Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role?
Abstract
1. Introduction
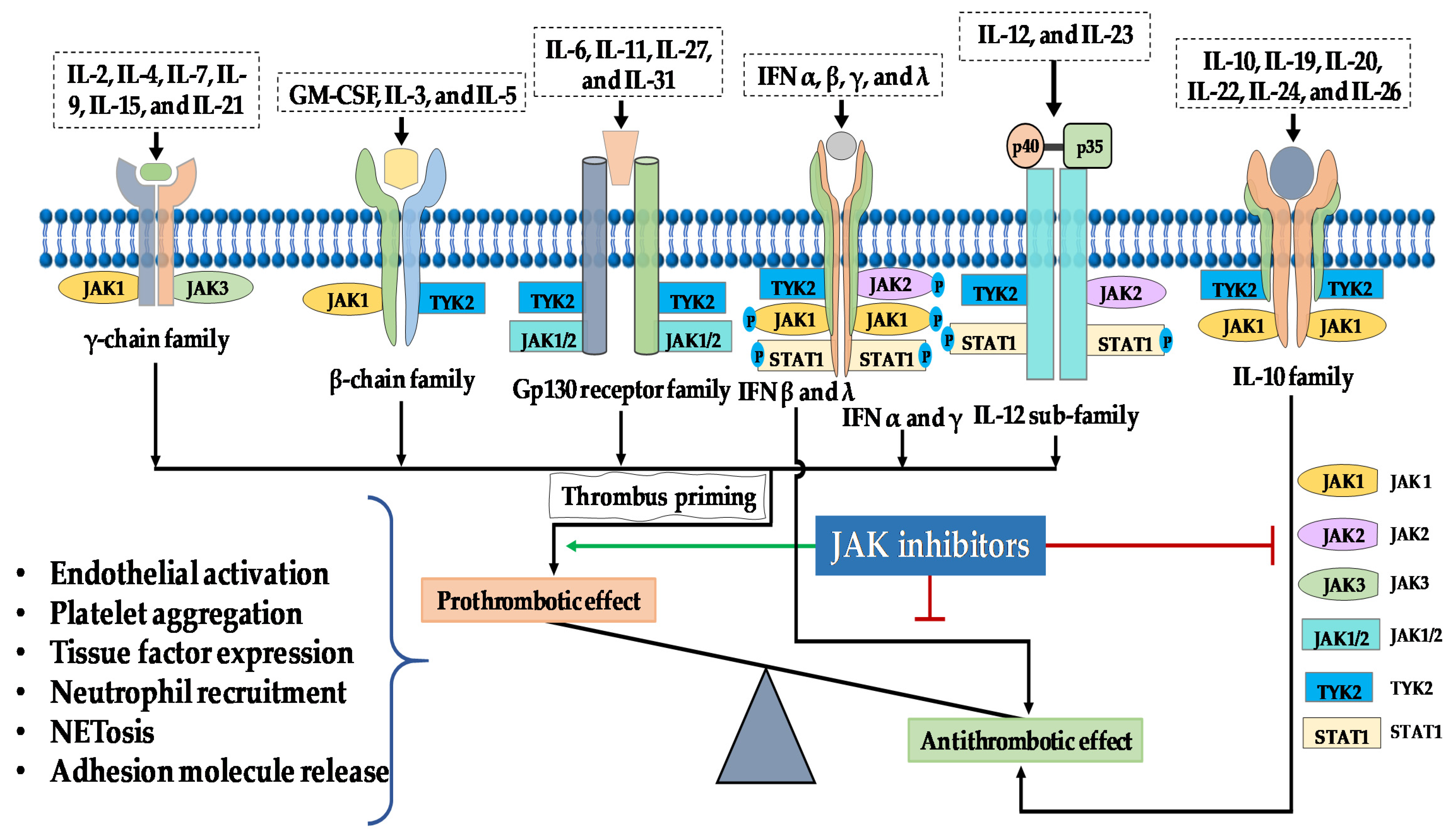
2. JAK/STAT Pathway
3. Thromboembolic Mechanisms
4. The Role of the JAK/STAT Pathway in Thrombus Formation
JAK/STAT and Platelet Function
5. The Role of Cytokines in Thrombus Formation
5.1. Interferons
5.2. Interleukin-6
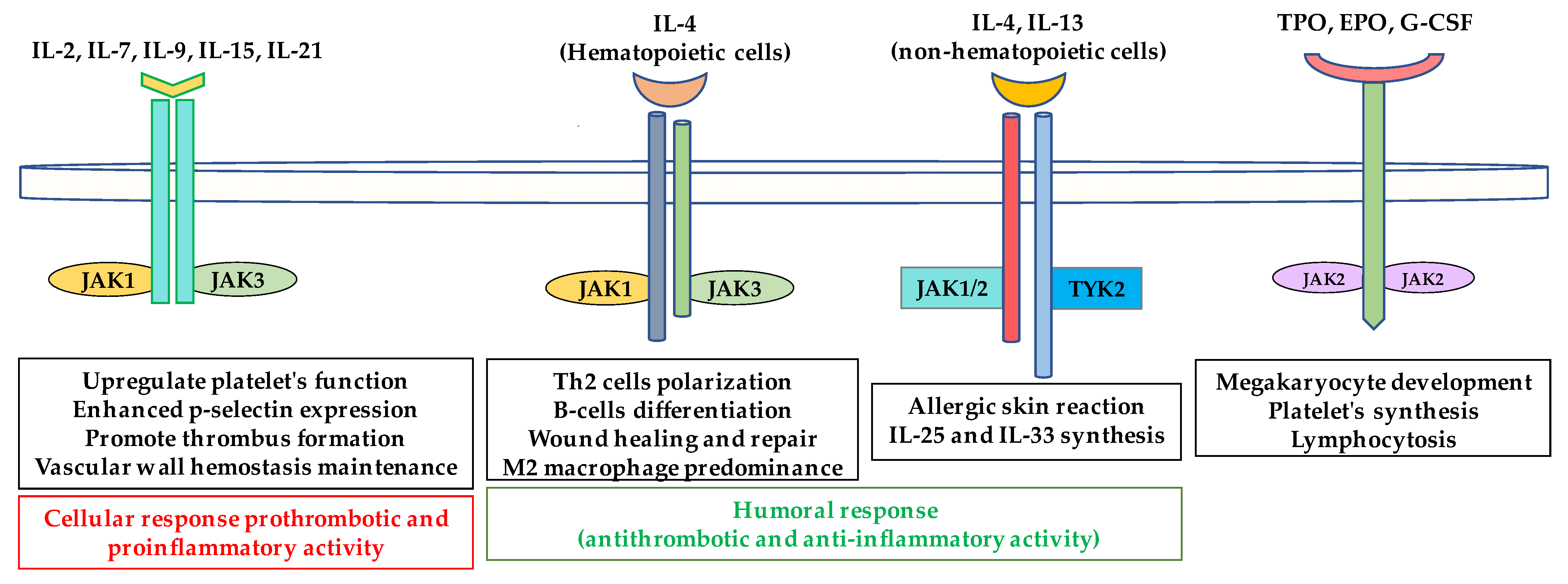
5.3. Interleukin-9
5.4. Interleukin-12/23
5.5. Interleukin-10
6. Thromboembolic Mechanisms of Jakinibs
7. Clinical Data on Thromboembolic Complications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| DMARDs | Disease-modifying antirheumatic drugs |
| JAK | Janus kinases |
| STAT | Signal transducers and activators of transcription |
| PE | Pulmonary embolism |
| DVT | Deep vein thrombosis |
| IFN | Interferon |
| IL | Interleukin |
| JH | JAK homology |
| SOCS | Suppressor of cytokine signaling |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| MACE | Major adverse cardiovascular events |
| VTE | Venous thromboembolic event |
References
- Macy, E. Immune-related adverse drug reactions and immunologically mediated drug hypersensitivity. Immunol. Allergy Clin. N. Am. 2020, 40, 635–647. [Google Scholar] [CrossRef]
- Kotyla, P.J. Bimodal function of anti-tnf treatment: Shall we be concerned about anti-tnf treatment in patients with rheumatoid arthritis and heart failure? Int. J. Mol. Sci. 2018, 19, 1739. [Google Scholar] [CrossRef]
- Kotyla, P.J.; Sliwinska-Kotyla, B.; Kucharz, E.J. Treatment with infliximab may contribute to the development of peripheral neuropathy among the patients with rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 1595–1596. [Google Scholar] [CrossRef]
- Kotyla, P.; Jankiewicz-Ziobro, K.; Owczarek, A.; Kucharz, E.J. Etanercept increases tumor necrosis factor-alpha level but not sfas level in patients with rheumatoid arthritis. Isr. Med. Assoc. J. 2015, 17, 14–18. [Google Scholar] [PubMed]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Dal Cin, L.; Brancati, S.; Scaglione, F. Jak-inhibitors for the treatment of rheumatoid arthritis: A focus on the present and an outlook on the future. Biomolecules 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Jamilloux, Y.; El Jammal, T.; Vuitton, L.; Gerfaud-Valentin, M.; Kerever, S.; Sève, P. Jak inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019, 18, 102390. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S. Jak-stat inhibitors: Immersing therapeutic approach for management of rheumatoid arthritis. Int. Immunopharmacol. 2020, 86, 106731. [Google Scholar] [CrossRef]
- Xu, P.; Shen, P.; Yu, B.; Xu, X.; Ge, R.; Cheng, X.; Chen, Q.; Bian, J.; Li, Z.; Wang, J. Janus kinases (jaks): The efficient therapeutic targets for autoimmune diseases and myeloproliferative disorders. Eur. J. Med. Chem. 2020, 192, 112155. [Google Scholar] [CrossRef]
- El Jammal, T.; Gerfaud-Valentin, M.; Sève, P.; Jamilloux, Y. Inhibition of jak/stat signaling in rheumatologic disorders: The expanding spectrum. Jt. Bone Spine 2020, 87, 119–129. [Google Scholar] [CrossRef]
- Kotyla, P.J. Are janus kinase inhibitors superior over classic biologic agents in ra patients? Biomed Res. Int. 2018, 2018, 7492904. [Google Scholar] [CrossRef]
- Kotyla, P.J.; Islam, M.A.; Engelmann, M. Clinical aspects of janus kinase (jak) inhibitors in the cardiovascular system in patients with rheumatoid arthritis. Int. J. Mol. Sci. 2020, 21, 7390. [Google Scholar] [CrossRef]
- Mease, P.; Charles-Schoeman, C.; Cohen, S.; Fallon, L.; Woolcott, J.; Yun, H.; Kremer, J.; Greenberg, J.; Malley, W.; Onofrei, A.; et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann. Rheum. Dis. 2020, 79, 1400–1413. [Google Scholar] [CrossRef]
- Yates, M.; Mootoo, A.; Adas, M.; Bechman, K.; Rampes, S.; Patel, V.; Qureshi, S.; Cope, A.P.; Norton, S.; Galloway, J.B. Venous thromboembolism risk with jak inhibitors: A meta-analysis. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Ibrahim, F.; Scott, D.L. Thromboembolism and janus kinase inhibitors. Drug Saf. 2020, 43, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Hammarén, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of jaks in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. Jaks and stats in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of jak–stat signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Kawamura, M.; McVicar, D.W.; Johnston, J.A.; Blake, T.B.; Chen, Y.Q.; Lal, B.K.; Lloyd, A.R.; Kelvin, D.J.; Staples, J.E.; Ortaldo, J.R.; et al. Molecular cloning of l-jak, a janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 6374–6378. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. Jak–stat signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Levy, D.E. Comparative evolutionary genomics of the stat family of transcription factors. Jak Stat 2012, 1, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zouein, F.A.; Duhé, R.J.; Booz, G.W. Jaks go nuclear: Emerging role of nuclear jak1 and jak2 in gene expression and cell growth. Growth Factors 2011, 29, 245–252. [Google Scholar] [CrossRef]
