Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
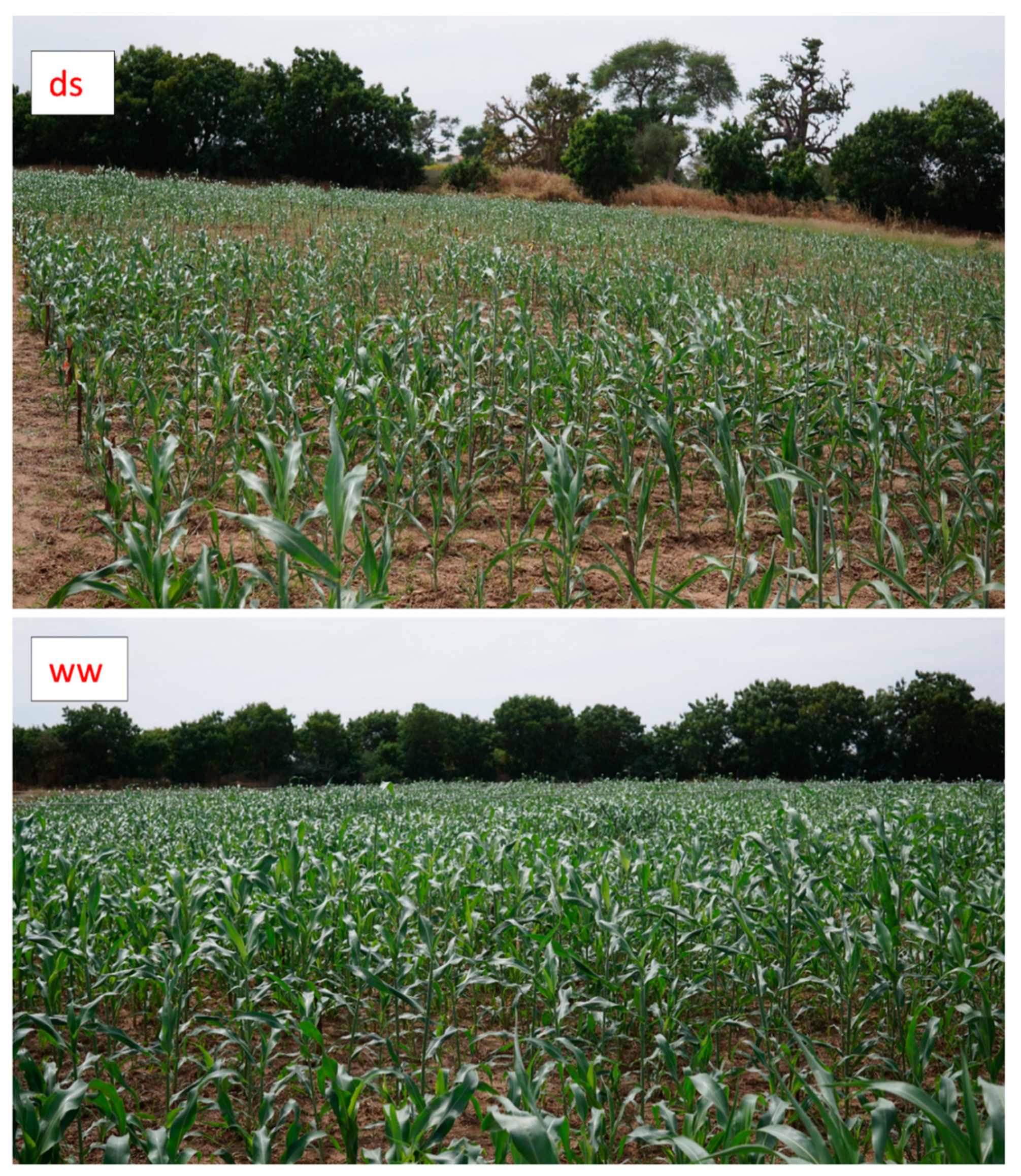
2.2.1. Experimental Conditions
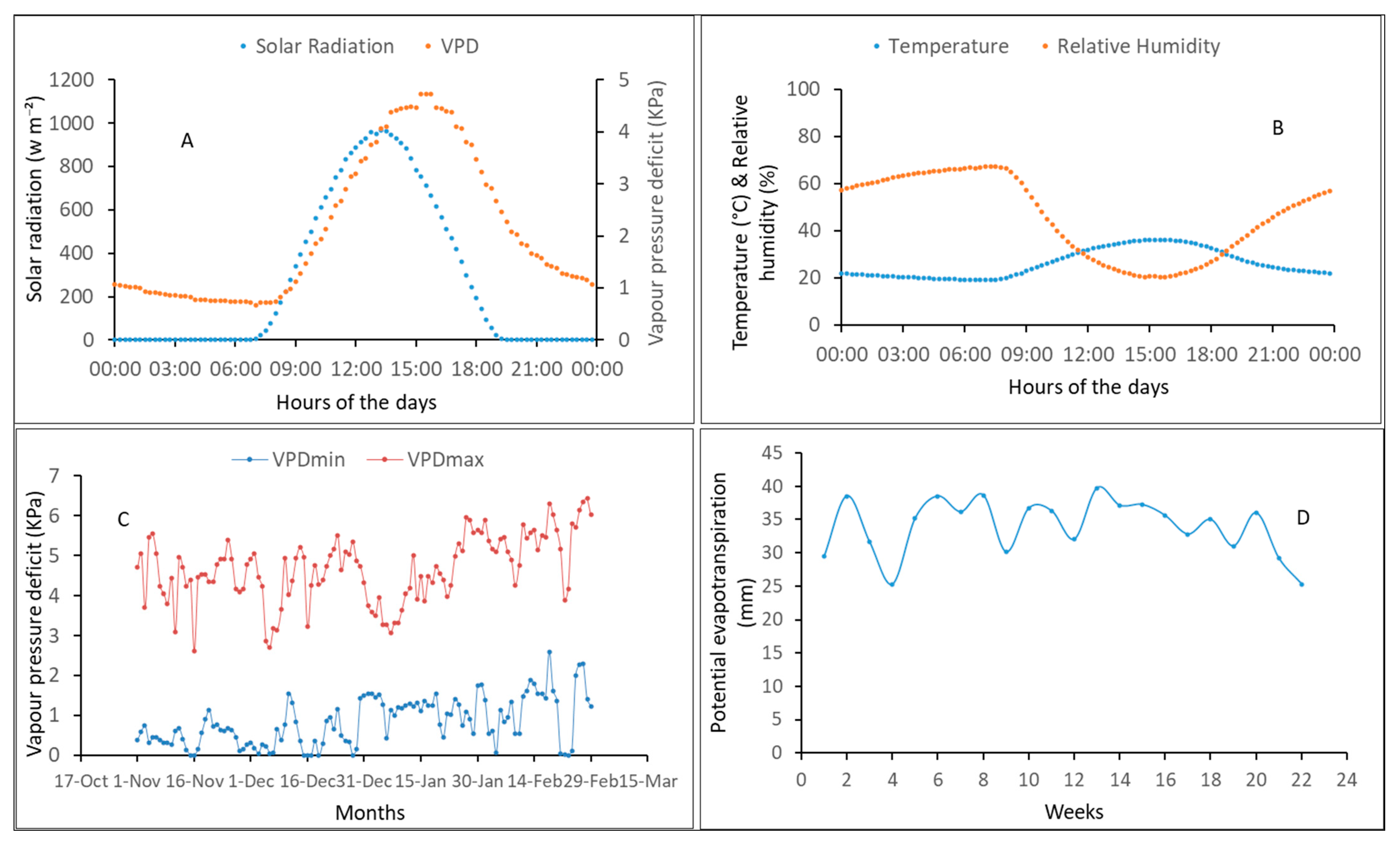
2.2.2. Weather Conditions
2.2.3. Characterization of the Drought Stress
2.2.4. Assessment of Agro-Physiological Traits
2.2.5. Roots Phenotyping
2.2.6. Statistical Analyses
3. Results
3.1. Measured Weather Conditions during the Experiments
3.2. Drought Stress Affected the Fraction of Transpirable Soil Water and Predawn Leaf Water Potential
3.3. Response of Sorghum Varieties to Early Water Deficit
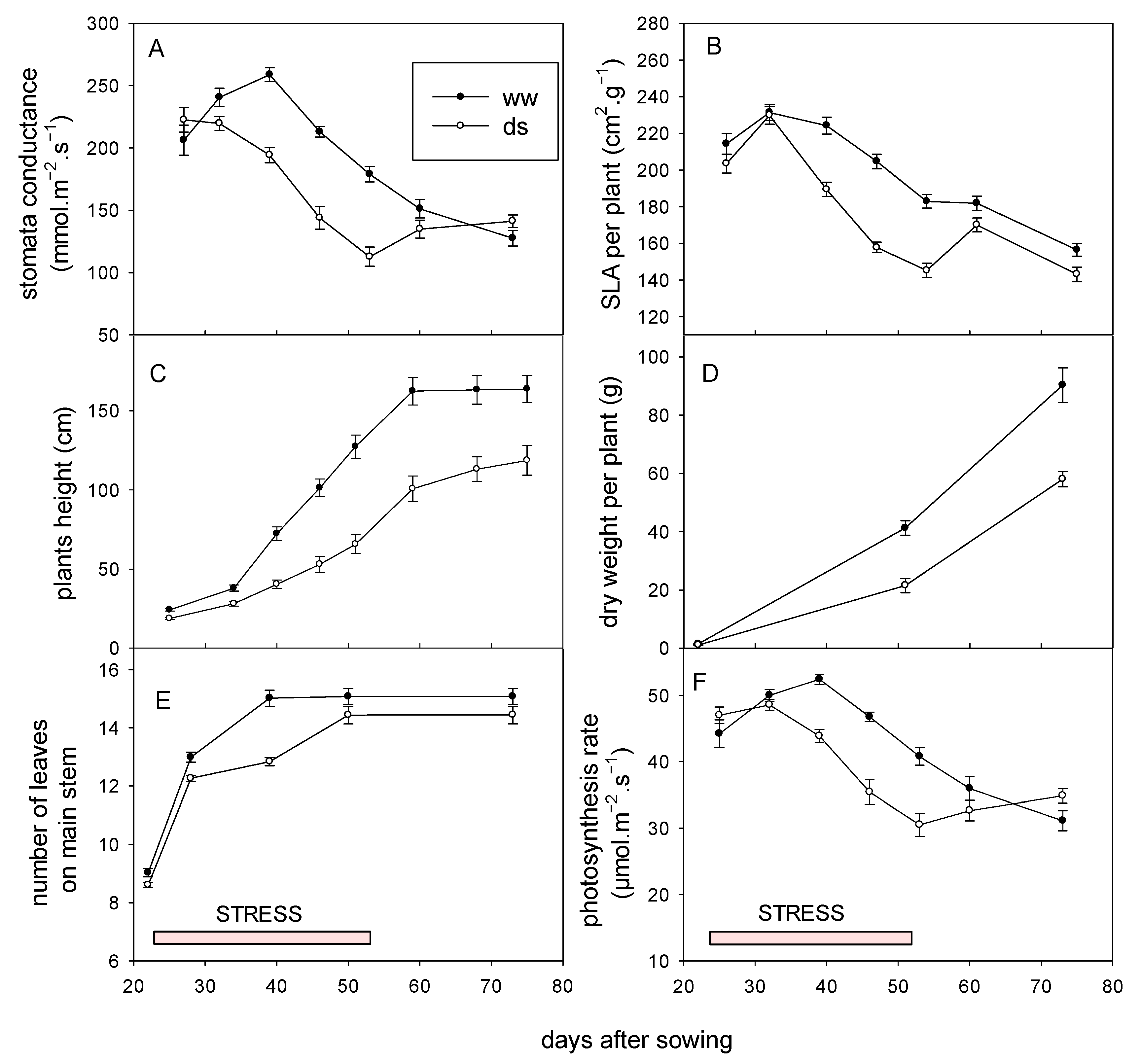
3.3.1. Effect on Growth, Recovery and Yield
3.3.2. Agro Morphological Adaptation of Sorghum to Early Water Deficit
3.3.3. Physiological Adaptation of Sorghum to Early Water Deficit
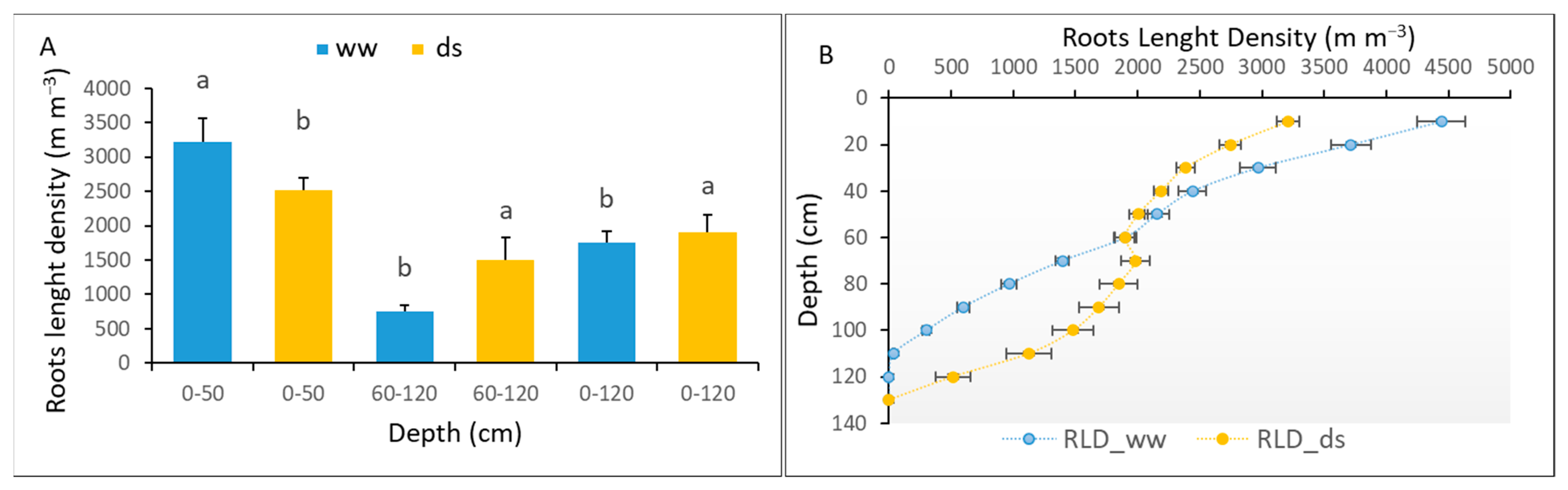
3.3.4. The Root Adaptation of Sorghum to Early Water Deficit
3.3.5. Traits Contribution to Early Drought Tolerance and Promising Varieties
4. Discussion
4.1. The Adaptive Behavior of Varieties
4.2. Interest for Breeding (Criteria to Privilege)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molden, D. Comprehensive assessment of water management in agriculture. In Water for Food Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Earthscan: London, UK, 2007; pp. 1–40. ISBN 9781849773799. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; Macdonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Agrama, H.A.; Tuinstra, M.R. Phylogenetic diversity and relationships among sorghum accessions using SSRs and RAPDs. Afr. J. Biotechnol. 2003, 2, 334–340. [Google Scholar] [CrossRef]
- Hadebe, S.T.; Modi, A.T.; Mabhaudhi, T. Drought Tolerance and Water Use of Cereal Crops: A Focus on Sorghum as a Food Security Crop in Sub-Saharan Africa. J. Agron. Crop. Sci. 2017, 203, 177–191. [Google Scholar] [CrossRef]
- FAOSTAT. Production/Yield Quantities of Sorghum in World (Rome). 2019. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 9 January 2021).
- Chaléard, J.-L. Les réponses de l’agriculture aux défis démo-économiques (The responses of agriculture to demo-economic challenges). Bull. Assoc. Geogr. Fr. 2010, 87, 40–55. [Google Scholar] [CrossRef]
- Sanchez, A.; Subudhi, P.; Rosenow, D.; Nguyen, H. Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol. Biol. 2002, 48, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Direction de l’Analyse de la Prévision et des Statistiques Agricoles (DAPSA). Rapport D’évaluation Préliminaire des Récoltes de la Campagne 2018/2019 et de la Sécurité Alimentaire et de la Nutrition; DAPSA: Dakar, Senegal, 2019. [Google Scholar]
- Salack, S.; Muller, B.; Gaye, A.T.; Hourdin, F.; Cisse, N. Analyses multi-échelles des pauses pluviométriques au Niger et au Sénégal. Sci. Chang. Planetaires Secher. 2012, 23, 3–13. [Google Scholar] [CrossRef]
