Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Standard Solutions
2.4. HS-SPME Multivariate Optimization Process
2.5. Gas Chromatography Quadrupole Mass Spectrometry (GC-qMS) Conditions
2.6. Method Validation
3. Results and Discussion
3.1. Optimization of HS-SPME Procedure
3.2. Method Validation
3.3. Quantification of Phthalates in Plastic-Based Food Packaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bošnir, J.; Puntarić, D.; Galić, A.; Škes, I.; Dijanić, T.; Klarić, M.; Grgić, M.; Čurković, M.; Šmit, Z. Migration of Phthalates from Plastic Containers into Soft Drinks and Mineral Water. Food Technol. Biotechnol. 2007, 45, 91–95. [Google Scholar]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Fierens, T.; Vanermen, G.; Van Holderbeke, M.; De Henauw, S.; Sioen, I. Effect of cooking at home on the levels of eight phthalates in foods. Food Chem. Toxicol. 2012, 50, 4428–4435. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Nie, X.P.; Wang, H.S.; Wong, M.H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int. 2013, 57–58, 75–80. [Google Scholar] [CrossRef]
- Kickham, P.; Otton, S.V.; Moore, M.M.; Ikonomou, M.G.; Gobas, F.A.P.C. Relationship between biodegradation and sorption of phthalate esters and their metabolites in natural sediments. Environ. Toxicol. Chem. 2012, 31, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- GIbarra, V.G.; De Quirós, A.R.B.; Losada, P.P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ferguson, K.K. Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in u.s. adults and adolescents from the national health and nutrition examination survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, L.; Zhu, J.; Liu, T.; Ye, L. Role of the 17β-hydroxysteroid dehydrogenase signalling pathway in di-(2-ethylhexyl) phthalate-induced ovarian dysfunction: An in vivo study. Sci. Total Environ. 2020, 712, 134406. [Google Scholar] [CrossRef]
- Van’T Erve, T.J.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Milne, G.L.; Calafat, A.M.; Swan, S.H.; Ferguson, K.K. Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environ. Sci. Technol. 2019, 53, 3258–3267. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Hyland, C.; Mora, A.M.; Kogut, K.; Calafat, A.M.; Harley, K.; Deardorff, J.; Holland, N.; Eskenazi, B.; Sagiv, S.K. Prenatal Exposure to Phthalates and Neurodevelopment in the CHAMACOS Cohort. Environ. Health Perspect. 