- Boulay, J.-L.; O’Shea, J.J.; Paul, W.E. Molecular phylogeny within type i cytokines and their cognate receptors. Immunity 2003, 19, 159–163. [Google Scholar] [CrossRef]
- Liongue, C.; Taznin, T.; Ward, A.C. Signaling via the cytor/jak/stat/socs pathway: Emergence during evolution. Mol. Immunol. 2016, 71, 166–175. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type i/ii cytokines, jaks, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc. Natl. Acad. Sci. USA 1990, 87, 6934–6938. [Google Scholar] [CrossRef]
- Waickman, A.T.; Park, J.-Y.; Park, J.-H. The common γ-chain cytokine receptor: Tricks-and-treats for t cells. Cell. Mol. Life Sci. 2016, 73, 253–269. [Google Scholar] [CrossRef]
- Nicola, N.A.; Babon, J.J. Leukemia inhibitory factor (lif). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef]
- Boulanger, M.J.; Bankovich, A.J.; Kortemme, T.; Baker, D.; Garcia, K.C. Convergent mechanisms for recognition of divergent cytokines by the shared signaling receptor gp130. Mol. Cell 2003, 12, 577–589. [Google Scholar] [CrossRef]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and specificity: Insights from the interleukin 6 family of cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The jak-stat pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Esch, A.; Masiarz, A.; Mossner, S.; Moll, J.M.; Grötzinger, J.; Schröder, J.; Scheller, J.; Floss, D.M. Deciphering site 3 interactions of interleukin 12 and interleukin 23 with their cognate murine and human receptors. J. Biol. Chem. 2020, 295, 10478–10492. [Google Scholar] [CrossRef]
- Reddy, V.; Cohen, S. Jak inhibitors: What is new? Curr. Rheumatol. Rep. 2020, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Gadina, M.; Johnson, C.; Schwartz, D.; Bonelli, M.; Hasni, S.; Kanno, Y.; Changelian, P.; Laurence, A.; O’Shea, J.J. Translational and clinical advances in jak-stat biology: The present and future of jakinibs. J. Leukoc. Biol. 2018, 104, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Tokumasa, N.; Suto, A.; Kagami, S.-I.; Furuta, S.; Hirose, K.; Watanabe, N.; Saito, Y.; Shimoda, K.; Iwamoto, I.; Nakajima, H. Expression of tyk2 in dendritic cells is required for il-12, il-23, and ifn-γ production and the induction of th1 cell differentiation. Blood 2007, 110, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Chopard, R.; Albertsen, I.E.; Piazza, G. Diagnosis and treatment of lower extremity venous thromboembolism: A review. JAMA 2020, 324, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A. The epidemiology of venous thromboembolism in the community. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 370–372. [Google Scholar] [CrossRef]
- Liederman, Z.; Chan, N.; Bhagirath, V. Current challenges in diagnosis of venous thromboembolism. J. Clin. Med. 2020, 9, 3509. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Risk factors for venous thromboembolism. J. Am. Coll. Cardiol. 2010, 56, 1–7. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Sasongko, T.H.; Gan, S.H. Antiphospholipid antibody-mediated thrombotic mechanisms in antiphospholipid syndrome: Towards pathophysiology-based treatment. Curr. Pharm. Des. 2016, 22, 4451–4469. [Google Scholar] [CrossRef]
- Islam, M.A. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Semin. Cancer Biol. 2020, 64, 108–113. [Google Scholar] [CrossRef]
- Bovill, E.G.; van der Vliet, A. Venous valvular stasis–associated hypoxia and thrombosis: What is the link? Annu. Rev. Physiol. 2011, 73, 527–545. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Wong, K.K.; Kamal, M.A.; Gan, S.H. Thrombotic management of antiphospholipid syndrome: Towards novel targeted therapies. Curr. Vasc. Pharm. 2017, 15, 313–326. [Google Scholar] [CrossRef]
- Budnik, I.; Brill, A. Immune factors in deep vein thrombosis initiation. Trends Immunol. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- Campos, J.; Brill, A. The role of bone marrow-derived cells in venous thromboembolism. Int. J. Biochem. Cell Biol. 2020, 128, 105850. [Google Scholar] [CrossRef]
- Pober, J.S.; Tellides, G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012, 33, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.S.; Branch, D.W.; Rauch, J. The antiphospholipid syndrome. N. Engl. J. Med. 2002, 346, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, F.; Kamal, M.A.; Gan, S.H. Genetic risk factors in thrombotic primary antiphospholipid syndrome: A systematic review with bioinformatic analyses. Autoimmun. Rev. 2018, 17, 226–243. [Google Scholar] [CrossRef] [PubMed]
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 107, 4–8. [Google Scholar] [CrossRef]
- Arruda, V.R.; von Zuben, P.M.; Chiaparini, L.C.; Annichino-Bizzacchi, J.M.; Costa, F.F. The mutation ala677 → Val in the methylene tetrahydrofolate reductase gene: A risk factor for arterial disease and venous thrombosis. Thromb. Haemost. 1997, 77, 818–821. [Google Scholar] [CrossRef]
