K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains, Plasmids and Growth Conditions
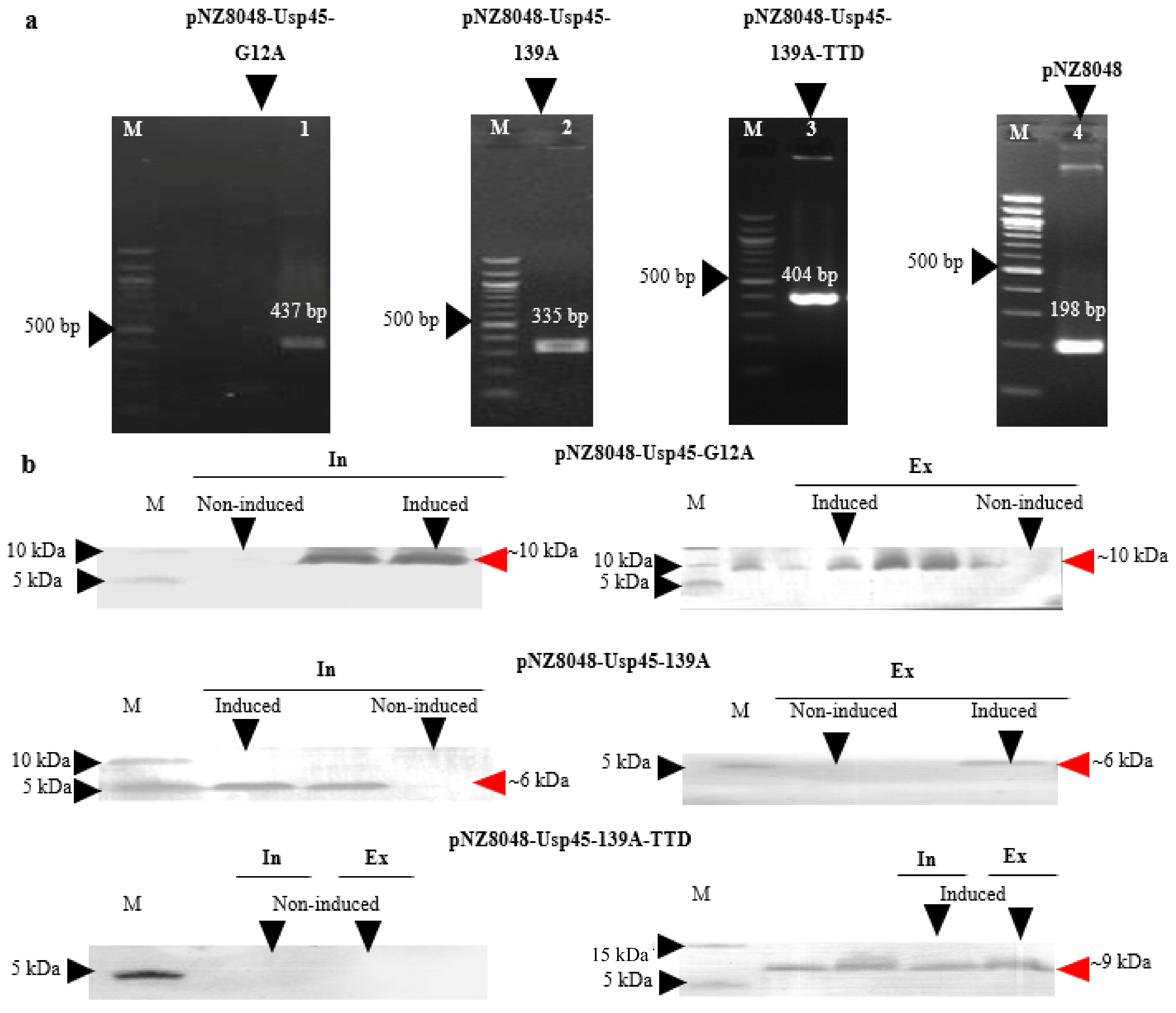
2.2. Cloning and Expression of Recombinant L. lactis
2.3. Protein Extraction and Western Blotting
2.4. Ni-NTA-HRP ELISA Specific Quantification of K-Ras Mimotope
2.5. Mice Strains
2.6. Oral Immunization
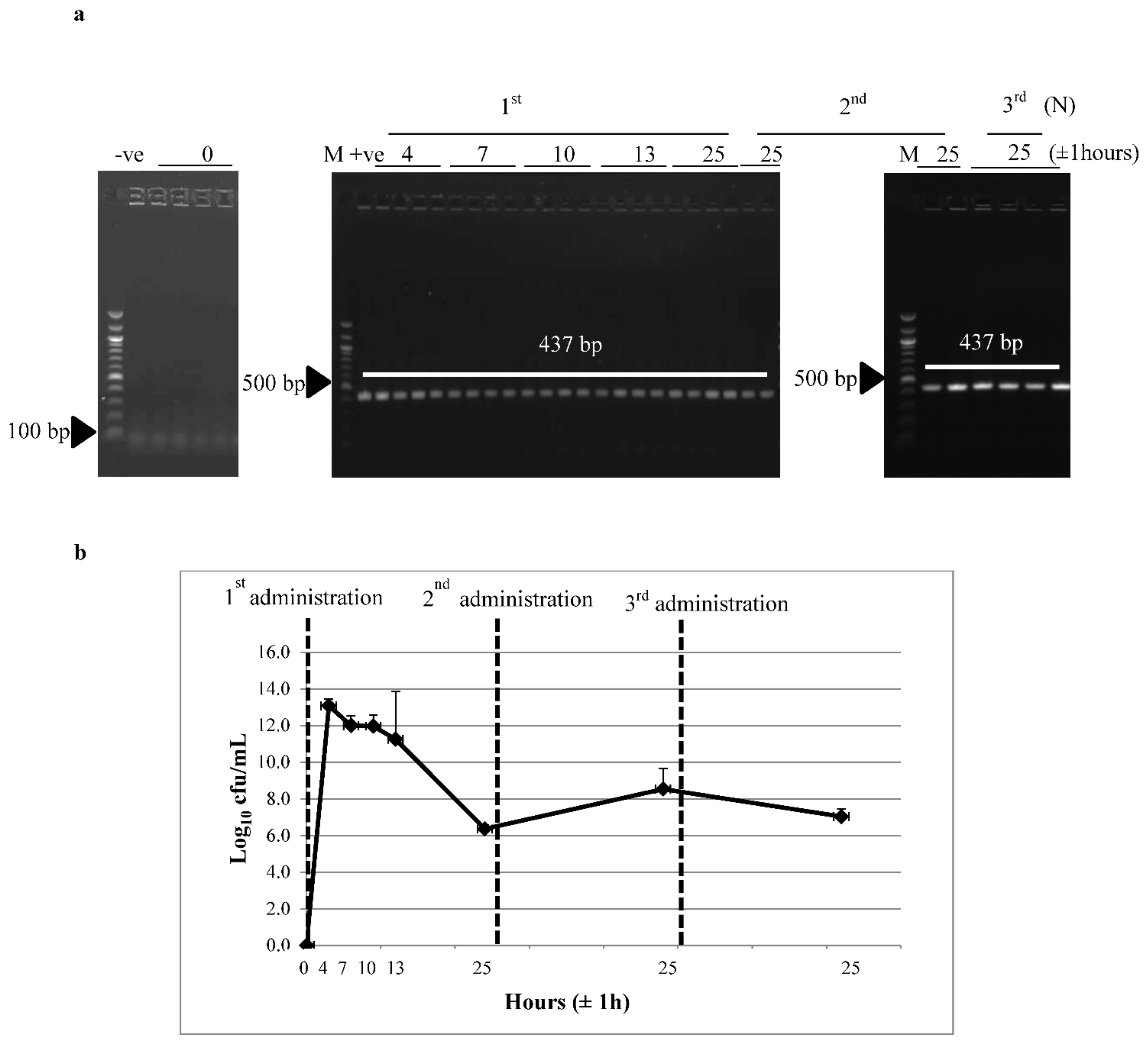
2.7. Detection of Recombinant L. lactis from Mice Feces
2.8. Isolation of Blood, Sera, Intestinal Wash Samples and Splenocytes
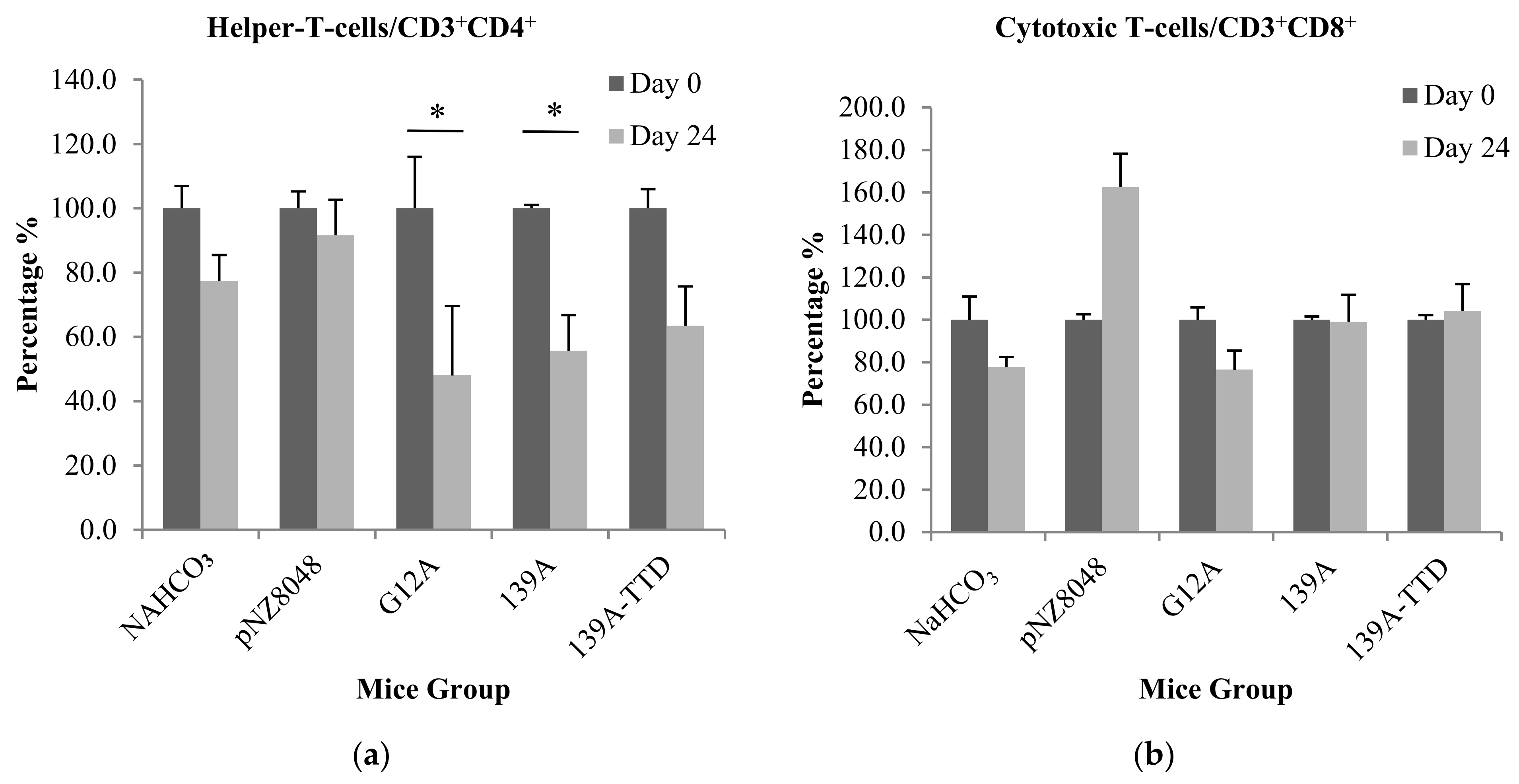
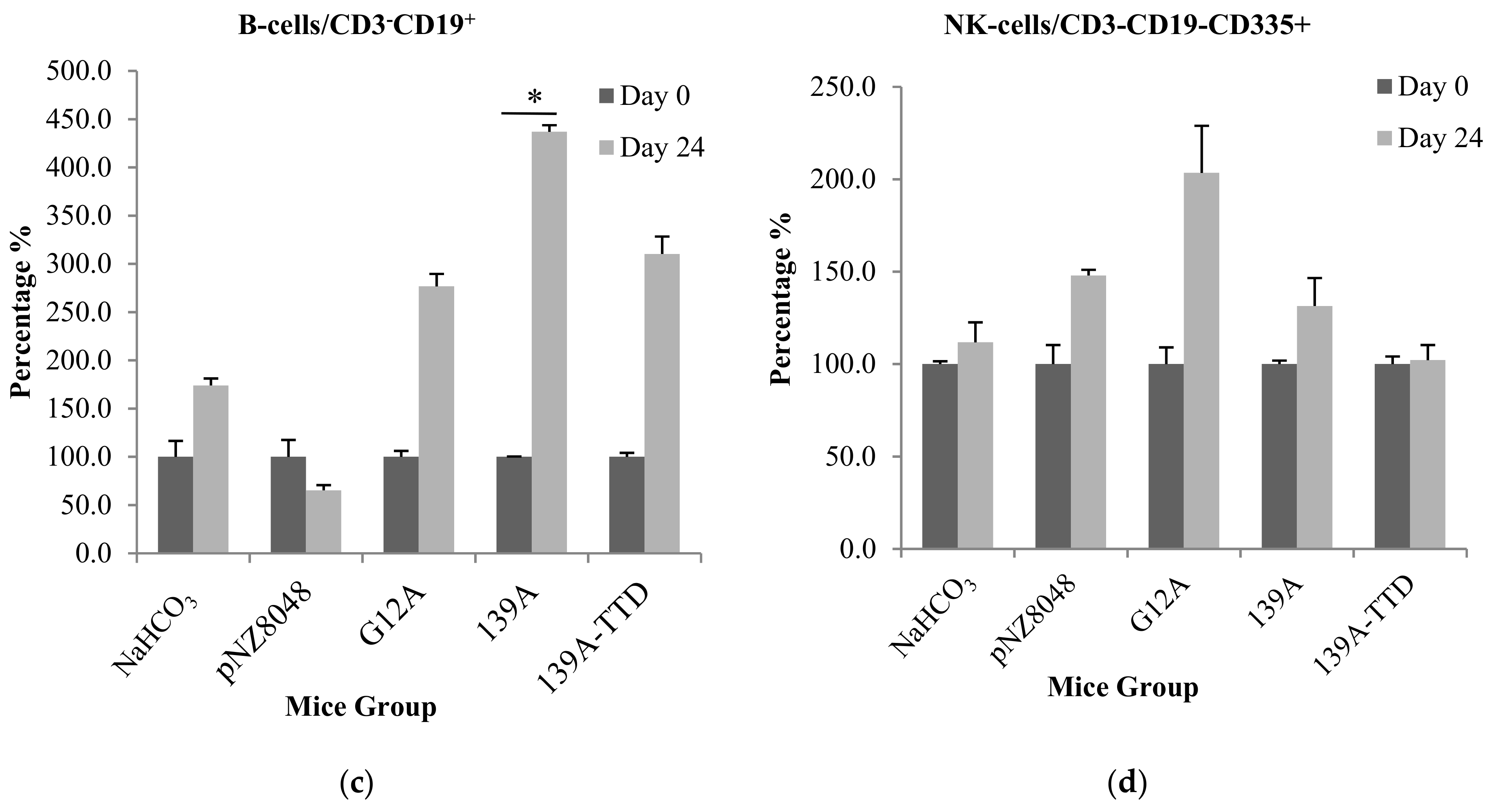
2.9. Immunophenotyping of T and B-Cell Populations
2.10. Detection of K-Ras-Specific Serum IgG and Intestinal IgA
2.11. Ex Vivo Antigen Stimulation and Detection of IFN-γ Producing T-Cells