- Elagib, N.A. Drought risk during the early growing season in Sahelian Sudan. Nat. Hazards 2015, 79, 1549–1566. [Google Scholar] [CrossRef]
- Eggen, M.; Özdoğan, M.; Zaitchik, B.F.; Ademe, D.; Foltz, J.; Simane, B. Vulnerability of sorghum production to extreme, sub-seasonal weather under climate change. Environ. Res. Lett. 2019, 14, 045005. [Google Scholar] [CrossRef]
- Duku, C.; Zwart, S.J.; Hein, L. Impacts of climate change on cropping patterns in a tropical, sub-humid watershed. PLoS ONE 2018, 13, e0192642. [Google Scholar] [CrossRef]
- Tovignan, T.K.; Fonceka, D.; Ndoye, I.; Cisse, N.; Luquet, D. The sowing date and post-flowering water status affect the sugar and grain production of photoperiodic, sweet sorghum through the regulation of sink size and leaf area dynamics. Field Crop. Res. 2016, 192, 67–77. [Google Scholar] [CrossRef]
- Tovignan, T.K.; Adoukonou-Sagbadja, H.; Diatta, C.; Clément-Vidal, A.; Soutiras, A.; Cisse, N.; Luquet, D. Terminal drought effect on sugar partitioning and metabolism is modulated by leaf stay-green and panicle size in the stem of sweet sorghum (Sorghum bicolor L. Moench). CABI Agric. Biosci. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Ghate, T.; Barvkar, V.; Deshpande, S.; Bhargava, S. Role of ABA Signaling in Regulation of Stem Sugar Metabolism and Transport under Post- Flowering Drought Stress in Sweet Sorghum. Plant Mol. Biol. Rep. 2019, 37, 303–313. [Google Scholar] [CrossRef]
- Kamal, N.M.; Gorafi, Y.S.A.; Tsujimoto, H.; Ghanim, A.M.A. Stay-Green QTLs Response in Adaptation to Post-Flowering Drought Depends on the Drought Severity. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Perotti, E.; Ortiz, R. Towards molecular breeding of reproductive traits in cereal crops. Plant Biotechnol. J. 2008, 6, 529–559. [Google Scholar] [CrossRef] [PubMed]
- Craufurd, P.Q.; Peacock, J.M. Effect of Heat and Drought Stress on Sorghum (Sorghum Bicolor) II. Grain Yield. Exp. Agric. 1993, 29, 77–86. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poor, P. Response of Sorghum to Abiotic Stresses: A Review. J. Agron. Crop. Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Boil. 2019, 43, 58–69. [Google Scholar] [CrossRef]
- Radhouane, L.; Aissa, N.; Romdhane, L. Effets d’un stress hydrique appliqué à différents stades de développement sur l’aspect quantitatif et qualitatif des semences chez un écotype autochtone de sorgho grain (Sorghum bicolor). J. Appl. Biosci. 2014, 74, 6149. [Google Scholar] [CrossRef]
- Soundharajan, B.; Sudheer, K. Deficit irrigation management for rice using crop growth simulation model in an optimization framework. Paddy Water Environ. 2009, 7, 135–149. [Google Scholar] [CrossRef]