- Albagoush, S.A.; Chakraborty, R.K.; Schmidt, A.E. Factor v leiden deficiency. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kropf, J.; Cheyney, S.; Vachon, J.; Flaherty, P.; Vo, M.; Carlan, S.J. Extensive catastrophic thromboses from elevation of factor viii. Clin. Pract. 2020, 10, 59–61. [Google Scholar] [CrossRef]
- Michels, A.; Dwyer, C.N.; Mewburn, J.; Nesbitt, K.; Kawecki, C.; Lenting, P.; Swystun, L.L.; Lillicrap, D. Vwf (von willebrand factor) is a critical mediator of deep vein thrombosis in a mouse model of diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Mackie, I.; Nallamilli, S.; Pires, T.; Moll, R.; Pericleous, C.; Isenberg, D.A.; Cohen, H.; Efthymiou, M. Anti-protein c antibodies and acquired protein c resistance in sle: Novel markers for thromboembolic events and disease activity? Rheumatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Senst, B.; Tadi, P.; Goyal, A.; Jan, A. Hypercoagulability. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lichtman, M.A. Clonal hematopoiesis: A “chip” off the old block. Blood 2015, 126, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Perner, F.; Perner, C.; Ernst, T.; Heidel, F.H. Roles of jak2 in aging, inflammation, hematopoiesis and malignant transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Mead, A.J.; Rugless, M.J.; Jacobsen, S.E.W.; Schuh, A. Germline jak2 mutation in a family with hereditary thrombocytosis. N. Engl. J. Med. 2012, 366, 967–969. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef]
- Roumen-Klappe, E.M.; den Heijer, M.; van Uum, S.H.; van der Ven-Jongekrijg, J.; van der Graaf, F.; Wollersheim, H. Inflammatory response in the acute phase of deep vein thrombosis. J. Vasc. Surg. 2002, 35, 701–706. [Google Scholar] [CrossRef]
- Shbaklo, H.; Holcroft, C.A.; Kahn, S.R. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb. Haemost. 2009, 101, 505–512. [Google Scholar] [CrossRef]
- Gupta, N.; Sahu, A.; Prabhakar, A.; Chatterjee, T.; Tyagi, T.; Kumari, B.; Khan, N.; Nair, V.; Bajaj, N.; Sharma, M. Activation of nlrp3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. USA 2017, 114, 4763–4768. [Google Scholar] [CrossRef]
- Oikonomou, E.; Leopoulou, M.; Theofilis, P.; Antonopoulos, A.S.; Siasos, G.; Latsios, G.; Mystakidi, V.C.; Antoniades, C.; Tousoulis, D. A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: Clinical and therapeutic implications. Atherosclerosis 2020, 309, 16–26. [Google Scholar] [CrossRef]
- Ashorobi, D.; Ameer, M.A.; Fernandez, R. Thrombosis. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Massberg, S. Blood coagulation in immunothrombosis-at the frontline of intravascular immunity. Semin. Immunol. 2016, 28, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Najem, M.Y.; Couturaud, F.; Lemarié, C.A. Cytokine and chemokine regulation of venous thromboembolism. J. Thromb. Haemost. 2020, 18, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; Peterson, D.; Ramseier, J.; Al-Bawardy, B.; Chun, H.; Proctor, D.; Strand, V.; Flavell, R.A.; King, B. The emerging role of janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- You, H.; Xu, D.; Zhao, J.; Li, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Jak inhibitors: Prospects in connective tissue diseases. Clin. Rev. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Clere-Jehl, R.; Mariotte, A.; Meziani, F.; Bahram, S.; Georgel, P.; Helms, J. Jak-stat targeting offers novel therapeutic opportunities in sepsis. Trends Mol. Med. 2020, 26, 987–1002. [Google Scholar] [CrossRef]
- Coppola, C.; Hopkins, B.; Huhn, S.; Du, Z.; Huang, Z.; Kelly, W.J. Investigation of the impact from il-2, il-7, and il-15 on the growth and signaling of activated cd4+ t cells. Int. J. Mol. Sci. 2020, 21, 7814. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, B.; Anton, O.M.; Yao, Z.; Dubois, S.; Ju, W.; Sato, N.; DiLillo, D.J.; Bamford, R.N.; Ravetch, J.V. Il-15 enhanced antibody-dependent cellular cytotoxicity mediated by nk cells and macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E10915–E10924. [Google Scholar] [CrossRef]
- Naradikian, M.S.; Myles, A.; Beiting, D.P.; Roberts, K.J.; Dawson, L.; Herati, R.S.; Bengsch, B.; Linderman, S.L.; Stelekati, E.; Spolski, R. Cutting edge: Il-4, il-21, and ifn-γ interact to govern t-bet and cd11c expression in tlr-activated b cells. J. Immunol. 2016, 197, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Laffargue, M.; Ragab-Thomas, J.M.; Ragab, A.; Tuech, J.; Missy, K.; Monnereau, L.; Blank, U.; Plantavid, M.; Payrastre, B.; Raynal, P. Phosphoinositide 3-kinase and integrin signalling are involved in activation of bruton tyrosine kinase in thrombin-stimulated platelets. FEBS Lett. 1999, 443, 66–70. [Google Scholar] [CrossRef]
- Pasquet, J.-M.; Quek, L.; Pasquet, S.; Poole, A.; Matthews, J.R.; Lowell, C.; Watson, S.P. Evidence of a role for shp-1 in platelet activation by the collagen receptor glycoprotein vi. J. Biol. Chem. 2000, 275, 28526–28531. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Leonard, W.J. The jak-stat pathway. Mol. Immunol. 2000, 37, 1–11. [Google Scholar] [CrossRef]