2019, 127, 107010. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.P.; Vila, M.; Garcia-Jares, C.; Homem, V.; Ratola, N.; Dagnac, T.; Llompart, M. Determination of multiclass personal care products in continental waters by solid-phase microextraction followed by gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1607, 460398. [Google Scholar] [CrossRef]
- Tran-Lam, T.-T.; Dao, Y.; Nguyen, D.; Ma, H.; Pham, T.; Le, G. Optimization of Sample Preparation for Detection of 10 Phthalates in Non-Alcoholic Beverages in Northern Vietnam. Toxics 2018, 6, 69. [Google Scholar] [CrossRef]
- Fernández-González, V.; Moscoso-Pérez, C.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Reliable, rapid and simple method for the analysis of phthalates in sediments by ultrasonic solvent extraction followed by head space-solid phase microextraction gas chromatography mass spectrometry determination. Talanta 2017, 162, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Campillo, N.; Pastor-Belda, M.; Oller, A.; Hernández-Córdoba, M. Determination of phthalate esters in cleaning and personal care products by dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1376, 18–25. [Google Scholar] [CrossRef]
- Amanzadeh, H.; Yamini, Y.; Moradi, M.; Asl, Y.A. Determination of phthalate esters in drinking water and edible vegetable oil samples by headspace solid phase microextraction using graphene/polyvinylchloride nanocomposite coated fiber coupled to gas chromatography-flame ionization detector. J. Chromatogr. A 2016, 1465, 38–46. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonaccia, A.; Patrolecco, L.; Lambreva, M.D.; Litescuc, S.C.; Ghugea, S.A.; Rea, G. Analytical tools monitoring endocrine disrupting chemicals. TrAC Trends Anal. Chem. 2016, 80, 555–567. [Google Scholar] [CrossRef]
- Net, S.; Delmont, A.; Sempéré, R.; Paluselli, A.; Ouddane, B. Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Sci. Total Environ. 2015, 515–516, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-J.; Liu, S.-Z.; Li, H.-H.; He, J.; Feng, J.-T.; Zhang, X.; Yan, H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019, 147, 82–90. [Google Scholar] [CrossRef]
- Carnol, L.; Schummer, C.; Moris, G. Quantification of six phthalates and one adipate in Luxembourgish beer using HS-SPME-GC/MS. Food Anal. Methods 2017, 10, 298–309. [Google Scholar] [CrossRef]