- Devnarain, N.; Crampton, B.; Chikwamba, R.; Becker, J.; O’Kennedy, M.M. Physiological responses of selected African sorghum landraces to progressive water stress and re-watering. S. Afr. J. Bot. 2016, 103, 61–69. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Photosynthetic response of sweet sorghum to drought and re-watering at different growth stages. Physiol. Plant. 2012, 149, 56–66. [Google Scholar] [CrossRef]
- Perrier, L.; Rouan, L.; Jaffuel, S.; Clément-Vidal, A.; Roques, S.; Soutiras, A.; Baptiste, C.; Bastianelli, D.; Fabre, D.; Dubois, C.; et al. Plasticity of Sorghum Stem Biomass Accumulation in Response to Water Deficit: A Multiscale Analysis from Internode Tissue to Plant Level. Front. Plant Sci. 2017, 8, 1516. [Google Scholar] [CrossRef]
- Taiwo, A.F.; Daramola, O.; Sow, M.; Semwal, V.K. Ecophysiology and Responses of Plants under Drought. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 231–268. [Google Scholar]
- Henry, A. IRRI’s drought stress research in rice with emphasis on roots: Accomplishments over the last 50 years. Plant Root 2013, 7, 92–106. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Masi, C.E.A.; Maranville, J.W. Evaluation of sorghum root branching using fractals. J. Agric. Sci. 1998, 131, 259–265. [Google Scholar] [CrossRef]
- Lynch, J.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef]
- Firezer, G.; Firew, M.; Taye, T.; Temesgen, M.; Kassahun, B. Phenotyping sorghum [Sorghum bicolor (L.) Moench] for drought tolerance with special emphasis to root angle. Afr. J. Agric. Res. 2020, 16, 1213–1222. [Google Scholar] [CrossRef]
- Chopart, J.-L.; Sine, B.; Dao, A.; Muller, B. Root orientation of four sorghum cultivars: Application to estimate root length density from root counts in soil profiles. Plant Root 2008, 2, 67–75. [Google Scholar] [CrossRef][Green Version]
- Gregory, P.J. Roots, rhizosphere and soil: The route to a better understanding of soil science? Eur. J. Soil Sci. 2005, 57, 2–12. [Google Scholar] [CrossRef]
- Faye, A.; Sine, B.; Chopart, J.-L.; Grondin, A.; Lucas, M.; Diedhiou, A.G.; Gantet, P.; Cournac, L.; Min, D.; Audebert, A.; et al. Development of a model estimating root length density from root impacts on a soil profile in pearl millet (Pennisetum glaucum (L.) R. Br). Application to measure root system response to water stress in field conditions. PLoS ONE 2019, 14, e0214182. [Google Scholar] [CrossRef]
- Böhm, W. In situ estimation of root length at natural soil profiles. J. Agric. Sci. 1976, 87, 365–368. [Google Scholar] [CrossRef]
- Tardieu, F. Analysis of the spatial variability of maize root density. Plant Soil 1988, 107, 259–266. [Google Scholar] [CrossRef]
