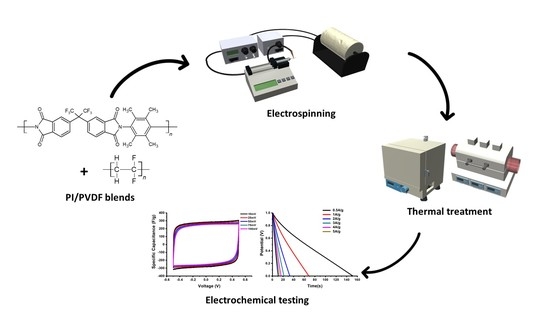
Preparation of Porous Carbon Nanofiber Electrodes Derived from 6FDA-Durene/PVDF Blends and Their Electrochemical Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
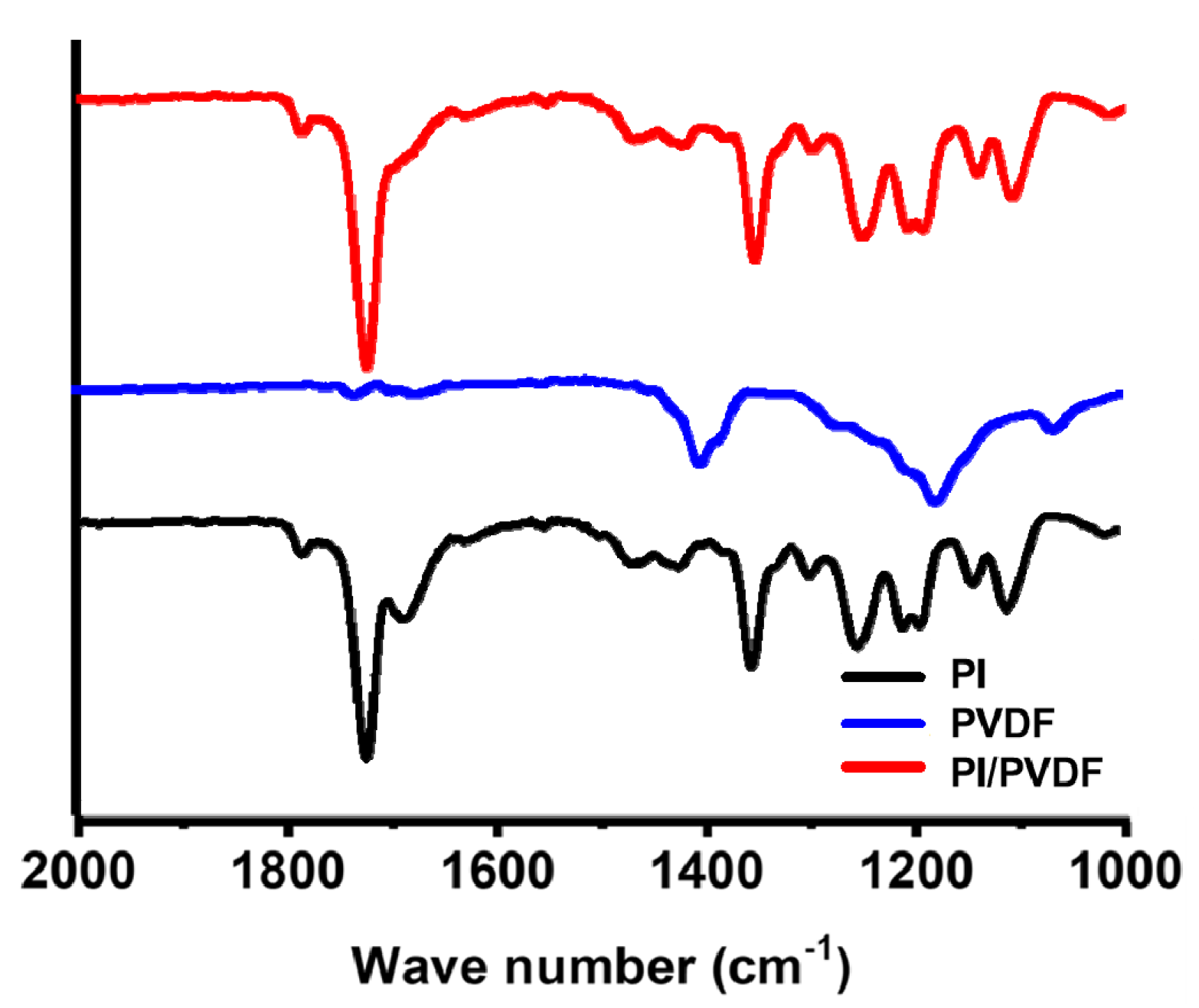
3.1. Characterization of CNFs Derived from 6FDA–Durene/PVDF
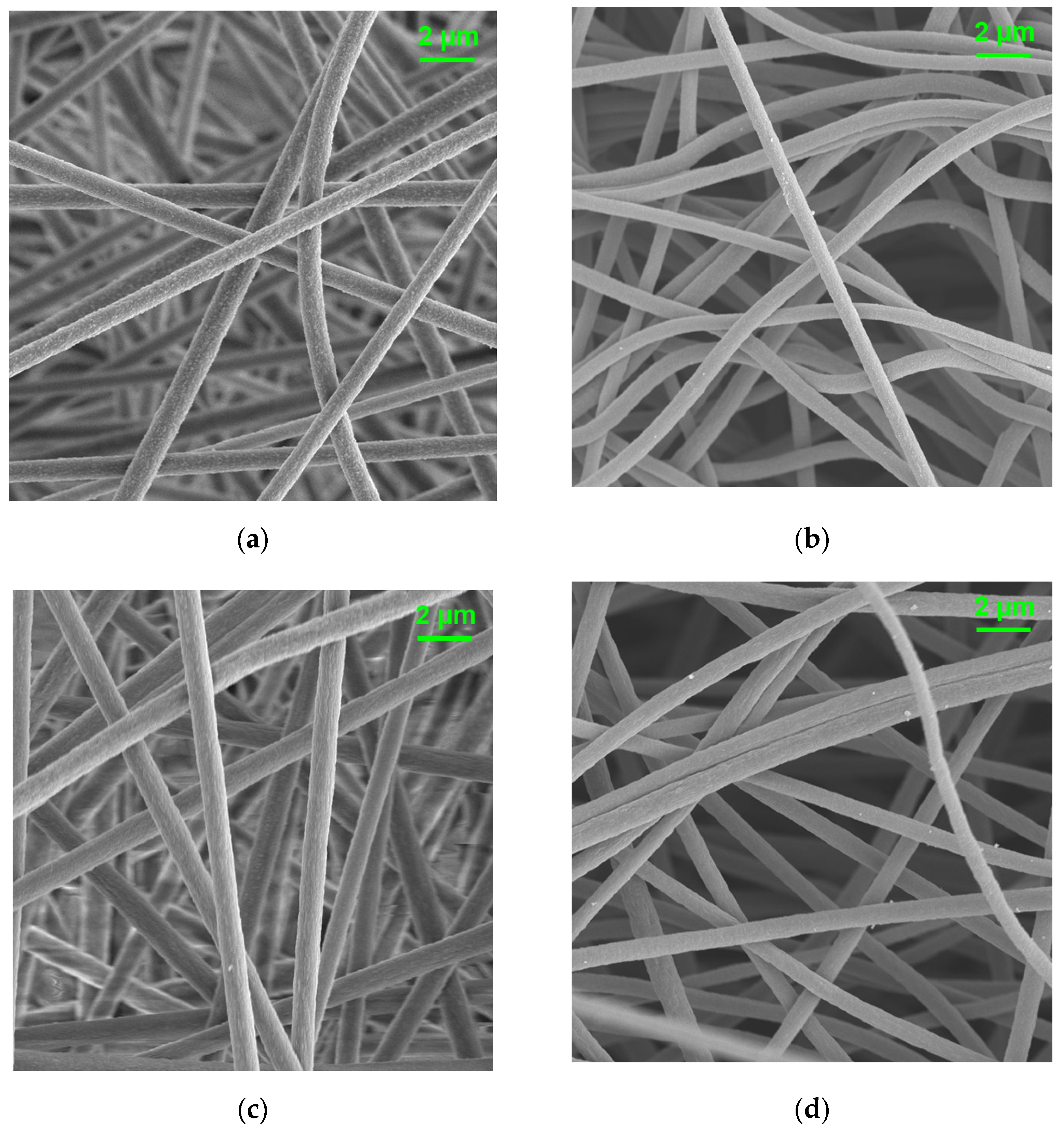
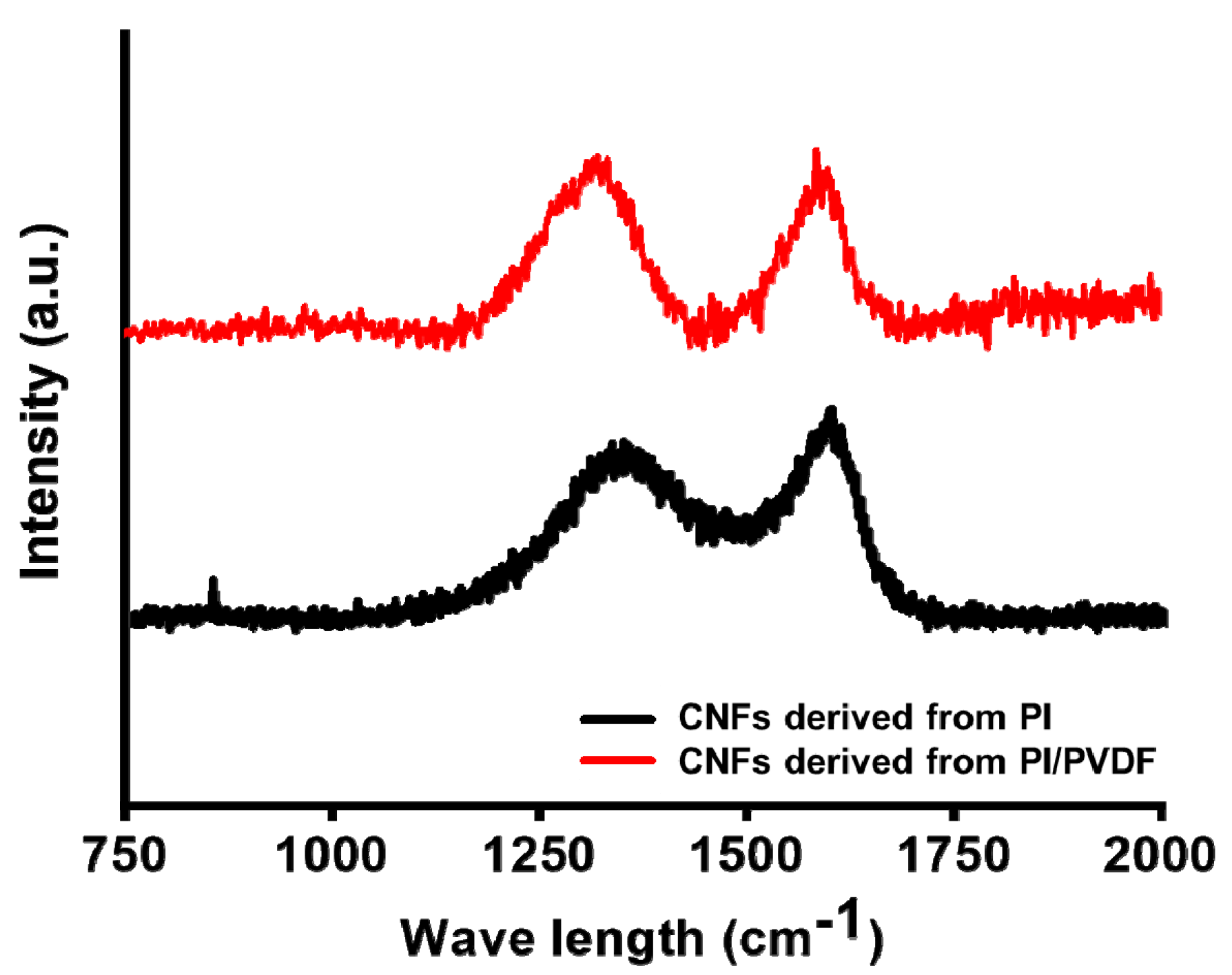
3.2. Surface Properties of CNFs Derived from 6FDA–Durene/PVDF
3.3. Electrochemical Performance of CNFs Derived from 6FDA–Durene/PVDF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun nanofiber-based anodes, cathodes, and separators for advanced lithium-ion batteries. Polym. Rev. 2011, 51, 239–264. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatti, T. A review on electrochemical double-layer capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef]
- De, B.; Banerjee, S.; Verma, K.D.; Pal, T.; Manna, P.; Kar, K.K. Carbon Nanofiber as Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Simitzis, J.; Soulis, S.K. Polyacrylonitrile. In Handbook of Thermoplastics; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Minus, M.L.; Kumar, S. Carbon Fibers. In Kirk-Othmer Encyclopedia of Chemical Technology; Interscience: Geneva, Switzerland, 2000. [Google Scholar]
- Kim, C.; Yang, K. Electrochemical properties of carbon nanofiber web as an electrode for supercapacitor prepared by electrospinning. Appl. Phys. Lett. 2003, 83, 1216–1218. [Google Scholar] [CrossRef]
- Kim, C. Electrochemical characterization of electrospun activated carbon nanofibres as an electrode in supercapacitors. J. Power Sources 2005, 142, 382–388. [Google Scholar] [CrossRef]
