, 129–149. [Google Scholar] [CrossRef]
- Economidou, D.; Hansson, A.C.; Weiss, F.; Terasmaa, A.; Sommer, W.H.; Cippitelli, A.; Fedeli, A.; Martin-Fardon, R.; Massi, M.; Ciccocioppo, R.; et al. Dysregulation of Nociceptin/Orphanin FQ Activity in the Amygdala Is Linked to Excessive Alcohol Drinking in the Rat. Biol. Psychiatry 2008, 64, 211–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansson, A.C.; Cippitelli, A.; Sommer, W.H.; Ciccocioppo, R.; Heilig, M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict. Biol. 2007, 12, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cippitelli, A.; Ayanwuyi, L.O.; Barbier, E.; Domi, E.; Lerma-Cabrera, J.M.; Carvajal, F.; Scuppa, G.; Li, H.; Ubaldi, M.; Heilig, M.; et al. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology 2015, 232, 1083–1093. [Google Scholar] [CrossRef]
- Pandey, S.C. A Critical Role of Brain-Derived Neurotrophic Factor in Alcohol Consumption. Biol. Psychiatry 2016, 79, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Jeanblanc, J.; He, D.-Y.; Carnicella, S.; Kharazia, V.; Janak, P.H.; Ron, D. Endogenous BDNF in the Dorsolateral Striatum Gates Alcohol Drinking. J. Neurosci. 2009, 29, 13494–13502. [Google Scholar] [CrossRef]
- Moonat, S.; Sakharkar, A.J.; Zhang, H.; Pandey, S.C. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict. Biol. 2010, 16, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hensler, J.G.; Ladenheim, E.E.; Lyons, W.E. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/-) mice. J. Neurochem. 2003, 85, 1139–1147. [Google Scholar] [CrossRef]
- McGough, N.N.H.; He, D.-Y.; Logrip, M.L.; Jeanblanc, J.; Phamluong, K.; Luong, K.; Kharazia, V.; Janak, P.H.; Ron, R. RACK1 and Brain-Derived Neurotrophic Factor: A Homeostatic Pathway That Regulates Alcohol Addiction. J. Neurosci. 2004, 24, 10542–10552. [Google Scholar] [CrossRef]
- Prakash, A.; Zhang, H.; Pandey, S.C. Innate Differences in the Expression of Brain-Derived Neurotrophic Factor in the Regions Within the Extended Amygdala Between Alcohol Preferring and Nonpreferring Rats. Alcohol. Clin. Exp. Res. 2008, 32, 909–920. [Google Scholar] [CrossRef]
- Marco, E.M.; Peñasco, S.; Hernández, M.-D.; Gil, A.; Borcel, E.; Moya, M.; Giné, E.; López-Moreno, J.A.; Guerri, C.; López-Gallardo, M.; et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Front. Behav. Neurosci. 2017, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Augier, E.; Flanigan, M.; Dulman, R.S.; Pincus, A.; Schank, J.R.; Rice, K.C.; Kejun, C.; Heilig, M.; Tapocik, J.D. Wistar rats acquire and maintain self-administration of 20 % ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology 2014, 231, 4561–4568. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Cippitelli, A.; Economidou, D.; Fedeli, A.; Massi, M. Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol. Behav. 2004, 82, 63–68. [Google Scholar] [CrossRef]
- Rodi, D.; Zucchini, S.; Simonato, M.; Cifani, C.; Massi, M.; Polidori, C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: Evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology 2007, 196, 523–531. [Google Scholar] [CrossRef]
- Scott, H.; Tjernström, N.; Roman, E. Effects of pair housing on voluntary alcohol intake in male and female Wistar rats. Alcohol 2020, 86, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Heimer, L.; Alheid, G.F. Piecing together the Puzzle of Basal Forebrain Anatomy. Adv. Exp. Med. Biol. 1991, 295, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 2009, 56, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Millan, E.Z.; Reese, R.M.; Grossman, C.D.; Chaudhri, N.; Janak, P.H. Nucleus Accumbens and Posterior Amygdala Mediate Cue-Triggered Alcohol Seeking and Suppress Behavior During the Omission of Alcohol-Predictive Cues. Neuropsychopharmacology 2015, 40, 2555–2565. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Panocka, I.; Polidori, C.; Regoli, M.; Massi, M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology 1999, 141, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.M.; Brothers, S.; Sartor, G.; Holm, L.; Heilig, M.; Wahlestedt, C.; Thorsell, A. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: Validation in rat models. Psychopharmacology 2016, 233, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.; Sandin, J.; Terenius, L.; Ögren, S.O. Acquisition, Expression, and Reinstatement of Ethanol-Induced Conditioned Place Preference in Mice: Effects of Opioid Receptor-Like 1 Receptor Agonists and Naloxone. J. Pharmacol. Exp. Ther. 2003, 304, 310–318. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Economidou, D.; Fedeli, A.; Angeletti, S.; Weiss, F.; Heilig, M.; Massi, M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology 2003, 172, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; De Guglielmo, G.; Hansson, A.C.; Ubaldi, M.; Kallupi, M.; Cruz, M.T.; Oleata, C.S.; Heilig, M.; Roberto, M. Restraint Stress Alters Nociceptin/Orphanin FQ and CRF Systems in the Rat Central Amygdala: Significance for Anxiety-Like Behaviors. J. Neurosci. 2014, 34, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Jenck, F.; Moreau, J.-L.; Martin, J.R.; Kilpatrick, G.J.; Reinscheid, R.K.; Monsma, F.J.; Nothacker, H.-P.; Civelli, O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. USA 1997, 94, 14854–14858. [Google Scholar] [CrossRef]
- Filaferro, M.; Ruggieri, V.; Novi, C.; Calò, G.; Cifani, C.; Di Bonaventura, M.M.; Sandrini, M.; Vitale, G. Functional antagonism between nociceptin/orphanin FQ and corticotropin-releasing factor in rat anxiety-related behaviors: Involvement of the serotonergic system. Neuropeptides 2014, 48, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Arletti, R.; Ruggieri, V.; Cifani, C.; Massi, M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides 2006, 27, 2193–2200. [Google Scholar] [CrossRef]
- Aujla, H.; Cannarsa, R.; Romualdi, P.; Ciccocioppo, R.; Martin-Fardon, R.; Weiss, F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict. Biol. 2013, 18, 467–479. [Google Scholar] [CrossRef]
- Borruto, A.M.; Fotio, Y.; Stopponi, S.; Brunori, G.; Petrella, M.; Caputi, F.F.; Romualdi, P.; Candeletti, S.; Narendran, R.; Rorick-Kehn, L.M.; et al. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br. J. Pharmacol. 2020, 177, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Brunori, G.; Weger, M.; Schoch, J.; Targowska-Duda, K.; Barnes, M.; Borruto, A.M.; Rorick-Kehn, L.M.; Zaveri, N.T.; Pintar, J.E.; Ciccocioppo, R.; et al. NOP Receptor Antagonists Decrease Alcohol Drinking in the Dark in C57BL/6J Mice. Alcohol. Clin. Exp. Res. 2019, 43, 2167–2178. [Google Scholar] [CrossRef]
- Silva, A.I.; Holanda, V.A.; Neto, J.G.A.; Junior, E.D.S.; Soares-Rachetti, V.P.; Calo, G.; Ruzza, C.; Gavioli, E.C. Blockade of NOP receptor modulates anxiety-related behaviors in mice exposed to inescapable stress. Psychopharmacology 2020, 237, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.E.; Rainnie, D.G. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology 2016, 41, 103–125. [Google Scholar] [CrossRef]
- Dabrowska, J.; Hazra, R.; Guo, J.D.; Dewitt, S.; Rainnie, D.G. Central CRF neurons are not created equal: Phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front. Neurosci. 2013, 7, 156. [Google Scholar] [CrossRef]
- Economidou, D.; Cippitelli, A.; Stopponi, S.; Braconi, S.; Clementi, S.; Ubaldi, M.; Martin-Fardon, R.; Weiss, F.; Massi, M.; Ciccocioppo, R. Activation of Brain NOP Receptors Attenuates Acute and Protracted Alcohol Withdrawal Symptoms in the Rat. Alcohol. Clin. Exp. Res. 2011, 35, 747–755. [Google Scholar] [CrossRef][Green Version]