- Zhang, N.; Lei, X.; Huang, T.; Su, L.; Zhang, L.; Xie, Z.; Wu, X. Guanidyl-functionalized polyhedral oligomeric silsesquioxane porous hybrid polymer coating for specific solid phase microextraction of phthalate esters in foodstuff. Chem. Eng. J. 2020, 386, 124003. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.I.; Kretzschmar, T. Simultaneous Determination of Polycyclic Aromatic Hydrocarbons, Alkylphenols, Phthalate Esters and Polychlorinated Biphenyls in Environmental Waters Based on Headspace-Solid Phase Microextraction Followed by Gas Chromatography-Tandem Mass Spectrometry. J. Environ. Anal. Chem. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef]
- Moreira, M.; André, L.; Cardeal, Z. Analysis of Phthalate Migration to Food Simulants in Plastic Containers during Microwave Operations. Int. J. Environ. Res. Public Health 2013, 11, 507–526. [Google Scholar] [CrossRef]
- Razavi, N.; Yazdi, A.S. New application of chitosan-grafted polyaniline in dispersive solid-phase extraction for the separation and determination of phthalate esters in milk using high-performance liquid chromatography. J. Sep. Sci. 2017, 40, 1739–1746. [Google Scholar] [CrossRef]
- Zaater, M.F.; Tahboub, Y.R.; Al Sayyed, A.N. Determination of phthalates in Jordanian bottled water using GC-MS and HPLC-UV: Environmental study. J. Chromatogr. Sci. 2014, 52, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.H.; Yue, Q.; Huang, Y.Y.; Yang, C.; Shen, X.F. Facile magnetization of covalent organic framework for solid-phase extraction of 15 phthalate esters in beverage samples. Talanta 2020, 206, 120194–120204. [Google Scholar] [CrossRef]
- Yue, Q.; Huang, Y.Y.; Shen, X.F.; Yang, C.; Pang, Y.H. In situ growth of covalent organic framework on titanium fiber for headspace solid-phase microextraction of 11 phthalate esters in vegetables. Food Chem. 2020, 318, 126507. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Fabrication of UMCM-1 based monolithic and hollow fiber—Metal-organic framework deep eutectic solvents/molecularly imprinted polymers and their use in solid phase microextraction of phthalate esters in yogurt, water and edible oil by GC-FID. Food Chem. 2020, 314, 126179. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.D.; Küplülü, Ö. Determination of phthalates in some milk products by liquid chromatography/tandem mass spectrometry. Ank. Univ. Vet. Fak. Derg. 2019, 66, 231–236. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Ho, C.-H.; Chang, H.-Y.; Yang, W.-C.; Lin, C.-F.; Lin, C.-T.; Xue, Y.-J.; Lai, J.-M.; Wang, J.-H.; Chang, G.-R. Analysis of Pollution of Phthalates in Pork and Chicken in Taiwan Using Liquid Chromatography–Tandem Mass Spectrometry and Assessment of Health Risk. Molecules 2019, 24, 3817. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Rodríguez-Ramos, R.; Rodríguez-Delgado, M.Á. A green and simple procedure based on deep eutectic solvents for the extraction of phthalates from beverages. Food Chem. 2020, 312, 125798. [Google Scholar] [CrossRef]
- Notardonato, I.; Protano, C.; Vitali, M.; Bhattacharya, B.; Avino, P. A Method Validation for Simultaneous Determination of Phthalates and Bisphenol A Released from Plastic Water Containers. Appl. Sci. 2019, 9, 2945. [Google Scholar] [CrossRef]
- Nlu, Y.; Gao, W.; Li, H.; Zhang, J.; Llan, Y. Rapid determination of 17 phthalate esters in capsanthin by QuEChERS coupled with gas chromatography-mass spectrometry. Anal. Sci. 2020, 36, 485–490. [Google Scholar] [CrossRef]
- Fan, J.C.; Ren, R.; Jin, Q.; He, H.L.; Wang, S.T. Detection of 20 phthalate esters in breast milk by GC-MS/MS using QuEChERS extraction method. Food Addit. Contam. Part A Chem. Anal. Controlexposure Risk Assess. 2019, 36, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Q.; Yuan, Y.; Wang, H.; Tong, Y.; Zhan, Y.; Sheng, X.; Sun, Y.; Zhou, X. Enrichment and sensitive determination of phthalate esters in environmental water samples: A novel approach of MSPE-HPLC based on PAMAM dendrimers-functionalized magnetic-nanoparticles. Talanta 2020, 206, 120213. [Google Scholar] [CrossRef]
- Zaytseva, N.V.; Ulanova, T.S.; Karnazhitskaya, T.D.; Zorina, A.S.; Permyakova, T.S. Determination of phthalates in juice product by high-performance liquid chromatography/mass spectrometry. Vopr. Pitan. 2018, 87, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Phthalates in Beverages and Plastic Bottles: Sample Preparation and Determination. Food Anal. Methods 2018, 11, 48–61. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Y.; Wu, H.; Diao, Q.; Tian, F.; Li, Y. Simultaneous determination of trace migration of phthalate esters in honey and royal jelly by GC-MS. J. Sep. Sci. 2014, 37, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, B.; Sabel, C.E.; Thomsen, M.; Gao, X.; Zhong, M.; Chen, Z.; Feng, P. Oral intake exposure to phthalates in vegetables produced in plastic greenhouses and its health burden in Shaanxi province, China. Sci. Total Environ. 