- Witthuhn, B.A.; Williams, M.D.; Kerawalla, H.; Uckun, F.M. Differential substrate recognition capabilities of janus family protein tyrosine kinases within the interleukin 2 receptor (i12r) system: Jak3 as a potential molecular target for treatment of leukemias with a hyperactive jak-stat signaling machinery. Leuk. Lymphoma 1999, 32, 289–297. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: Rationale, progress, and caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, M.; Zhu, F.; Zhang, S.; Ding, P.; Wang, M. Il-9 promotes the development of deep venous thrombosis by facilitating platelet function. Thromb. Haemost. 2018, 118, 1885–1894. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Benbarche, S.; Strassel, C.; Angénieux, C.; Mallo, L.; Freund, M.; Gachet, C.; Lanza, F.; De La Salle, H. Dual role of il-21 in megakaryopoiesis and platelet homeostasis. Haematologica 2017, 102, 637. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cunningham, A.; Houston, K.; Sharma, A.M.; Chen, L.; Dokun, A.O.; Lye, R.J.; Spolski, R.; Leonard, W.J.; Annex, B.H. Endothelial interleukin-21 receptor up-regulation in peripheral artery disease. Vasc. Med. 2016, 21, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gushiken, F.C.; Bolgiano, D.; Salsbery, B.J.; Aghakasiri, N.; Jing, N.; Wu, X.; Vijayan, K.V.; Rumbaut, R.E.; Adachi, R.; et al. Signal transducer and activator of transcription 3 (stat3) regulates collagen-induced platelet aggregation independently of its transcription factor activity. Circulation 2013, 127, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Houck, K.L.; Tian, Y.; Bharadwaj, U.; Hull, K.; Zhou, Z.; Zhou, M.; Wu, X.; Tweardy, D.J.; Romo, D. Piperlongumine blocks jak2-stat3 to inhibit collagen-induced platelet reactivity independent of reactive oxygen species. PLoS ONE 2015, 10, e0143964. [Google Scholar] [CrossRef]
- Houck, K.L.; Yuan, H.; Tian, Y.; Solomon, M.; Cramer, D.; Liu, K.; Zhou, Z.; Wu, X.; Zhang, J.; Oehler, V.; et al. Physical proximity and functional cooperation of glycoprotein 130 and glycoprotein vi in platelet membrane lipid rafts. J. Thromb. Haemost. 2019, 17, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Hannoodee, S.; Nasuruddin, D.N. Acute inflammatory response. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Vazquez-Garza, E.; Jerjes-Sanchez, C.; Navarrete, A.; Joya-Harrison, J.; Rodriguez, D. Venous thromboembolism: Thrombosis, inflammation, and immunothrombosis for clinicians. J. Thromb. Thrombolysis 2017, 44, 377–385. [Google Scholar] [CrossRef]
- Mosevoll, K.A.; Johansen, S.; Wendelbo, Ø.; Nepstad, I.; Bruserud, Ø.; Reikvam, H. Cytokines, adhesion molecules, and matrix metalloproteases as predisposing, diagnostic, and prognostic factors in venous thrombosis. Front. Med. 2018, 5, 147. [Google Scholar] [CrossRef]
- Apostolou, E.; Tzioufas, A.G. Type-iii interferons in sjögren’s syndrome. Clin. Exp. Rheumatol. 2020, 38, 245–252. [Google Scholar]
- Cheng, C.W.; Fang, W.F.; Tang, K.T.; Lin, J.D. Serum interferon levels associated with the disease activity in women with overt graves’ disease. Cytokine 2020, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.R.; Gerriets, V. Interferon. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Liu, W.; Li, M.; Wang, Z.; Wang, J. Ifn-γ mediates the development of systemic lupus erythematosus. Biomed Res. Int. 2020, 2020, 7176515. [Google Scholar] [PubMed]
- Chrysanthopoulou, A.; Kambas, K.; Stakos, D.; Mitroulis, I.; Mitsios, A.; Vidali, V.; Angelidou, I.; Bochenek, M.; Arelaki, S.; Arampatzioglou, A. Interferon lambda1/il-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J. Pathol. 2017, 243, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xin, L.; Chan, B.P.; Teoh, R.; Tang, B.L.; Tan, Y. Interferon-β administration confers a beneficial outcome in a rabbit model of thromboembolic cerebral ischemia. Neurosci. Lett. 2002, 327, 146–148. [Google Scholar] [CrossRef]
- Ladislau, L.; Suárez-Calvet, X.; Toquet, S.; Landon-Cardinal, O.; Amelin, D.; Depp, M.; Rodero, M.P.; Hathazi, D.; Duffy, D.; Bondet, V. Jak inhibitor improves type i interferon induced damage: Proof of concept in dermatomyositis. Brain 2018, 141, 1609–1621. [Google Scholar] [CrossRef]
- Lauwerys, B.R.; Ducreux, J.; Houssiau, F.A. Type i interferon blockade in systemic lupus erythematosus: Where do we stand? Rheumatology 2014, 53, 1369–1376. [Google Scholar] [CrossRef]
- Welcher, A.A.; Boedigheimer, M.; Kivitz, A.J.; Amoura, Z.; Buyon, J.; Rudinskaya, A.; Latinis, K.; Chiu, K.; Oliner, K.S.; Damore, M.A. Blockade of interferon-γ normalizes interferon-regulated gene expression and serum cxcl10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015, 67, 2713–2722. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Mukaida, N.; Kondo, T. Absence of ifn-γ accelerates thrombus resolution through enhanced mmp-9 and vegf expression in mice. J. Clin. Investig. 2011, 121, 2911–2920. [Google Scholar] [CrossRef]