- Zheng, Z.; Hey, S.; Jubery, T.Z.; Liu, H.; Yang, Y.; Coffey, L.; Miao, C.; Sigmon, B.; Schnable, J.C.; Hochholdinger, F.; et al. Shared Genetic Control of Root System Architecture between Zea mays and Sorghum bicolor. Plant Physiol. 2019, 182, 977–991. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Ndiaye, M.; Adam, M.; Muller, B.; Guisse, A.; Cisse, N. Performances agronomiques et stabilité phénotypique de génotypes de Sorgho (Sorghum bicolor (L.) Moench) au Sénégal: Une étude des interactions génotypes-environnement. J. Appl. Biosci. 2018, 125, 12617. [Google Scholar] [CrossRef][Green Version]
- Ndiaye, M.; Adam, M.; Ganyo, K.K.; Guisse, A.; Cisse, N.; Muller, B. Genotype-Environment Interaction: Trade-Offs between the Agronomic Performance and Stability of Dual-Purpose Sorghum (Sorghum bicolor L. Moench) Genotypes in Senegal. Agronomy 2019, 9, 867. [Google Scholar] [CrossRef]
- Joseph, S.B.D.; Boubacar, G.; Michel, V.; Mamoutou, K.; Léonce, L.D.; Mohamed, D.; Niaba, T.; Diaga, D.; Audebert, A. Response of eight sorghum varieties to plant density and nitrogen fertilization in the Sudano-Sahelian zone in Mali. Afr. J. Agric. Res. 2020, 16, 1401–1410. [Google Scholar] [CrossRef]
- Debieu, M.; Sine, B.; Passot, S.; Grondin, A.; Akata, E.; Gangashetty, P.; Vadez, V.; Gantet, P.; Foncéka, D.; Cournac, L.; et al. Response to early drought stress and identification of QTLs controlling biomass production under drought in pearl millet. PLoS ONE 2018, 13, e0201635. [Google Scholar] [CrossRef]
- Sinclair, T.; Ludlow, M. Influence of Soil Water Supply on the Plant Water Balance of Four Tropical Grain Legumes. Funct. Plant Biol. 1986, 13, 329–341. [Google Scholar] [CrossRef]
- Peyrano, G.; Taleisnik, E.; Quiroga, M.; De Forchetti, S.M.; Tigier, H. Salinity effects on hydraulic conductance, lignin content and peroxidase activity in tomato roots. Plant Physiol. Biochem. 1997, 35, 387–393. [Google Scholar]
- Strauss, A.; Krüger, G.; Strasser, R.; Van Heerden, P. Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Environ. Exp. Bot. 2006, 56, 147–157. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Dusserre, J.; Audebert, A.; Radanielson, A.M.; Chopart, J.-L. Towards a simple generic model for upland rice root length density estimation from root intersections on soil profile. Plant Soil 2009, 325, 277–288. [Google Scholar] [CrossRef]
- McIntosh, M.S. Analysis of Combined Experiments 1. Agron. J. 1983, 75, 153–155. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. Lond. Ser. A 1937, 160, 268–282. [Google Scholar] [CrossRef]
- Sebastien, L.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/web/packages/factoextra/index.htmlb (accessed on 18 October 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 November 2020).