2019, 696, 133921. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Kim, Y.Y.; Cho, H.D.; Kim, J.; Lee, J.Y.; Lee, Y.; Jo, E.; Lee, J.; Cha, S.; Han, S.B. Development and investigation of a QuEChERS-based method for determination of phthalate metabolites in human milk. J. Pharm. Biomed. Anal. 2020, 181, 113092. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef]
- Du, L.; Ma, L.; Qiao, Y.; Lu, Y.; Xiao, D. Determination of phthalate esters in teas and tea infusions by gas chromatography-mass spectrometry. Food Chem. 2016, 197, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, M.; Pepe, A.; Sacchetti, G.; Compagnone, D.; Mastrocola, D.; Cichelli, A. Determination of phthalate esters in wine using solid-phase extraction and gas chromatography-mass spectrometry. Food Chem. 2008, 111, 771–777. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran, Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z.; Liu, Y. Migration and potential risk of trace phthalates in bottled water: A global situation. Water Res. 2018, 147, 362–372. [Google Scholar] [CrossRef]
- Overgaard, L.E.K.; Bonefeld, C.M.; Frederiksen, H.; Main, K.M.; Thyssen, J.P. The association between phthalate exposure and atopic dermatitis with a discussion of phthalate induced secretion of interleukin-1β and thymic stromal lymphopoietin. Expert Rev. Clin. Immunol. 2016, 12, 609–616. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.I.; Lee, C.W. Development and validation of a modified QuEChERS method coupled with LC-MS/MS to determine arbutin in pear peels. Food Sci. Biotechnol. 2016, 25, 987–992. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Liu, L.; Li, Y.; Ren, N.; Kannan, K. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J. Agric. Food Chem. 2012, 60, 6913–6919. [Google Scholar] [CrossRef] [PubMed]
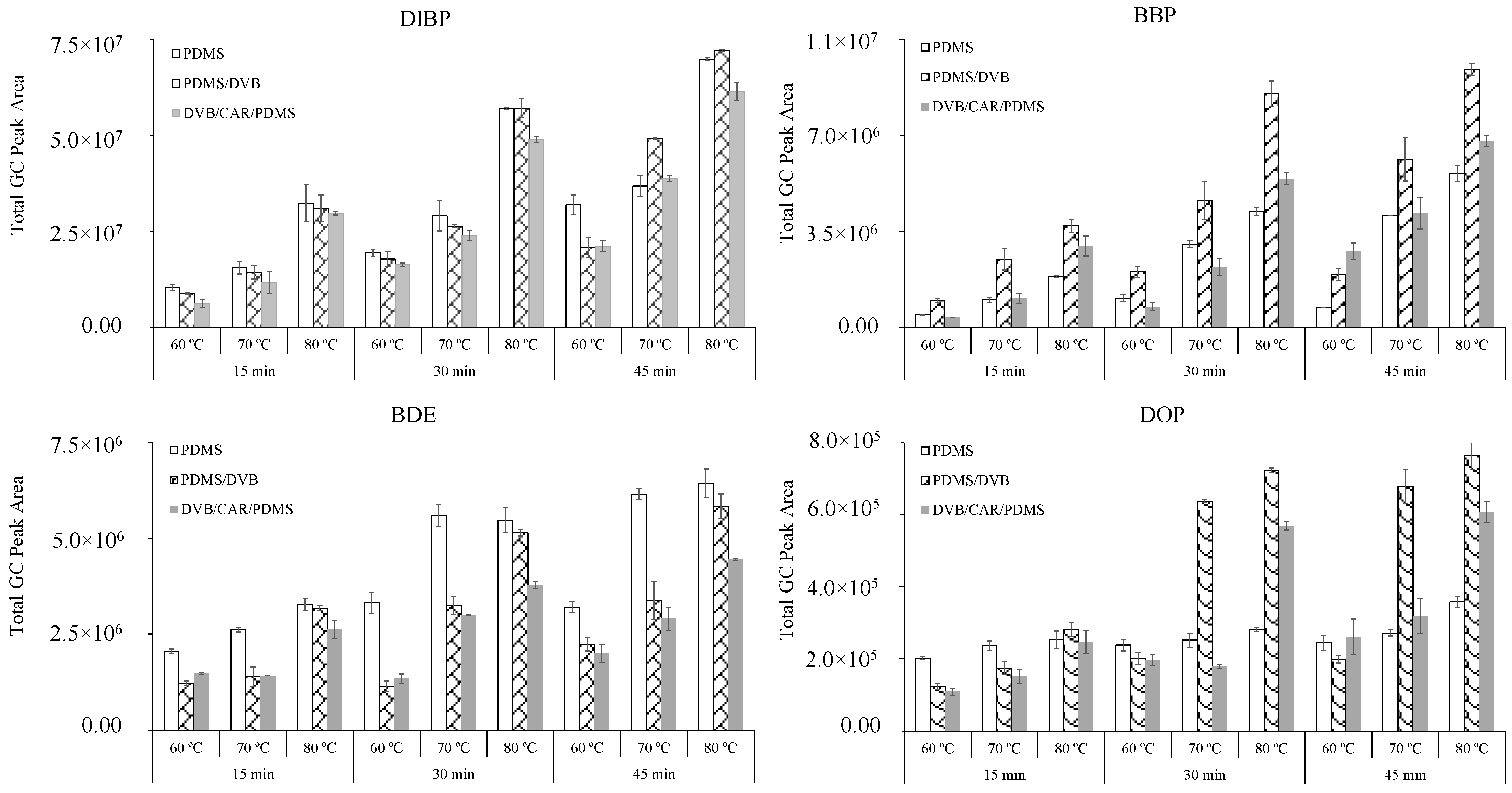
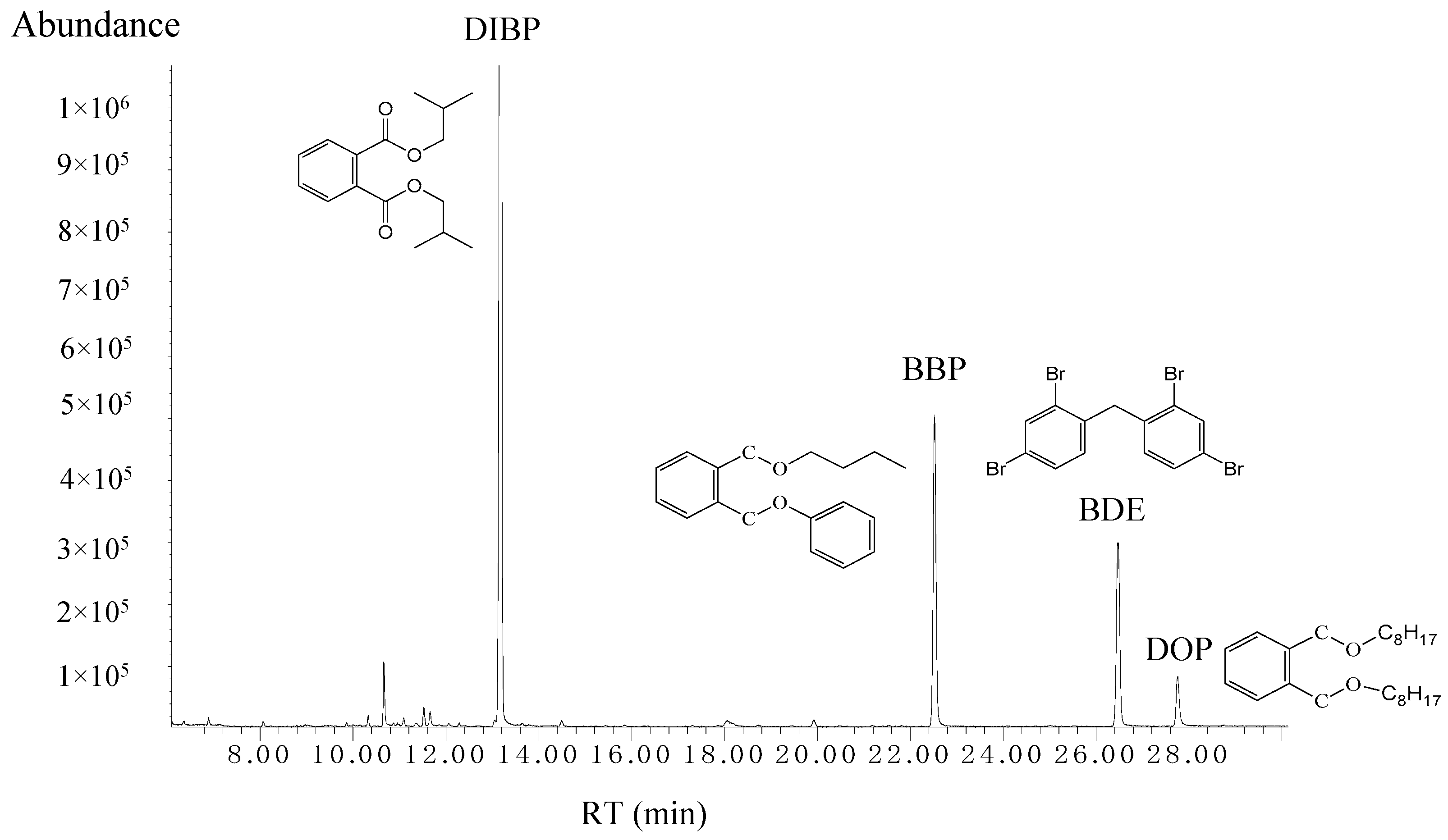
| RT (min) | PAEs | Linear Range (µg/L) | Equation | R2 | LOD (µg/L) | LOQ (µg/L) |
|---|---|---|---|---|---|---|
| 13.18 | DIBP | 0.5–60 | y = 817,649x + 106 | 0.996 | 0.06 | 0.21 |
| 22.55 | BBP | 0.5–60 | y = 66,577x + 116,115 | 0.999 | 0.03 | 0.10 |
| 26.49 | BDE | 1–60 | y = 124,317x + 755,468 | 0.995 | 0.08 | 0.24 |