- Luther, N.; Shahneh, F.; Brähler, M.; Krebs, F.; Jäckel, S.; Subramaniam, S.; Stanger, C.; Schönfelder, T.; Kleis-Fischer, B.; Reinhardt, C.; et al. Innate effector-memory t-cell activation regulates post-thrombotic vein wall inflammation and thrombus resolution. Circ. Res. 2016, 119, 1286–1295. [Google Scholar] [CrossRef]
- Chetoui, N.; Boisvert, M.; Gendron, S.; Aoudjit, F. Interleukin-7 promotes the survival of human cd4+ effector/memory t cells by up-regulating bcl-2 proteins and activating the jak/stat signalling pathway. Immunology 2010, 130, 418–426. [Google Scholar] [CrossRef]
- Bertin, F.R.; Rys, R.; Mathieu, C.; Laurance, S.; Lemarié, C.; Blostein, M. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.S.; Buggins, P.; Hughes, E.; Lip, G.Y. Relation of interleukin-6, c-reactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am. J. Cardiol. 2004, 93, 1368–1373. [Google Scholar] [CrossRef]
- Conway, D.S.; Buggins, P.; Hughes, E.; Lip, G.Y. Relationship of interleukin-6 and c-reactive protein to the prothrombotic state in chronic atrial fibrillation. Am. J. Cardiol. 2004, 43, 2075–2082. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Blann, A.D.; García, A.; Marco, P.; Sogorb, F.; Lip, G.Y. Interleukin-6, endothelial activation and thrombogenesis in chronic atrial fibrillation. Eur. Heart J. 2003, 24, 1373–1380. [Google Scholar] [CrossRef][Green Version]
- Wojcik, B.M.; Wrobleski, S.K.; Hawley, A.E.; Wakefield, T.W.; Myers, D.D., Jr.; Diaz, J.A. Interleukin-6: A potential target for post-thrombotic syndrome. Ann. Vasc. Surg. 2011, 25, 229–239. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Noelle, R.J.; Nowak, E.C. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 2010, 10, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kubatzky, K.F.; Mitra, D.K. An update on interleukin-9: From its cellular source and signal transduction to its role in immunopathogenesis. Int. J. Mol. Sci. 2019, 20, 2113. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, J.; Yu, M.; Fan, H.; Guo, Z.Q.; Yang, R.; Guo, H.P.; Liao, Y.H.; Wang, M. Il-17a facilitates platelet function through the erk2 signaling pathway in patients with acute coronary syndrome. PLoS ONE 2012, 7, e40641. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Lai, J.-H. New insights into the il-12 and il-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem. Pharmacol. 2020, 175, 113928. [Google Scholar] [CrossRef]
- Schönfelder, T.; Brandt, M.; Kossmann, S.; Knopp, T.; Münzel, T.; Walter, U.; Karbach, S.H.; Wenzel, P. Lack of t-bet reduces monocytic interleukin-12 formation and accelerates thrombus resolution in deep vein thrombosis. Sci. Rep. 2018, 8, 3013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Golden, J.B.; Camhi, M.I.; Zhang, X.; Fritz, Y.; Diaconu, D.; Ivanco, T.L.; Simon, D.I.; Kikly, K.; McCormick, T.S. Protection from psoriasis-related thrombosis after inhibition of il-23 or il-17a. J. Investig. Dermatol. 2018, 138, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Popovic-Kuzmanovic, D.; Novakovic, I.; Stojanovich, L.; Aksentijevich, I.; Zogovic, N.; Tovilovic, G.; Trajkovic, V. Increased activity of interleukin-23/interleukin-17 cytokine axis in primary antiphospholipid syndrome. Immunobiology 2013, 218, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Blbulyan, A.; Martirosyan, A.; Petrek, M.; Navratilova, Z.; Manukyan, G. Antiphospholipid syndrome and monocytes: New aspects. Georgian Med. News 2017, 12–17. [Google Scholar]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Austin, J.C.; Hare, B.D.; Burdick, M.D.; Greenfield, L.J.; Wakefield, T.W. Il-10 regulates thrombus-induced vein wall inflammation and thrombosis. J. Immunol. 1998, 161, 1471–1476. [Google Scholar] [PubMed]
- Van der Poll, T.; Levi, M.; Hack, C.E.; Ten Cate, H.; Van Deventer, S.; Eerenberg, A.; De Groot, E.; Jansen, J.; Gallati, H.; Büller, H. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J. Exp. Med. 1994, 179, 1253–1259. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Andreadou, I.; Triantafyllidi, H.; Tsoumani, M.; Varoudi, M.; Vlastos, D.; Makavos, G.; Kostelli, G.; et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin. Res. Cardiol. 2019, 108, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Jerjes-Sanchez, C. Venous and Arterial Thrombosis: A Continuous Spectrum of the Same Disease? Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eklund, C.M. Proinflammatory cytokines in crp baseline regulation. Adv. Clin. Chem. 2009, 48, 111–136. [Google Scholar]
- Burstein, S.A.; Mei, R.L.; Henthorn, J.; Friese, P.; Turner, K. Leukemia inhibitory factor and interleukin-11 promote maturation of murine and human megakaryocytes in vitro. J. Cell. Physiol. 1992, 153, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- England, R.N.; Autieri, M.V. Anti-inflammatory effects of interleukin-19 in vascular disease. Int. J. Inflam. 2012, 2012, 253583. [Google Scholar] [CrossRef]