- Habyarimana, E.; Laureti, D.; Ninno, M.; Lorenzoni, C. Performances of biomass sorghum [Sorghum bicolor (L.) Moench] under different water regimes in Mediterranean region. Ind. Crop. Prod. 2004, 20, 23–28. [Google Scholar] [CrossRef]
- Hud, A.A.; Abuali, A.I.; Elnahsikh, M.H.; Ahmed, M.I.; Idris, A.E.; Farah, A.M.; Ali, H.A.; Mohamed, B.B.; Babiker, A.E.; Mohamed, E.A. Effect of water stress on vegetative growth, yield and yield components of sweet sorghum (Sorghum bicolor L.) genotypes. Environ. Nat. Resour. Int. J. 2016, 7755, 33–38. [Google Scholar]
- Kholová, J.; Hash, C.T.; Kakkera, A.; Kočová, M.; Vadez, V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. J. Exp. Bot. 2009, 61, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kholová, J.; Hash, C.T.; Kumar, P.L.; Yadav, R.S.; Kočová, M.; Vadez, V. Terminal drought-tolerant pearl millet [Pennisetum glaucum (L.) R. Br.] have high leaf ABA and limit transpiration at high vapour pressure deficit. J. Exp. Bot. 2010, 61, 1431–1440. [Google Scholar] [CrossRef]
- Belko, N.; Zaman-Allah, M.; Cisse, N.; Diop, N.N.; Zombre, G.; Ehlers, J.D.; Vadez, V. Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought-tolerant cowpea. Funct. Plant Biol. 2012, 39, 306–322. [Google Scholar] [CrossRef]
- Verma, R.; Kumar, R.; Nath, A. Drought Resistance Mechanism and Adaptation to Water Stress in Sorghum [Sorghum bicolor (L.) Moench]. Int. J. Bio-Resour. Stress Manag. 2018, 9, 167–172. [Google Scholar] [CrossRef]
- Banks, J.M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Tingting, X.; Peixi, S.; Lishan, S. Photosynthetic characteristics and water use efficiency of sweet sorghum underdifferent watering regimes. Pak. J. Bot. 2010, 42, 3981–3994. [Google Scholar]
- Harris, K.; Subudhi, P.K.; Borrell, A.; Jordan, D.; Rosenow, D.; Nguyen, H.; Klein, P.; Klein, R.; Mullet, J. Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J. Exp. Bot. 2006, 58, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kapanigowda, M.H.; Perumal, R.; Djanaguiraman, M.; Aiken, R.M.; Tesso, T.; Prasad, P.V.V.; Little, C. Genotypic variation in sorghum [Sorghum bicolor (L.) Moench] exotic germplasm collections for drought and disease tolerance. SpringerPlus 2013, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Panneerselvam, R. Growth and photosynthetic pigments responses of two varieties of Catharanthus roseus to triadimefon treatment. Comptes Rendus Biol. 2008, 331, 272–277. [Google Scholar] [CrossRef]
- Shao, H.; Chu, L.-Y.; Jaleel, C.A.; Changxing, Z. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef]
- Araus, J.L.; Tapia, L.; Alegre, L. The Effect of Changing Sowing Date on Leaf Structure and Gas Exchange Characteristics of Wheat Flag Leaves Grown under Mediterranean Climate Conditions. J. Exp. Bot. 1989, 40, 639–646. [Google Scholar] [CrossRef]
- Blanc, E. The Impact of Climate Change on Crop Yields in Sub-Saharan Africa. Am. J. Clim. Chang. 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Vadez, V.; Hash, T.; Bidinger, F.R.; Kholová, J. II.1.5 Phenotyping pearl millet for adaptation to drought. Front. Physiol. 2012, 3, 386. [Google Scholar] [CrossRef] [PubMed]
- Elramlawi, H.R.; Mohammed, H.I.; Elamin, A.W.; Abdallah, O.A.; Taha, A.A. Adaptation of Sorghum (Sorghum bicolor L. Moench) Crop Yield to Climate Change in Eastern Dryland of Sudan. In Handbook of Climate Change Resilience; Springer: Cham, Switzerland, 2019; pp. 2549–2573. [Google Scholar]
- Gregory, P.J. Plant Roots: Growth, Activity and Interactions with the Soil. Clim. Chang. Phys. Sci. Basis 2013, 53, 1–30. [Google Scholar]
- Kothari, K.; Ale, S.; Bordovsky, J.P.; Porter, D.O.; Munster, C.L.; Hoogenboom, G. Potential benefits of genotype-based adaptation strategies for grain sorghum production in the Texas High Plains under climate change. Eur. J. Agron. 2020, 117, 126037. [Google Scholar] [CrossRef]