2.12. Ethics Approval and Consent to Participate
3. Results
3.1. Expression and Extracellular Secretion of K-Ras Peptide Mimotopes by L. lactis NZ9000
3.2. Survivability of Recombinant L. lactis through the GI Tract
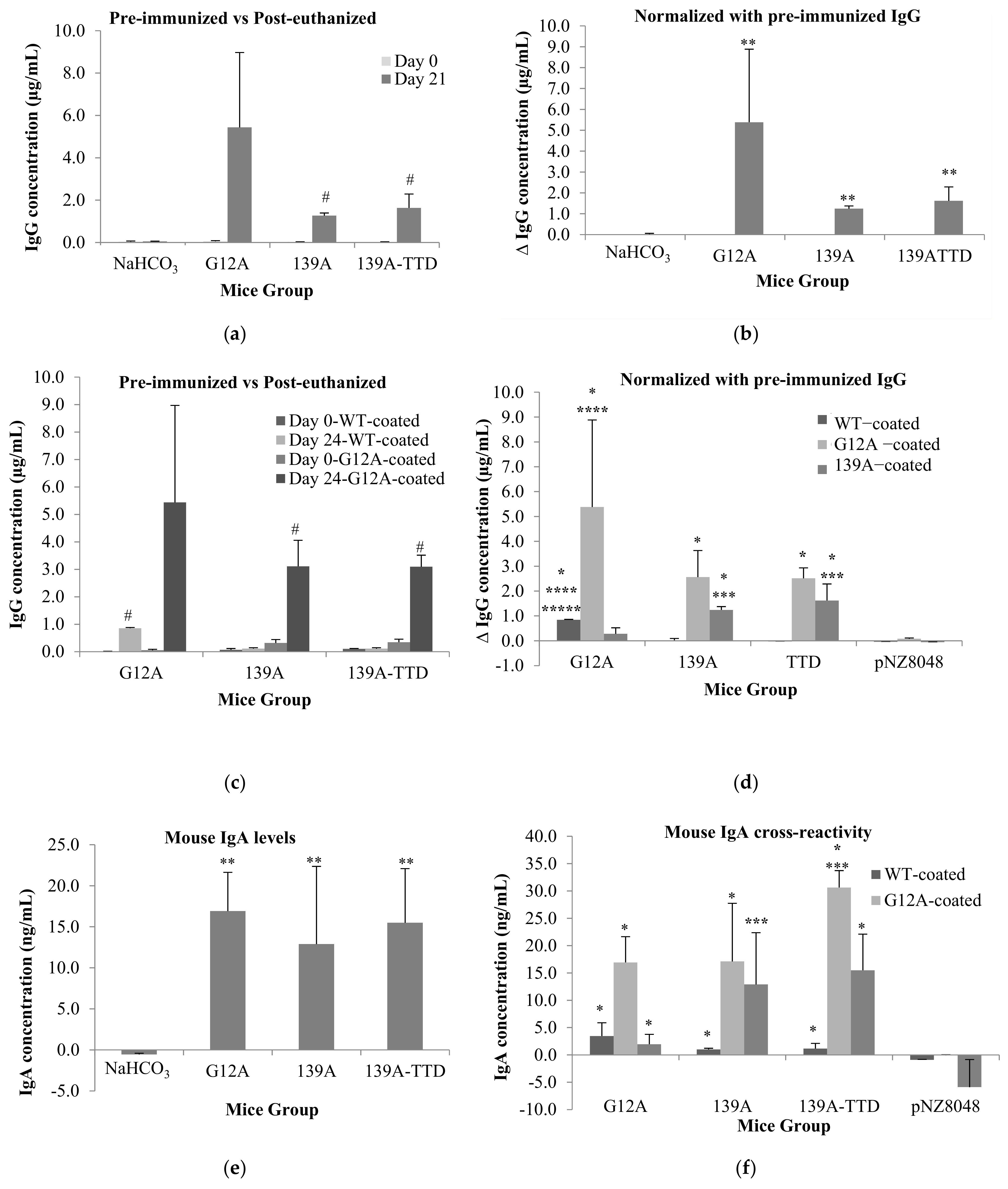
3.3. Oral Immunization of 139A-Secreting L. lactis Induces Elevation of B-Cell Population
3.4. Recombinant L. lactis Secreting 139A-TTD Enhances G12A-Mutant K-Ras-Specific Humoral Response in BALB/c Mice
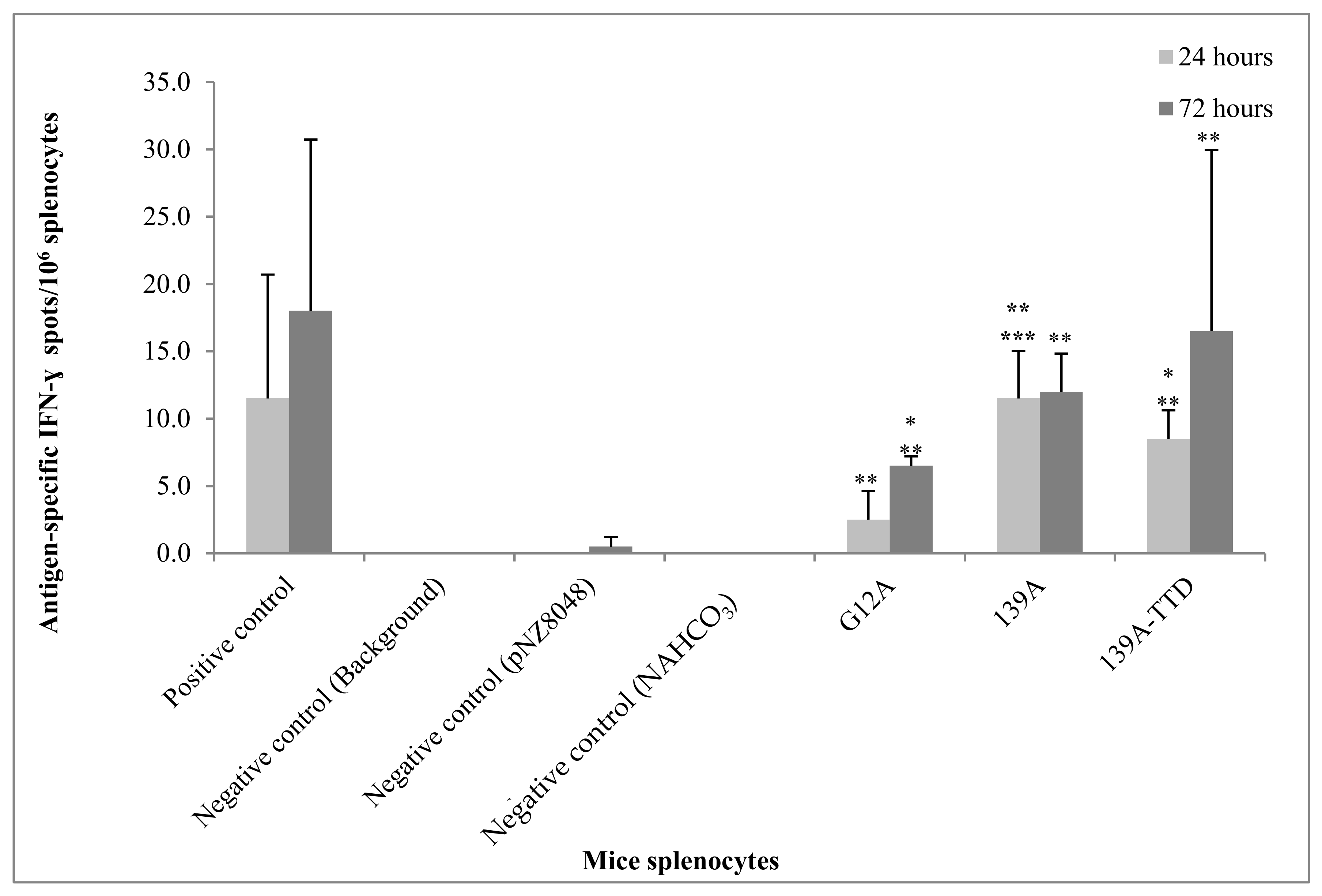
3.5. Re-Stimulation of 139A-TTD Immunized Mice Splenocytes Induces G12A-Mutant K-Ras-Specific T-Cell Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jančík, S.; Drabek, J.; Radzioch, D.; Hajduch, M. Clinical relevance of KRAS in human cancers. J. Biomed. Biotechnol. 2010, 2010, 150960. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.K.; Raman, T. Ras and Ras mutations in cancer. Cent. Eur. J. Biol. 2013, 8, 609–624. [Google Scholar] [CrossRef]
- Van Krieken, J.H.; Jung, A.; Kirchner, T.; Carneiro, F.; Seruca, R.; Bosman, F.T.; Quirke, P.; Fléjou, J.F.; Hansen, T.P.; De Hertogh, G.; et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: Proposal for an European quality assurance program. Virchows Arch. 2008, 453, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Jessup, J.M.; Somerfield, M.R.; Hamilton, S.R.; Hammond, E.H.; Hayes, D.F.; McAllister, P.K.; Morton, R.F.; Schilsky, R.L. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009, 27, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Du, X. KRAS mutation testing in metastatic colorectal cancer. World J. Gastroenterol. 2012, 18, 5171–5180. [Google Scholar]
- Tong, J.H.; Lung, R.W.; Sin, F.M.; Law, P.P.; Kang, W.; Chan, A.W.; Ma, B.B.; Mak, T.W.; Ng, S.S.; To, K.F. Characterization of rare transforming KRAS mutations in sporadic colorectal cancer. Cancer Biol. Ther. 2014, 15, 768–776. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Li, K.; Yin, H.; Zheng, J.N. Composite peptide-based vaccines for cancer immunotherapy (Review). Int. J. Mol. Med. 2015, 35, 17–23. [Google Scholar] [CrossRef]
- Katsuda, M.; Yamaue, H. Cancer vaccine therapy based on peptides. Trends Immunother. 2017, 1, 10–18. [Google Scholar] [CrossRef]
- Wada, S.; Yada, E.; Ohtake, J.; Sasada, T. Personalized peptide vaccines for cancer therapy: Current progress and state of the art. Expert Rev. Precis. Med. Drug Dev. 2017, 2, 371–381. [Google Scholar] [CrossRef]