- Jung, K.-H.; Panapitiya, N.; Ferraris, J.P. Electrochemical energy storage performance of carbon nanofiber electrodes derived from 6FDA-durene. Nanotechnology 2018, 29, 275701. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Ha, T.; Lee, D.Y.; Choi, M.-S.; Lee, S.W.; Jung, K.-H. Preparation and Electrochemical Properties of Porous Carbon Nanofiber Electrodes Derived from New Precursor Polymer: 6FDA-TFMB. Polymers 2020, 12, 1851. [Google Scholar] [CrossRef]
- Hong, S.-M.; Kim, S.H.; Jeong, B.G.; Jo, S.M.; Lee, K.B. Development of porous carbon nanofibers from electrospun polyvinylidene fluoride for CO 2 capture. RSC Adv. 2014, 4, 58956–58963. [Google Scholar] [CrossRef]
- Yang, Y.; Centrone, A.; Chen, L.; Simeon, F.; Hatton, T.A.; Rutledge, G.C. Highly porous electrospun polyvinylidene fluoride (PVDF)-based carbon fiber. Carbon 2011, 49, 3395–3403. [Google Scholar] [CrossRef]
- Hong, S.E.; Kim, D.-K.; Jo, S.M.; Kim, D.Y.; Chin, B.D. Graphite nanofibers prepared from catalytic graphitization of electrospun poly (vinylidene fluoride) nanofibers and their hydrogen storage capacity. Catal. Today 2007, 120, 413–419. [Google Scholar] [CrossRef]
- Jung, K.-H.; Deng, W.; Smith Jr, D.W.; Ferraris, J.P. Carbon nanofiber electrodes for supercapacitors derived from new precursor polymer: Poly (acrylonitrile-co-vinylimidazole). Electrochem. Commun. 2012, 23, 149–152. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Zhang, X.; Huo, L.; Liang, J.; Wu, L.; Liu, Y.; Gao, J.; Pang, H.; Xue, H. Copolymer derived micro/meso-porous carbon nanofibers with vacancy-type defects for high-performance supercapacitors. J. Mater. Chem. A 2020, 8, 2463–2471. [Google Scholar] [CrossRef]