- Naoura, G.; Sawadogo, N.; Atchozou, E.A.; Emendack, Y.; Hassan, M.A.; Reoungal, D.; Amos, D.N.; Djirabaye, N.; Tabo, R.; Laza, H. Assessment of agro-morphological variability of dry-season sorghum cultivars in Chad as novel sources of drought tolerance. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Pradhan, D.; Shekoofa, A. Inheritance of limited-transpiration trait in peanut: An update. J. Crop. Improv. 2018, 32, 281–286. [Google Scholar] [CrossRef]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Grain yield, harvest index, and water use of wheat. J. Aust. Inst. Agric. Sci. 1977, 43, 117–120. [Google Scholar]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; Van Herwaarden, A.F. Breeding Opportunities for Increasing the Efficiency of Water Use and Crop Yield in Temperate Cereals. Crop. Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef]
- Venuprasad, R.; Lafitte, H.R.; Atlin, G.N. Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crop. Sci. 2007, 47, 285–293. [Google Scholar] [CrossRef]


| Variety | Code | Type | Maturity (Days) | Height (cm) | Potential Yield (t/ha) | Panicle Form | Photoperiod-Sensitivity | Isohyet (mm) | Origin |
|---|---|---|---|---|---|---|---|---|---|
| Fadda | V1 | Guinea (hybrid) | 128 | 200–300 | 4.5 | non compact | moderate | 700–1000 | Mali (IER/ICRISAT) |
| NIELENI | V2 | Guinea (hybrid) | 115 | 300 | 4 | semi compact | low | 700–800 | Mali (IER/ICRISAT) |
| IS15401 | V3 | Guinea-Caudatum | 115 | 400–450 | 2 | semi compact | high | 900–1200 | Mali (IER/ICRISAT) |
| PABLO | V4 | Guinea (hybrid) | 125 | 400 | 4 | non compact | moderate | 700–1000 | Mali (IER/ICRISAT) |
| CSM63E | V5 | Guinea | 90 | 400 | 2 | non compact | low | 600–1000 | Mali (IER/ICRISAT) |
| SK5912 | V6 | Caudatum | 170 | 200 | 2.5–3.5 | semi compact | high | 700–900 | Nigeria |
| GRINKAN | V7 | Caudatum | 90 | 120 | 4 | semi compact | non | 500–800 | Mali (IER/ICRISAT) |
| SOUMBA | V8 | Caudatum | 115 | 250 | 2.5 | semi compact | low | 600–1000 | Mali (IER/ICRISAT) |
| 621B | V9 | Caudatum | 105 | 175 | 2.5–3 | semi compact | non | 600–900 | Senegal (ISRA) |
| F2-20 | V10 | Caudatum | 110 | 210 | 3–5.3 | semi compact | low | 600–900 | Senegal (ISRA) |
| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| Traits | Mean ww | Mean ds | ΔWS | Signif. | Mean ww | Mean ds | ΔWS | Signif. |
| NLA | 15.33 a | 13.92 b | −9.1 | *** | 15.30 a | 14.26 b | −6.7 | *** |
| PHS | 143.89 a | 120.33 b | −16.3 | *** | 130.55 a | 66.59 b | −48.9 | *** |
| DLW | 13.65 b | 19.56 a | 43.2 | *** | 14.22 b | 16.37 a | 15.1 | *** |
| PHT | 174.83 a | 148.58 b | −15.0 | *** | 165.75 a | 127.24 b | −23.2 | *** |
| SDW | 453.38 a | 370.61 b | −18.2 | *** | 427.35 a | 308.60 b | −27.7 | *** |
| Yield | 3271.85 a | 2526.21 b | −22.7 | *** | 2419.84 a | 1738.64 b | −28.1 | *** |
| Pn | 39.32 a | 34.43 b | −12.4 | *** | 41.38 a | 29.90 b | −27.7 | *** |
| C | 184.94 a | 150.97 b | −18.3 | *** | 179.14 a | 115.88 b | −35.3 | *** |
| E | 7.43 a | 5.47 b | −26.3 | *** | 7.04 a | 5.22 b | −25.9 | *** |
| Tleaf | 39.02 b | 39.53 a | 1.2 | ** | 39.16 b | 42.15 a | 7.6 | *** |
| Drought Recovery Index (DRI) | ||||
|---|---|---|---|---|
| Varieties | NLA | PHS | DWP | Pn |
| V1 | −0.04 | −0.54 | −0.32 | −0.22 |
| V2 | −0.10 | −0.43 | −0.60 | −0.24 |
| V3 | −0.12 | −0.33 | −0.75 | −0.08 |
| V4 | −0.13 | −0.81 | −0.98 | 0.07 |
| V5 | −0.10 | −0.68 | −0.64 | 0.35 |
| V6 | −0.09 | −0.80 | −0.97 | −0.16 |
| V7 | −0.13 | −0.55 | −0.93 | −0.05 |
| V8 | −0.11 | −0.77 | −0.57 | 0.12 |
| V9 | −0.12 | −0.62 | −0.44 | −0.18 |
| V10 | −0.12 | −0.69 | −0.32 | 0.00 |
| MEAN | −0.11 | −0.62 | −0.65 | −0.04 |
| NLA | PHS | DLW | PHT | SDW | YIELD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds | ww | ds | ww | ds | ww | ds | |
| Year 2018 | V1 | 14.6 cd | 13.5 bc | 133.7 bc | 111.7 bcd | 5.7 e | 13.9 cd | 172.5 cd | 148.9 cd | 427.6 bc | 358.7 abc | 4183.4 abc | 3182.3 b |
| V2 | 15.3 bc | 13.6 bc | 125.7 bc | 101.6 bcd | 16.8 ab | 28.7 a | 162.8 d | 140.4 cde | 470.2 abc | 349.3 abc | 3715.9 d | 3359.3 b | |
| V3 | 15.5 abc | 13.2 bc | 204.3 a | 179.3 a | 12.6 bcd | 25.8 a | 191.2 bc | 193.6 ab | 498.7 ab | 409.0 abc | 2001.5 e | 1817.6 de | |
| V4 | 15.5 abc | 13.8 bc | 200.6 a | 137.5 b | 10.7 cde | 17.3 bc | 205.6 b | 161.3 bc | 530.0 ab | 449.8 ab | 1606.4 ef | 1540.3 e | |
| V5 | 15.0 bc | 13.5 bc | 186.3 a | 193.5 a | 6.0 e | 16.6 bc | 235.3 a | 215.6 a | 603.2 a | 461.4 a | 1473.1 f | 1168 f | |
| V6 | 16.6 a | 15.9 a | 108.5 cd | 91.2 cd | 7.8 de | 9.9 de | 159.8 d | 133.6 cde | 408.0 bc | 424.4 ab | 3926 abcd | 2598.3 c | |