- Wei, C.-C.; Hsu, Y.-H.; Li, H.-H.; Wang, Y.-C.; Hsieh, M.-Y.; Chen, W.-Y.; Hsing, C.-H.; Chang, M.-S. Il-20: Biological functions and clinical implications. J. Biomed. Sci. 2006, 13, 601–612. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Andersen, T.; Heftdal, L.D.; Hvid, M.; Gerwien, J.; Sivakumar, P.; Taylor, P.C.; Senolt, L.; Deleuran, B. The il-20 cytokine family in rheumatoid arthritis and spondyloarthritis. Front. Immunol. 2018, 9, 2226. [Google Scholar] [CrossRef] [PubMed]
- Larochette, V.; Miot, C.; Poli, C.; Beaumont, E.; Roingeard, P.; Fickenscher, H.; Jeannin, P.; Delneste, Y. Il-26, a cytokine with roles in extracellular DNA-induced inflammation and microbial defense. Front. Immunol. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T. The contrasting roles of il-2 and il-15 in the life and death of lymphocytes: Implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin e2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Ollivier, V.; Ternisien, C.; Vu, T.; Elbim, C.; Hakim, J.; Prost, D.D. Interleukin 4 prevents the induction of tissue factor mrna in human monocytes in response to lps or pma stimulation. Br. J. Haematol. 1993, 85, 462–468. [Google Scholar] [CrossRef]
- Iwase, R.; Ishiguro, T.; Fujita, K.; Ishibashi, S.; Yokota, T. Dupilumab for atopic dermatitis, a possible risk factor of juvenile ischemic stroke: A case report. J. Stroke Cerebrovasc. Dis. 2020, 29, 104763. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (il)-4 and il-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Rutz, S.; Wang, X.; Ouyang, W. The il-20 subfamily of cytokines—from host defence to tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 783–795. [Google Scholar] [CrossRef]
- Bosmann, M.; Ward, P.A. Modulation of inflammation by interleukin-27. J. Leukoc. Biol. 2013, 94, 1159–1165. [Google Scholar] [CrossRef]
- Hermanns, H.M. Oncostatin m and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef]
- Faille, D.; Lamrani, L.; Loyau, S.; Huisse, M.-G.; Bourrienne, M.-C.; Alkhaier, S.; Cassinat, B.; Boulaftali, Y.; Debus, J.; Jandrot-Perrus, M.; et al. Interferon alpha therapy increases pro-thrombotic biomarkers in patients with myeloproliferative neoplasms. Cancers 2020, 12, 992. [Google Scholar] [CrossRef]
- Shah, M.; Capanu, M.; Soff, G.; Asmis, T.; Kelsen, D. Risk factors for developing a new venous thromboembolism in ambulatory patients with non-hematologic malignancies and impact on survival for gastroesophageal malignancies. J. Thromb. Haemost. 2010, 8, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Fusté, B.; Serradell, M.; Escolar, G.; Cases, A.; Mazzara, R.; Castillo, R.; Ordinas, A.; Díaz-Ricart, M. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb. Haemost. 2002, 88, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J. The biology of thrombopoietin and thrombopoietin receptor agonists. Int. J. Hematol. 2013, 98, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Jiang, X.-M.; Gong, J.-P. Recombinant human granulocyte colony-stimulating factor enhanced the resolution of venous thrombi. J. Vasc. Surg. 2008, 47, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Zoch-Zwierz, W.M.; Tomaszewska, B.; Zelazowska, B. Relationship of serum interleukin-7 concentration and the coagulation state in children with nephrotic syndrome. Pediatr. Int. 2005, 47, 424–429. [Google Scholar] [CrossRef]
- Van Anh Do-Thi, J.-O.; Lee, H.L. Crosstalk between the producers and immune targets of il-9. Immune Netw. 2020, 20, 1–16. [Google Scholar]
- Gerstenberger, B.S.; Ambler, C.; Arnold, E.P.; Banker, M.E.; Brown, M.F.; Clark, J.D.; Dermenci, A.; Dowty, M.E.; Fensome, A.; Fish, S.; et al. Discovery of tyrosine kinase 2 (tyk2) inhibitor (pf-06826647) for the treatment of autoimmune diseases. J. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Zhang, H.; Xie, T.; Ye, X.-Y. Selective tyk2 inhibitors as potential therapeutic agents: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Tanaka, Y.; Petri, M.A.; Smolen, J.S.; Wallace, D.J.; Dow, E.R.; Higgs, R.E.; Rocha, G.; Crowe, B.; Benschop, R.J.; et al. Baricitinib-associated changes in global gene expression during a 24-week phase ii clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci. Med. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Ferreira, S.; Guttman-Yassky, E.; Torres, T. Selective jak1 inhibitors for the treatment of atopic dermatitis: Focus on upadacitinib and abrocitinib. Am. J. Clin. Dermatol. 2020, 21, 783–798. [Google Scholar] [CrossRef]
- Genovese, M.C.; Greenwald, M.; Codding, C.; Zubrzycka-Sienkiewicz, A.; Kivitz, A.J.; Wang, A.; Shay, K.; Wang, X.; Garg, J.P.; Cardiel, M.H. Peficitinib, a jak inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Statistical Review and Evaluation. Clinical Studies NDA Number 50-679. Drug Name: Maxipime 04/22/2009. Available online: https://www.fda.gov/media/77126/download (accessed on 27 February 2021).