- Ciccocioppo, R.; Economidou, D.; Rimondini, R.; Sommer, W.; Massi, M.; Heilig, M. Buprenorphine Reduces Alcohol Drinking Through Activation of the Nociceptin/Orphanin FQ-NOP Receptor System. Biol. Psychiatry 2007, 61, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Calo, G.; Rizzi, A.; Rizzi, D.; Bigoni, R.; Guerrini, R.; Marzola, G.; Marti, M.; McDonald, J.; Morari, M.; Lambert, D.G.; et al. [Nphe1,Arg14,Lys15 ]Nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002, 136, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bregola, G.; Candeletti, S.; Romualdi, P.; Simonato, M. Limbic seizures increase pronociceptin mRNA levels in the thalamic reticular nucleus. NeuroReport 1999, 10, 541–546. [Google Scholar] [CrossRef]
- Vermehren-Schmaedick, A.; Khanjian, R.A.; Balkowiec, A. Cellular mechanisms of activity-dependent BDNF expression in primary sensory neurons. Neuroscience 2015, 310, 665–673. [Google Scholar] [CrossRef]
- Laflamme, N.; Barden, N.; Rivest, S. Corticotropin-releasing factor and glucocorticoid receptor (GR) gene expression in the paraventricular nucleus of immune-challenged transgenic mice expressing type II GR antisense ribonucleic acid. J. Mol. Neurosci. 1997, 8, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lyon, E. Mutation detection using fluorescent hybridization probes and melting curve analysis. Expert Rev. Mol. Diagn. 2001, 1, 92–101. [Google Scholar] [CrossRef]
| Brain Area | Genotype/Treatment | Gene | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pN/OFQ | NOP | pDYN | KOP | CRF | CRFR1 | BDNF | TrkB | ||
| AMY | Wistar EtOH vs. Wistar vehicle | = | = | = | = | = | = | = | ↑ |
| msP vehicle vs. Wistar vehicle | ↑ | ↑ | = | = | ↑ | = | = | ↑ | |
| msP EtOH vs. msP vehicle | ↓ | ↓ | ↓ | ↓ | ↓ | = | ↑ | = | |
| BNST | Wistar EtOH vs. Wistar vehicle | = | = | = | = | ↓ | = | = | = |
| msP vehicle vs. Wistar vehicle | ↑ | ↑ | = | = | ↑ | ↑ | ↑ | = | |
| msP EtOH vs. msP vehicle | ↓ | ↑ | = | ↑ | ↑ | ↑ | = | = | |
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| pN/OFQ | TGCAGCACCTGAAGA GAATG | CAACTTCCGGGCTGACTTC |
| NOP | AGCTTCTGAAGAGGCTGTGT | GACCTCCCAGTATGGAGCAG |
| CRF | GCAGCGGGACTTCTGTTGA | CGCAGCCGTTGAATTTCTTG |
| Pdyn | CCTGTCCTTGTGTTCCCTGT | AGAGGCAGTCAGGGTGAGAA |
| KOP | TTGGCTACTGGCATCATCTG | ACACTCTTCAAGCGCAGGAT |
| CRF1R | TGCCAGGAGATTCTCAACGAA | AAAGCCGAGATGAGGTTCCAG |
| BDNF | AAGTCTGCATTACATTCCTCGA | GTTTTCTGAAAGAGGGACAGTTTAT |
| TrkB | AAGTTCTACGGTGTCTGTGTG | TTCTCTCCTACCAAGCAGTTC |
| GAPDH | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputi, F.F.; Stopponi, S.; Rullo, L.; Palmisano, M.; Ubaldi, M.; Candeletti, S.; Ciccocioppo, R.; Romualdi, P. Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats. Int. J. Mol. Sci. 2021, 22, 2448. https://doi.org/10.3390/ijms22052448
Caputi FF, Stopponi S, Rullo L, Palmisano M, Ubaldi M, Candeletti S, Ciccocioppo R, Romualdi P. Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats. International Journal of Molecular Sciences. 2021; 22(5):2448. https://doi.org/10.3390/ijms22052448
Chicago/Turabian StyleCaputi, Francesca Felicia, Serena Stopponi, Laura Rullo, Martina Palmisano, Massimo Ubaldi, Sanzio Candeletti, Roberto Ciccocioppo, and Patrizia Romualdi. 2021. "Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats" International Journal of Molecular Sciences 22, no. 5: 2448. https://doi.org/10.3390/ijms22052448
APA StyleCaputi, F. F., Stopponi, S., Rullo, L., Palmisano, M., Ubaldi, M., Candeletti, S., Ciccocioppo, R., & Romualdi, P. (2021). Dysregulation of Nociceptin/Orphanin FQ and Dynorphin Systems in the Extended Amygdala of Alcohol Preferring Marchigian Sardinian (msP) Rats. International Journal of Molecular Sciences, 22(5), 2448. https://doi.org/10.3390/ijms22052448