- Abeykoon, N.C.; Mahmood, S.F.; Yang, D.J.; Ferraris, J.P. Electrospun poly (acrylonitrile-co-itaconic acid) as a porous carbon precursor for high performance supercapacitor: Study of the porosity induced by in situ porogen activity of itaconic acid. Nanotechnology 2019, 30, 435401. [Google Scholar] [CrossRef]
- Abeykoon, N.C.; Bonso, J.S.; Ferraris, J.P. Supercapacitor performance of carbon nanofiber electrodes derived from immiscible PAN/PMMA polymer blends. RSC Adv. 2015, 5, 19865–19873. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 2009, 20, 155705. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Ferraris, J.P. Preparation of porous carbon nanofibers derived from PBI/PLLA for supercapacitor electrodes. Nanotechnology 2016, 27, 425708. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, J.; Xie, Z.; Wang, X.; Lin, T. Preparation, structure and supercapacitance of bonded carbon nanofiber electrode materials. Carbon 2011, 49, 2380–2388. [Google Scholar] [CrossRef]
- Jung, K.-H.; Ferraris, J.P. Preparation and electrochemical properties of carbon nanofibers derived from polybenzimidazole/polyimide precursor blends. Carbon 2012, 50, 5309–5315. [Google Scholar] [CrossRef]
- Abeykoon, N.C.; Garcia, V.; Jayawickramage, R.A.; Perera, W.; Cure, J.; Chabal, Y.J.; Balkus, K.J.; Ferraris, J.P. Novel binder-free electrode materials for supercapacitors utilizing high surface area carbon nanofibers derived from immiscible polymer blends of PBI/6FDA-DAM: DABA. RSC Adv. 2017, 7, 20947–20959. [Google Scholar] [CrossRef]