| V7 | 13.5 d | 12.4 c | 92.5 d | 86.2 cd | 14.2 bc | 19.2 b | 127.2 e | 108.9 e | 315.0 c | 294.5 bc | 3812.1 cd | 2006.9 d | |
| V8 | 16.0 ab | 14.4 ab | 147.5 b | 111.8 bcd | 22.4 a | 26.9 a | 168.1 d | 126.0 de | 496.8 ab | 339.9 abc | 3922 bcd | 3132.8 b | |
| V9 | 15.7 abc | 14.9 ab | 98.4 d | 74.0 d | 18.3 ab | 8.6 e | 152.2 d | 117.1 de | 321.5 c | 259.3 c | 4246.0 ab | 3851.7 a | |
| V10 | 15.1 bc | 13.8 bc | 141.0 b | 116.1 bc | 21.4 a | 28.3 a | 169.3 cd | 140.0 cde | 451.0 abc | 359.3 abc | 4349.3 a | 2643.02 c | |
| Grand mean | 15.3 a | 13.9 b | 143.8 a | 120.3 b | 13.6 b | 19.5 a | 174.8 a | 148.5 b | 453.3 a | 370.6 b | 3271.85 a | 2526.21 b | |
| Anova | |||||||||||||
| V | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | |
| E | *** | *** | *** | *** | *** | *** | |||||||
| V×E | ns | ** | *** | * | Ns | *** | |||||||
| Year 2019 | V1 | 15.7 ab | 13.7 a | 120.4 a | 77.5 a | 13.7 d | 15.1 e | 173.2 cd | 127 abcd | 494.2 bc | 377.3 abc | 3876.8 a | 2424 a |
| V2 | 16.5 a | 14.2 a | 140.6 a | 83.5 a | 14.9 ab | 16 abcd | 149.4 de | 120.1 bcd | 404.5 bcd | 259.0 bc | 2127.7 cd | 2070.3 abc | |
| V3 | 15.0 ab | 13.8 a | 163.1 a | 103.1 a | 11.9 f | 15.7 de | 216.7 ab | 170.4 abc | 329.6 cd | 323.0 abc | 1886.4 d | 1752.5 bc | |
| V4 | 15.2 ab | 14.3 a | 107.3 a | 38.3 a | 15.3 a | 16.1 cd | 204.6 bc | 183.2 ab | 791.2 a | 399.4 ab | 1233.9 e | 1124.5 de | |
| V5 | 15.5 ab | 14.3 a | 154.6 a | 68.5 a | 14.8 ab | 16.3 bcd | 250.8 a | 198.2 a | 564.5 b | 428.4 a | 771.2 f | 817.4 e | |
| V6 | 16.0 a | 15.3 a | 107.1 a | 60.8 a | 12.6 e | 16.1 cd | 137.9 ef | 88.4 d | 413.2 bcd | 274.0 abc | 3429.2 ab | 1582 bcd | |
| V7 | 13.5 b | 14.1 a | 106.5 a | 55.3 a | 14.5 bc | 16 abcd | 109.2 f | 93.4 d | 380.8 bcd | 246.5 c | 3166.7 b | 2027.6 abc | |
| V8 | 15.8 ab | 14.4 a | 124.5 a | 49.0 a | 15.1 ab | 17.2 a | 143 def | 106.1 cd | 330.4 cd | 267.8 abc | 2514.8 c | 2087.6 ab | |
| V9 | 15.1 ab | 14.4 a | 99.4 a | 53.5 a | 15.0 ab | 17.1 ab | 131.4 ef | 101.8 cd | 303.7 d | 272.0 abc | 2105.5 cd | 2037 abc | |
| V10 | 14.3 ab | 13.7 a | 149.4 a | 66.3 a | 14.0 cd | 16.7 abc | 140 def | 102.6 cd | 382.6 bcd | 278.3 abc | 3085.8 b | 1462.9 cd | |
| Grand mean | 15.3a | 14.2b | 130.5a | 66.5 b | 14.2 b | 16.3 a | 165.7 a | 127.2 b | 427.3 a | 308.6 b | 2419.84 a | 1738.64 b | |
| Anova | |||||||||||||
| V | * | ns | ns | ns | *** | *** | *** | *** | *** | ** | *** | *** | |
| E | *** | *** | *** | *** | *** | *** | |||||||
| V×E | ns | ns | *** | * | ** | *** | |||||||
| Both | Y | ns | *** | *** | *** | *** | *** | ||||||
| years | V×Y | * | *** | *** | *** | ** | *** | ||||||
| E×Y | ns | *** | *** | ns | ** | * | |||||||
| V×E×Y | ns | ** | * | ns | ns | *** | |||||||
| Pn | C | E | Tleaf | ||||||
|---|---|---|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds | ww | ds | |
| Year 2018 | V1 | 43.14 a | 31.92 abc | 213.06 a | 119.35 cd | 9.34 a | 5.06 ab | 36.34 cd | 40.70 ab |
| V2 | 41.57 ab | 33.29 abc | 189.14 ab | 101.85 d | 7.00 b | 4.00 b | 34.80 d | 39.12 abc | |
| V3 | 39.88 ab | 39.66 a | 179.04 ab | 169.84 ab | 7.12 b | 6.42 a | 40.30 ab | 39.83 abc | |
| V4 | 38.99 ab | 38.61 a | 169.08 b | 140.07 abcd | 7.38 b | 5.58 ab | 40.77 a | 39.77 abc | |
| V5 | 35.64 b | 26.05 bc | 169.11 b | 128.31 bcd | 7.13 b | 4.45 ab | 39.87 ab | 39.08 bc | |
| V6 | 42.17 ab | 39.83 a | 202.09 ab | 181.85 a | 7.56 ab | 6.16 a | 39.32 ab | 40.94 a | |
| V7 | 40.69 ab | 37.81 a | 194.62 ab | 181.45 a | 6.39 b | 5.89 a | 40.40 ab | 39.54 abc | |
| V8 | 38.16 ab | 38.02 a | 182.93 ab | 183.21 a | 7.68 ab | 6.36 a | 40.83 a | 38.51 c | |
| V9 | 36.05 ab | 35.74 ab | 166.88 b | 166.72 ab | 7.50 ab | 6.53 a | 39.34 ab | 39.02 bc | |
| V10 | 37.70 ab | 22.96 c | 186.72 ab | 147.17 abc | 7.22 b | 4.61 ab | 37.83 bc | 38.75 c | |
| Grand mean | 39.32 a | 34.43 b | 184.94 a | 150.97 b | 7.43 a | 5.47 b | 39.02 b | 39.53 a | |
| Anova | |||||||||
| V | * | *** | ** | *** | ** | *** | *** | ** | |
| E | *** | *** | *** | ** | |||||
| V×E | *** | *** | *** | *** | |||||
| Year 2019 | V1 | 47.03 a | 22.66 c | 193.03 a | 78.41 c | 7.28 ab | 4.00 b | 38.75 b | 42.86 ab |
| V2 | 42.37 abc | 29.28 abc | 189.03 a | 108.90 bc | 7.25 ab | 5.17 ab | 39.98 a | 42.72 ab | |