| 27.77 | DOP | 1–60 | y = 44,993x – 100,807 | 0.999 | 0.07 | 0.23 |
| RT (min) | Compounds | Spiked Level (µg/L) | % REC ± SD | Intra-Day (%) | Inter-Day (%) |
|---|---|---|---|---|---|
| 13.17 | DIBP | 0.5 | 90.20 ± 1.3 | 3.5 | 7.5 |
| 30 | 91.60 ± 0.9 | 1.9 | 5.8 | ||
| 60 | 104.9 ± 1.8 | 0.9 | 3.3 | ||
| 22.55 | BBP | 0.5 | 107.9 ± 2.0 | 5.9 | 8.5 |
| 30 | 111.3 ± 1.9 | 1.3 | 6.7 | ||
| 60 | 105.7 ± 3.2 | 0.9 | 1.7 | ||
| 26.49 | BDE | 1 | 98.30 ± 0.8 | 7.9 | 12.7 |
| 30 | 91.30 ± 1.4 | 3.7 | 10.6 | ||
| 60 | 94.00 ± 1.7 | 0.6 | 3.5 | ||
| 27.77 | DOP | 1 | 109.2 ± 4.3 | 1.5 | 8.2 |
| 30 | 99.20 ± 1.6 | 1.4 | 3.1 | ||
| 60 | 95.50 ± 0.8 | 0.7 | 2.2 |
| Target Analytes | Samples | Extraction Procedure | Analytical Method | LOD (µg/L) | LOQ (µg/L) | Rec (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 6 PAEs | Milk products | LLE | LC-MS/MS | - | 20–30 µg/kg | 84–96 | [30] |
| 5 PAEs | Meats | LLE | LC-MS/MS | - | 40 µg/kg | 96–103 | [31] |
| 8 PAEs | Tea, juices | DES-VA-EDLLME | HPLC-DAD | 5.1–17.8 | 17.2–59.4 | 84–120 | [32] |
| 6 PAEs, BPA | Waters | SB-DLLME | GC-MS | 0.001–0.008 | 0.005–0.014 | 95–99 | [33] |
| 17 PAEs | Capsanthin | QuEChERS | GC-MS | 0.2–0.5 µg/kg | 0.6–1.5 µg/kg | 83–118 | [34] |
| 20 PAEs | Breast milk | QuEChERS | GC-MS/MS | 0.004–1.3 µg/kg | 0.02–4.2 µg/kg | 83–123 | [35] |
| 3 PAEs | Waters | MSPE | HPLC-VWD | 0.025–0.16 | 0.082–0.54 | 93–102 | [36] |
| 15 PAEs | Beverages | MSPE | GC-MS/MS | 0.005–2.748 | 0.018–9.151 | 79–122 | [27] |
| 6 PAEs, BPA | Honey | UVA-DLLME | GC-MS | 3–13 µg/kg | 7–22 µg/kg | 71–100 | [20] |
| 6 PAEs, 1 Adipate | Beers | HS-SPME | GC-MS | 0.006–0.590 | 0.020–1.959 | 74–101 | [28] |
| 11 PAEs | Vegetables | HS-SPME | GC-MS/MS | 0.001–0.430 | - | - | [21] |
| 10 PAEs | Milk and rice | SPME | GC-MS | 0.054–2.51 ng/L | 0.18–8.37 ng/L | 89–114 | [24] |
| 4 PAEs | Yogurts, waters | HFLMP-SPME | GC-FID | 0.008–0.030 | 0.028–0.120 | 96–100 | [29] |
| 4 PAEs | Food packaging | HS-SPME | GC-MS | 0.03–0.08 | 0.10–0.24 | 90–111 | This study |
| Samples | Phthalates Concentration (µg/L) ± SD | |||
|---|---|---|---|---|
| DIBP | BBP | BDE | DOP | |
| Plastic 1 | <LOD | - | - | - |
| Plastic 2 | <LOD | - | - | - |
| Plastic 3 | <LOD | - | - | - |
| Plastic 4 | <LOD | 1.4 ± 0.01 | <LOD | 2.8 ± 0.04 |
| Plastic 5 | 4.8 ± 0.3 | - | - | 1.0 ± 0.01 |