- Fu, S.; Sanders, E.S.; Kulkarni, S.S.; Koros, W.J. Carbon molecular sieve membrane structure–property relationships for four novel 6FDA based polyimide precursors. J. Membr. Sci. 2015, 487, 60–73. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Solid polymer electrolyte based on PVDF-HFP and ionic liquid embedded with TiO2 nanoparticle for electric double layer capacitor (EDLC) application. J. Electrochem. Soc. 2017, 164, F1348. [Google Scholar] [CrossRef]
- Senthil, R.; Theerthagiri, J.; Madhavan, J.; Murugan, K.; Arunachalam, P.; Arof, A. Enhanced performance of dye-sensitized solar cells based on organic dopant incorporated PVDF-HFP/PEO polymer blend electrolyte with g-C3N4/TiO2 photoanode. J. Solid State Chem. 2016, 242, 199–206. [Google Scholar] [CrossRef]
- Jawaid, M.; Khan, M.M. Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Khasim, S.; Pasha, A.; Badi, N.; Lakshmi, M.; Mishra, Y.K. High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices. RSC Adv. 2020, 10, 10526–10539. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liu, P.; Baker, G.L. Sulfonated polyimide and PVDF based blend proton exchange membranes for fuel cell applications. J. Mater. Chem. A 2015, 3, 3847–3853. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Ma, Y.; Yang, W. Improved performance of lithium ion battery separator enabled by co-electrospinnig polyimide/poly (vinylidene fluoride-co-hexafluoropropylene) and the incorporation of TiO2-(2-hydroxyethyl methacrylate). J. Power Sources 2015, 273, 1127–1135. [Google Scholar] [CrossRef]