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. Eular recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Vallejo-Yagüe, E.; Weiler, S.; Micheroli, R.; Burden, A.M. Thromboembolic safety reporting of tofacitinib and baricitinib: An analysis of the who vigibase. Drug Saf. 2020, 43, 881–891. [Google Scholar] [CrossRef]
- Giménez Poderós, T.; Gallardo Borge, S.; Vazquez-Ferreiro, P. Risk of venous thromboembolism associated with tofacitinib and baricitinib: A systematic review and indirect meta-analysis. Pharmacotherapy 2020. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.C.; Islam, M.A.; Woon, P.Y.; Johan, M.F.; Hassan, R.; Ramli, M. Molecular genetics of thrombotic myeloproliferative neoplasms: Implications in precision oncology. Genes Dis. 2021. [Google Scholar] [CrossRef]
- Coltro, G.; Vannucchi, A.M. The safety of jak kinase inhibitors for the treatment of myelofibrosis. Expert Opin. Drug Saf. 2021. [Google Scholar] [CrossRef] [PubMed]
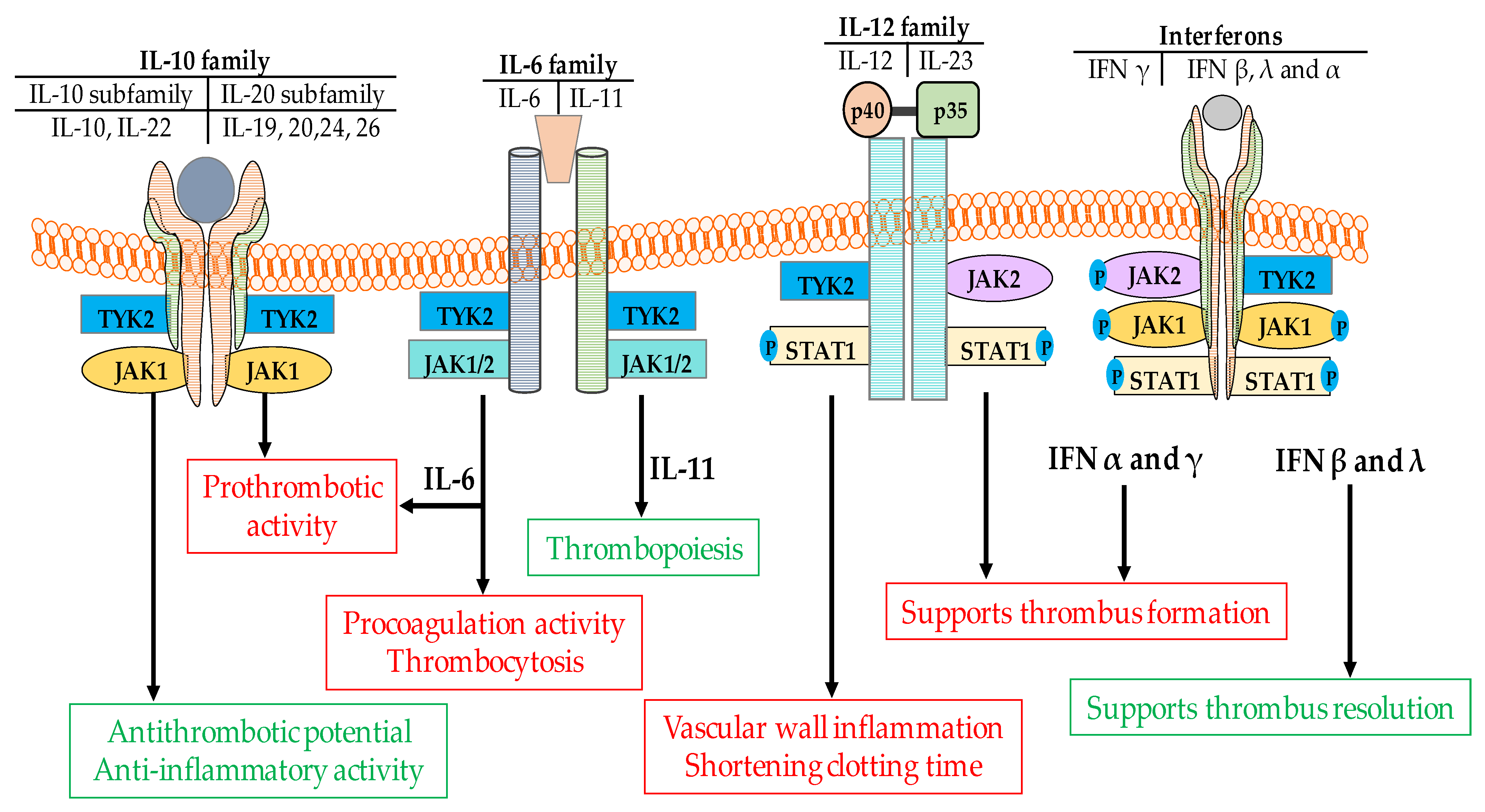
| Cytokines | Activities | References |
|---|---|---|
| Interleukin-6 family | ||
| IL-6 | Prothrombotic | [122] |
| Reduction of Vessel Dilation | [123] | |
| Induces the expression of TF | [124] | |
| Increases the level of fibrinogen | ||
| Higher expression of factor VIII | ||
| High expression of von Willebrand factor | ||
| Endothelial activation | [92] | |
| Endothelial cell damage | ||
| Increased platelet aggregation | ||
| Increased sensitivity to thrombin | ||
| Amplify inflammatory response (cytokine storm) | [125] | |
| Regulate acute phase reactant synthesis | [126] | |
| IL-11 | Thrombopoiesis | [127] |
| Interleukin-10 family | ||
| IL-10 | counteract pro-inflammatory cytokines | [121] |
| IL-22 | Dual (pro and anti-inflammatory activity) | [128] |
| Potential anti-thrombotic activity? | ||
| Inteleukin-20 subfamily | ||
| IL-19 | Expressed in response to vascular injury | [129] |
| Exerts a pronounced anti-inflammatory effect upon vascular cells | ||
| IL-20 | Strong proinflammatory activity (role in thrombus formation unknown) | [130] |
| IL-24 | Strong proinflammatory potential | [131] |
| IL-26 | Induces production of proinflammatory cytokines by innate immune cells | [132] |
| JAK Type | Cytokine Signaling | Thrombotic Effect | Active Jakinibs |
|---|---|---|---|
| JAK1, JAK2, TYK2 | IL-6 family | TOFA (pan-Jakinib) BARI (JAK1/JAK2) UPA (JAK1) RUX, MMD, FEDR, PAC (JAK2) | |
| IL-6 | Proinflammatory and prothrombotic [122] | ||
| IL-11 | Proinflammatory and prothrombotic [127] | ||
| IL-27 | Dual role in inflammation and prothrombotic (?) [139] | ||
| IL-31 | Anti-inflammatory and anti-thrombotic? [140] | ||
| JAK1, TYK2 | IL-10 family | TOFA (pan-Jakinib) BARI (JAK1/JAK2) UPA (JAK1) | |
| IL-10 | Anti-inflammatory and anti-thrombotic [119] | ||