| Plastic 6 | 10.6 ± 0.2 | - | - | 2.2 ± 0.06 |
| Plastic 7 | 9.0 ± 0.4 | - | - | 1.8 ± 0.07 |
| Plastic 8 | 6.1 ± 0.3 | - | - | 1.1 ± 0.08 |
| Plastic 9 | 8.2 ± 0.4 | - | - | - |
| Plastic 10 | 4.7 ± 0.05 | - | - | 2.2 ± 0.02 |
| Plastic 11 | 3.6 ± 0.7 | - | - | 1.9 ± 0.2 |
| Plastic 12 | 10.7 ± 0.6 | - | - | - |
| Plastic 13 | 6.8 ± 0.8 | - | - | 1.9 ± 0.4 |
| Plastic 14 | 4.3 ± 0.2 | - | <LOD | 2.5 ± 0.2 |
| Samples | Phthalates | Concentration Range | Ref. |
|---|---|---|---|
| Meats | DEHP (Pork and Chicken) | 0.62, 0.8 mg/kg | [31] |
| DEHP (Fruit jam, Salted meat); DnBP | 170 μg/kg, 2380 μg/kg; 1580 μg/kg | [42] | |
| Spices | DEHP; DiBP, DBP; BBzP | 2598 μg/kg; >300 μg/kg | [3,49] |
| Tea | DMP, DEP, DIBP; DBP; DEHP | 1.135–3.734 mg/kg | [43] |
| Wine | DMP, DEP/DBP/BBP | 0.024–0.029 μg/mL | [44] |
| Waters | DEHP, DBP, DEP, BOP DEHP, BBP, DBP, DEP, DMP (Bottled water) | 0.76/0.96/1.06/0.77 μg/L 3.42/2.89/13.99/5.35/1.15/2.07 μg/L | [46] |
| Capsanthin | DBP, DEHP | 0.872/0.992 μg/g | [34] |
| Beers | DMP, DEP, DBP, BBP, DEHP, DOP, DEHA | 0.588, 0.175, 0.118, 0.079, 0.009, 0.006, 0.009 μg/L | [20] |
| Juice | DOP, DBP, DIBP, DEHP, BBP (Juice) | 0.01–08 mg/dm3 | [37] |
| Beverages | DEHP, DEP | 0.580 /0.070 μg/L | [38] |
| Honey/Royal Jelly | DIBP, BBP, BDE, DOP, | 0.3/1.5; 0.8/3; 0.3/1.5; 1.2; 6 ng/g | [39] |
| Vegetables | DEHP, DnBP, DiBP, DEP, BBP | 1881–4664/985/338/9/2 μg/kg | [40] |
| Powdered and Human/Raw Milk | Mono-BP, mono-BzP, DnBP, BzBP, DEHP | 0.1–500 ng/mL 18/1.2/21 μg/kg | [3,41] |
| Yoghurt | DEHP, DBP, BBP | 170/112/63 μg/kg | [30] |
| Plastic Containers | DIBP, BBP, BDE, DOP | 4.39/1.42/<LOD/1.03 µg/L | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging. Appl. Sci. 2021, 11, 2130. https://doi.org/10.3390/app11052130
Perestrelo R, Silva CL, Algarra M, Câmara JS. Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging. Applied Sciences. 2021; 11(5):2130. https://doi.org/10.3390/app11052130
Chicago/Turabian StylePerestrelo, Rosa, Catarina L. Silva, Manuel Algarra, and José S. Câmara. 2021. "Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging" Applied Sciences 11, no. 5: 2130. https://doi.org/10.3390/app11052130
APA StylePerestrelo, R., Silva, C. L., Algarra, M., & Câmara, J. S. (2021). Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging. Applied Sciences, 11(5), 2130. https://doi.org/10.3390/app11052130