- Rajati, H.; Navarchian, A.H.; Tangestaninejad, S. Preparation and characterization of mixed matrix membranes based on Matrimid/PVDF blend and MIL-101 (Cr) as filler for CO2/CH4 separation. Chem. Eng. Sci. 2018, 185, 92–104. [Google Scholar] [CrossRef]
- Lin, S.-C.; Coates, M.; Pearce, E.M.; Huang, P.-T. Compatible Blends of Polyvinylidene Fluoride and Aromatic Polyimide. U.S. Patent US09/410,796, 6 November 2001. [Google Scholar]
- Kanehashi, S.; Nakagawa, T.; Nagai, K.; Duthie, X.; Kentish, S.; Stevens, G. Effects of carbon dioxide-induced plasticization on the gas transport properties of glassy polyimide membranes. J. Membr. Sci. 2007, 298, 147–155. [Google Scholar] [CrossRef]
- Mohamadi, S. Preparation and characterization of PVDF/PMMA/graphene polymer blend nanocomposites by using ATR-FTIR technique. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 1. [Google Scholar] [CrossRef]
- Inagaki, M.; Ohta, N.; Hishiyama, Y. Aromatic polyimides as carbon precursors. Carbon 2013, 61, 1–21. [Google Scholar] [CrossRef]
- Liao, C.; Zhao, J.; Yu, P.; Tong, H.; Luo, Y. Synthesis and characterization of SBA-15/poly (vinylidene fluoride)(PVDF) hybrid membrane. Desalination 2010, 260, 147–152. [Google Scholar] [CrossRef]
- Ouyang, Z.-W.; Chen, E.-C.; Wu, T.-M. Thermal stability and magnetic properties of polyvinylidene fluoride/magnetite nanocomposites. Materials 2015, 8, 4553–4564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Serrano, S.; Santiago-Avilés, J.J. Raman characterization of carbon nanofibers prepared using electrospinning. Synth. Met. 2003, 138, 423–427. [Google Scholar] [CrossRef]