| V3 | 41.56 abc | 35.05 ab | 191.45 a | 140.03 ab | 6.70 abc | 5.73 ab | 40.44 a | 41.68 bc | |
| V4 | 37.76 bc | 34.51 ab | 137.08 b | 135.79 ab | 6.12 c | 5.40 ab | 38.30 bc | 43.11 a | |
| V5 | 41.08 abc | 27.06 abc | 167.77 ab | 96.88 bc | 7.24 ab | 4.96 ab | 39.94 a | 40.73 c | |
| V6 | 38.79 abc | 26.58 abc | 165.52 ab | 94.97 bc | 6.89 abc | 5.23 ab | 40.50 a | 42.12 ab | |
| V7 | 43.72 ab | 35.59 ab | 194.77 a | 177.36 a | 7.65 a | 6.18 a | 37.36 c | 42.34 ab | |
| V8 | 34.15 c | 27.81 abc | 164.44 ab | 98.39 bc | 6.56 bc | 4.78 ab | 40.00 a | 40.62 c | |
| V9 | 43.24 ab | 25.57 bc | 190.44 a | 93.53 bc | 7.14 abc | 4.64 ab | 37.63 bc | 43.27 a | |
| V10 | 44.10 ab | 36.41 a | 197.92 a | 134.17 ab | 7.61 a | 6.11 a | 38.67 b | 42.06 ab | |
| Grand mean | 41.38 a | 29.90 b | 179.14 a | 115.88 b | 7.04 a | 5.22 b | 39.16 b | 42.15 a | |
| Anova | |||||||||
| V | ** | *** | *** | *** | *** | * | *** | *** | |
| E | *** | *** | *** | *** | |||||
| V×E | *** | *** | ** | *** | |||||
| Both | Y | ns | *** | ** | *** | ||||
| V×Y | *** | *** | *** | * | |||||
| years | E×Y | *** | *** | ns | *** | ||||
| V×E×Y | *** | *** | ** | ** | |||||
| NTR | RLD [0–120 cm] | RLD [0–50 cm] | RLD [60–120 cm] | |||||
|---|---|---|---|---|---|---|---|---|
| V | ww | ds | ww | ds | ww | ds | ww | ds |
| V1 | 63.00 a | 28.66 ab | 1922.80 a | 1687.83 g | 3457.35 c | 2764.00 a | 800.02 c | 1578.33 c |
| V2 | 32.00 f | 22.33 cd | 1760.68 bc | 2041.48 c | 3286.61 e | 2698.90 b | 654.07 f | 1571.89 c |
| V3 | 40.33 c | 26.00 bc | 1751.29 bc | 1769.92 f | 3358.45 d | 2435.41 f | 648.08 f | 1294.57 e |
| V4 | 50.33 b | 31.66 a | 1467.24 e | 1574.29 h | 2519.95 h | 2236.74 g | 715.29 e | 1090.53 f |
| V5 | 62.66 a | 28.66 ab | 1781.89 bc | 1945.37 e | 3245.57 e | 2641.44 c | 719.73 e | 1448.18 d |
| V6 | 40.00 cd | 28.66 ab | 1739.46 bc | 1993.00 d | 3564.50 b | 2520.00 e | 873.33 a | 1421.00 d |
| V7 | 36.00 e | 25.33 bc | 1827.72 b | 2190.90 b | 3311.3 de | 2598.55 d | 772.75 cd | 1807.34 b |
| V8 | 42.00 c | 30.66 a | 1991.88 a | 2453.95 a | 3659.27 a | 2704.23 b | 800.89 c | 2266.67 a |
| V9 | 37.00 de | 25.00 bcd | 1625.93 d | 1687.83 g | 2738.30 g | 2271.24 g | 831.38 b | 1271.11 e |
| V10 | 37.00 de | 21.33 d | 1696.45 cd | 1757.95 f | 3121.66 f | 2428.02 f | 763.37 d | 1279.32 e |
| Grand mean | 44.03 a | 26.83 b | 1758.61 b | 1897.27 a | 3214.64 a | 2522.88 b | 757.89 b | 1502.8 a |
| Anova | ||||||||
| V | *** | *** | *** | *** | *** | *** | *** | *** |
| E | *** | *** | *** | *** | ||||
| V×E | *** | *** | *** | *** | ||||
| Traits | Clusters | Stage | Phenotyping | Interest for Breeding |
|---|---|---|---|---|
| Roots Length Density | 1,3,4 | Growth | Metric | Related yield set up |
| Soil water stock | - | Growth | Metric | Related yield set up |
| Plant height | 3,1,2 | Growth, maturity | Metric | Biomass increasing |
| Dead leaves weight | 2,1 | Growth | Metric | Related yield set up |
| Photosynthesis rate | 1,3,4 | Growth | Metric | Related yield set up |
| Transpiration rate | 1,3,4 | Growth | Metric | Related yield set up |
| Grain weight (yield) | 2,4,3 | Maturity | Metric | Yield increasing |
| Straw dry weight | 3,1,2,3 | Growth, maturity | Metric | Biomass increasing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gano, B.; Dembele, J.S.B.; Tovignan, T.K.; Sine, B.; Vadez, V.; Diouf, D.; Audebert, A. Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy 2021, 11, 443. https://doi.org/10.3390/agronomy11030443
Gano B, Dembele JSB, Tovignan TK, Sine B, Vadez V, Diouf D, Audebert A. Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy. 2021; 11(3):443. https://doi.org/10.3390/agronomy11030443
Chicago/Turabian StyleGano, Boubacar, Joseph Sékou B. Dembele, Thierry Klanvi Tovignan, Bassirou Sine, Vincent Vadez, Diaga Diouf, and Alain Audebert. 2021. "Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties" Agronomy 11, no. 3: 443. https://doi.org/10.3390/agronomy11030443
APA StyleGano, B., Dembele, J. S. B., Tovignan, T. K., Sine, B., Vadez, V., Diouf, D., & Audebert, A. (2021). Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy, 11(3), 443. https://doi.org/10.3390/agronomy11030443