| IL-19 | Anti-thrombotic [129] | ||
| IL-20 | Proinflammatory and prothrombotic [130] | ||
| IL-22 | Dual (pro and anti-thrombotic) and proinflammatory [128] | ||
| IL-24 | Prothrombotic and proinflammatory [131] | ||
| IL-26 | Prothrombotic [132] | ||
| JAK1, TYK2 JAK1, JAK2 JAK1, TYK2 JAK1, TYK2 | Interferons | TOFA (pan-Jakinib) BARI (JAK1/JAK2) UPA (JAK1) | |
| IFN-λ | Anti-inflammatory and anti-thrombotic [98] | ||
| IFN-γ | Proinflammatory and prothrombotic [72] | ||
| IFN-α | Proinflammatory and prothrombotic [141] | ||
| IFN-β | Anti-inflammatory and anti-thrombotic [99] | ||
| JAK2, TYK2 | IL-12/23 | TOFA (pan-Jakinib) BARI (JAK/JAK2) RUX, MMD, FEDR, PAC (JAK2) | |
| IL-12 | Proinflammatory and prothrombotic [116,117] | ||
| IL-23 | Proinflammatory and prothrombotic [116,118] | ||
| JAK2 homodimer | Hematopoietins | TOFA (pan-Jakinib) BAR (JAK1/JAK2) RUX, MMD, FEDR, PAC (JAK2) | |
| GCSF | Proinflammatory and prothrombotic [142] | ||
| EPO | Proinflammatory and prothrombotic [143] | ||
| TPO | Proinflammatory and prothrombotic [144] | ||
| GMCSF | Proinflammatory and anti-thrombotic (?) [145] | ||
| JAK1, JAK3 | γ chain cytokine family | ||
| IL-2 | Possible dual role in inflammation, prothrombotic [146] | TOFA (pan-Jakinib) BAR (JAK1/JAK2) UPA (JAK1) | |
| IL-4 (IL-4Rα/IL13Rα receptor) | Anti-inflammatory and anti-thrombotic [136] | ||
| IL-7 | Proinflammatory and prothrombotic [147] | ||
| IL-9 | Dual (pro and anti-thrombotic) [148] | ||
| IL-15 | Proinflammatory prothrombotic (?) [146] | ||
| IL-21 | |||
| JAK1, JAK2 TYK2 | IL-4 (IL-4Rα/IL-2Rγ receptor) | Anti-thrombotic [136] | TOFA (pan-Jakinib) BARI (JAK1/JAK2) UPA (JAK1) RUX, MMD, FEDR PAC (JAK2) |
| JAK1, JAK2 TYK2 | IL-13 | Anti-thrombotic [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotyla, P.J.; Engelmann, M.; Giemza-Stokłosa, J.; Wnuk, B.; Islam, M.A. Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role? Int. J. Mol. Sci. 2021, 22, 2449. https://doi.org/10.3390/ijms22052449
Kotyla PJ, Engelmann M, Giemza-Stokłosa J, Wnuk B, Islam MA. Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role? International Journal of Molecular Sciences. 2021; 22(5):2449. https://doi.org/10.3390/ijms22052449
Chicago/Turabian StyleKotyla, Przemysław J., Małgorzata Engelmann, Joanna Giemza-Stokłosa, Bartosz Wnuk, and Md Asiful Islam. 2021. "Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role?" International Journal of Molecular Sciences 22, no. 5: 2449. https://doi.org/10.3390/ijms22052449
APA StyleKotyla, P. J., Engelmann, M., Giemza-Stokłosa, J., Wnuk, B., & Islam, M. A. (2021). Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role? International Journal of Molecular Sciences, 22(5), 2449. https://doi.org/10.3390/ijms22052449