- Ohta, N.; Nishi, Y.; Morishita, T.; Tojo, T.; Inagaki, M. Preparation of microporous carbon films from fluorinated aromatic polyimides. Carbon 2008, 46, 1350–1357. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Ku, B.-C.; Kim, J.; Joh, H.-I. Structural evolution of polyacrylonitrile fibers in stabilization and carbonization. Adv. Chem. Eng. Sci. 2012, 2, 275–282. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Kim, S.J.; Son, Y.J.; Jeon, B.; Han, Y.S.; Kim, Y.-J.; Jung, K.-H. Surface crosslinking of 6FDA-durene nanofibers for porous carbon nanofiber electrodes in electrochemical double layer capacitors. Nanotechnology 2020, 31, 215404. [Google Scholar] [CrossRef]
- Hashemi, M.; Rahmanifar, M.S.; El-Kady, M.F.; Noori, A.; Mousavi, M.F.; Kaner, R.B. The use of an electrocatalytic redox electrolyte for pushing the energy density boundary of a flexible polyaniline electrode to a new limit. Nano Energy 2018, 44, 489–498. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Jayawickramage, R.A.P.; Balkus, K.J.; Ferraris, J.P. Binder free carbon nanofiber electrodes derived from polyacrylonitrile-lignin blends for high performance supercapacitors. Nanotechnology 2019, 30, 355402. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, J.; Fan, Q.; Guo, J.; Liu, W.; Cao, E.; Shi, J.; Song, Y. Preparation and one-step activation of nanoporous ultrafine carbon fibers derived from polyacrylonitrile/cellulose blend for used as supercapacitor electrode. J. Mater. Sci. 2018, 53, 4527–4539. [Google Scholar] [CrossRef]
- Bard, A.J.; Abruna, H.D.; Chidsey, C.E.; Faulkner, L.R.; Feldberg, S.W.; Itaya, K.; Majda, M.; Melroy, O.; Murray, R.W. The electrode/electrolyte interface—A status report. J. Phys. Chem. 1993, 97, 7147–7173. [Google Scholar] [CrossRef]
- Pell, W.G.; Conway, B.E.; Adams, W.A.; de Oliveira, J. Electrochemical efficiency in multiple discharge recharge cycling of supercapacitors in hybrid EV applications. J. Power Sources 1999, 80, 134–141. [Google Scholar] [CrossRef]
- Eskusson, J.; Jänes, A.; Kikas, A.; Matisen, L.; Lust, E. Physical and electrochemical characteristics of supercapacitors based on carbide derived carbon electrodes in aqueous electrolytes. J. Power Sources 2011, 196, 4109–4116. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Karuppasamy, K.; Arunachalam, P.; Elakkiya, V.; Kuppusami, P.; Maiyalagan, T.; Kim, H.-S. Recent advances in 2-D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors–A brief review. J. Ind. Eng. Chem. 2018, 67, 12–27. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Li, Y.; Jiao, L.; Chen, J. Intercalation pseudocapacitance in flexible and self-standing V2O3 porous nanofibers for high-rate and ultra-stable K ion storage. Nano Energy 2018, 50, 462–467. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, D.; Gu, L.; Liu, B.; Guo, F.; Ren, Y.; Hao, S. Support-induced morphology and content tailored NiCo2O4 nanostructures on temperature-dependent carbon nanofibers with enhanced pseudocapacitive performance. Electrochim. Acta 2018, 286, 1–13. [Google Scholar] [CrossRef]
- Yi, C.-q.; Zou, J.-p.; Yang, H.-z.; Xian, L. Recent advances in pseudocapacitor electrode materials: Transition metal oxides and nitrides. Trans. Nonferr. Met. Soc. China 2018, 28, 1980–2001. [Google Scholar] [CrossRef]

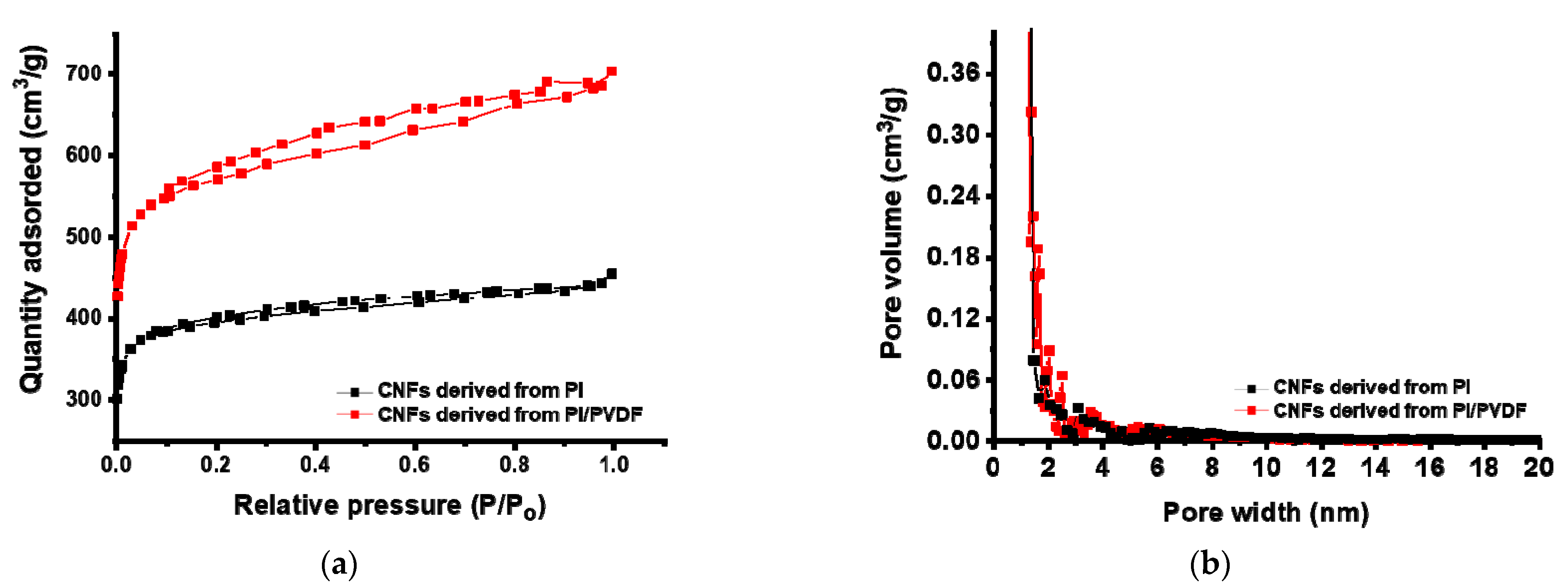

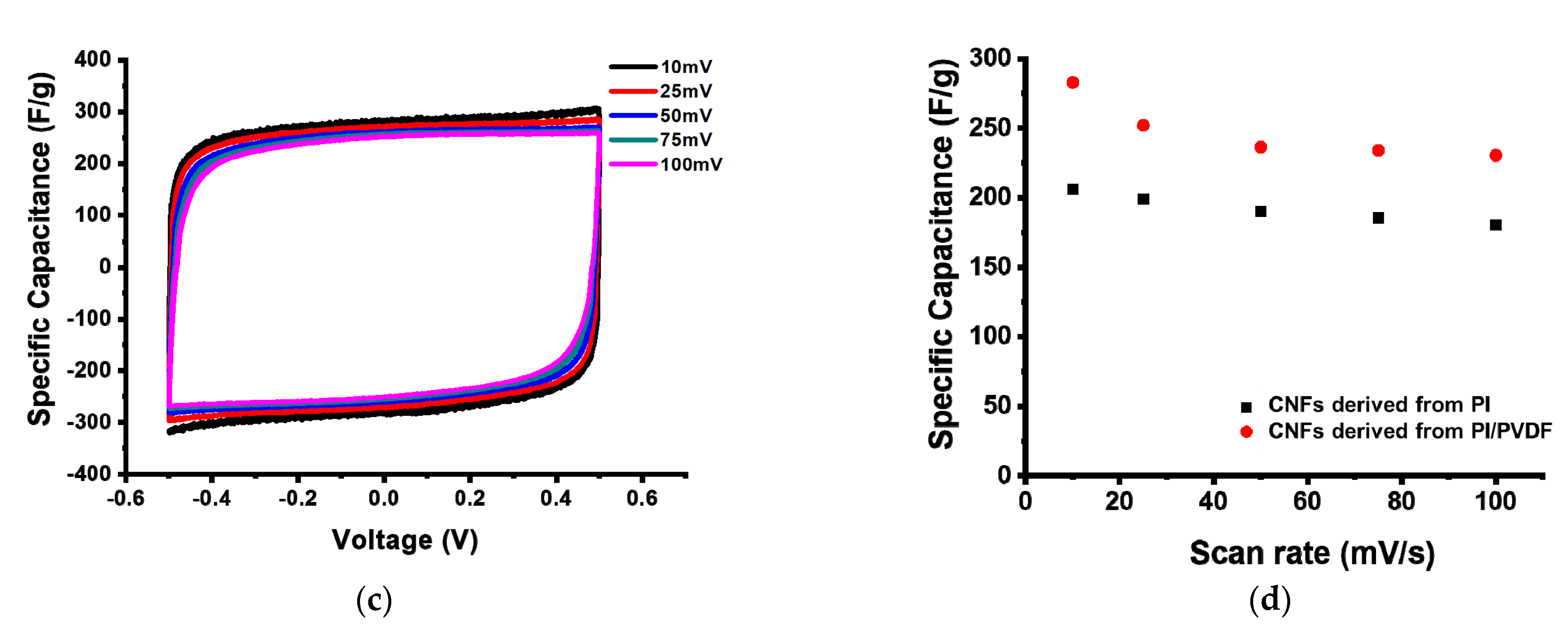
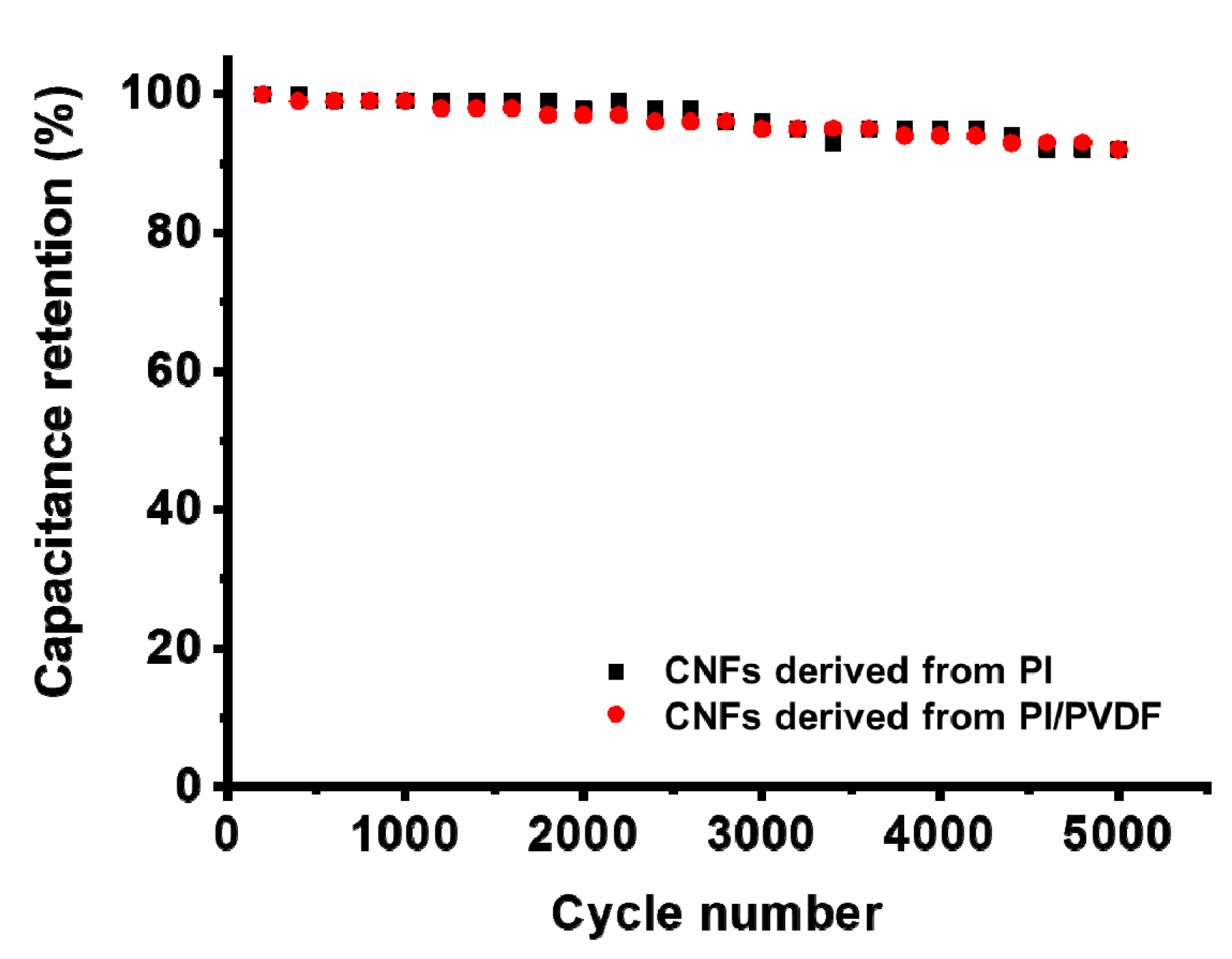

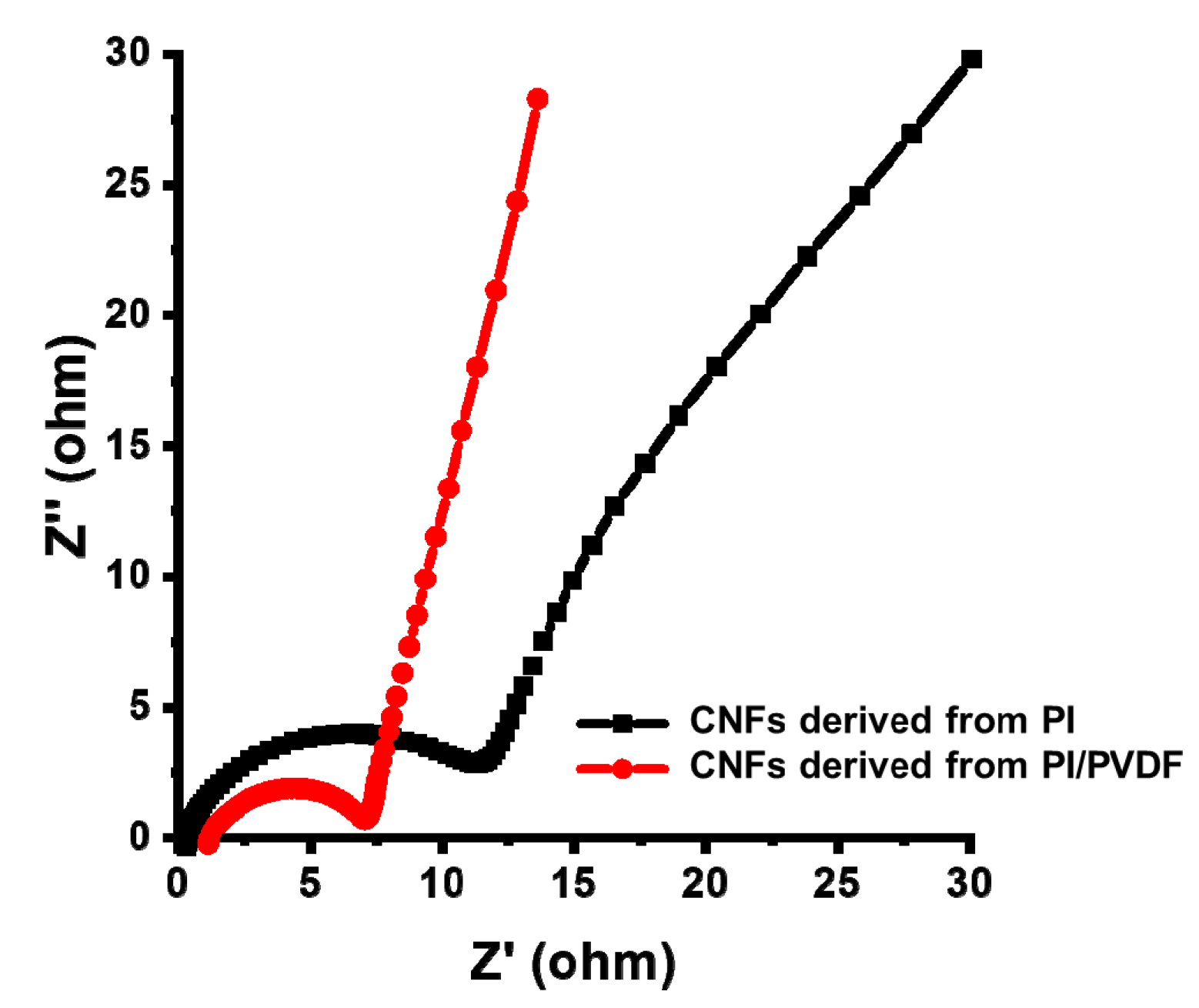
| SSA 1 (m2/g) | TPV 2 (cm3/g) | Vmicro 3 (m3/g) | Vmeso 4 (m3/g) | |
|---|---|---|---|---|
| PI | 1210 | 0.580 | 0.496 | 0.084 |
| PI/PVDF | 1559 | 0.684 | 0.565 | 0.119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.G.; Lee, B.C.; Jung, K.-H. Preparation of Porous Carbon Nanofiber Electrodes Derived from 6FDA-Durene/PVDF Blends and Their Electrochemical Properties. Polymers 2021, 13, 720. https://doi.org/10.3390/polym13050720
Lee DG, Lee BC, Jung K-H. Preparation of Porous Carbon Nanofiber Electrodes Derived from 6FDA-Durene/PVDF Blends and Their Electrochemical Properties. Polymers. 2021; 13(5):720. https://doi.org/10.3390/polym13050720
Chicago/Turabian StyleLee, Do Geun, Byeong Chul Lee, and Kyung-Hye Jung. 2021. "Preparation of Porous Carbon Nanofiber Electrodes Derived from 6FDA-Durene/PVDF Blends and Their Electrochemical Properties" Polymers 13, no. 5: 720. https://doi.org/10.3390/polym13050720
APA StyleLee, D. G., Lee, B. C., & Jung, K.-H. (2021). Preparation of Porous Carbon Nanofiber Electrodes Derived from 6FDA-Durene/PVDF Blends and Their Electrochemical Properties. Polymers, 13(5), 720. https://doi.org/10.3390/polym13050720