- Santos, P.M.; Butterfield, L.H. Dendritic cell-based cancer vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Knittelfelder, R.; Riemer, A.B.; Jensen-Jarolim, E. Mimotope vaccination—From allergy to cancer. Expert Opin. Biol. Ther. 2009, 9, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Buhrman, J.D.; Slansky, J.E. Mimotope vaccine efficacy gets a “boost” from native tumor antigens. Oncoimmunology 2013, 2, e23492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riemer, A.B.; Jensen-Jarolim, E. Mimotope vaccines: Epitope mimics induce anti-cancer antibodies. Immunol. Lett. 2007, 113, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Z.; Fan, D. Overview of mimotopes and related strategies in tumor vaccine development. Expert Rev. Vaccines 2008, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Buhrman, J.D.; Slansky, J.E. Improving T cell responses to modified peptides in tumor vaccines. Immunol. Res. 2013, 55, 34–47. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Salgaller, M.L.; Southwood, S.; Robbins, P.F.; Sette, A.; Rosenberg, S.A.; Kawakami, Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J. Immunol. 1996, 157, 2539–2548. [Google Scholar]
- Yu, Z.; Theoret, M.R.; Touloukian, C.E.; Surman, D.R.; Garman, S.C.; Feigenbaum, L.; Baxter, T.K.; Baker, B.M.; Restifo, N.P. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J. Clin. Investig. 2004, 114, 551–559. [Google Scholar] [CrossRef]
- Ng, A.W.; Tan, P.J.; Hoo, W.P.; Liew, D.S.; Teo, M.Y.; Siak, P.Y.; Ng, S.M.; Tan, E.W.; Rahim, R.A.; Lim, R.L.; et al. In silico-guided sequence modifications of K-ras epitopes improve immunological outcome against G12V and G13D mutant KRAS antigens. PeerJ 2018, 6, e5056. [Google Scholar] [CrossRef]
- Diethelm-Okita, B.M.; Okita, D.K.; Banaszak, L.; Conti-Fine, B.M. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 2000, 181, 1001–1009. [Google Scholar] [CrossRef]
- Yano, A.; Ito, K.; Miwa, Y.; Kanazawa, Y.; Chiba, A.; Iigo, Y.; Kashimoto, Y.; Kanda, A.; Murata, S.; Makino, M. The Peptide Vaccine Combined with Prior Immunization of a Conventional Diphtheria-Tetanus Toxoid Vaccine Induced Amyloid β Binding Antibodies on Cynomolgus Monkeys and Guinea Pigs. J. Immunol. Res. 2015, 2015, 786501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, Y.; Guo, L.; Lv, X.; Song, H.; Xi, T. Oral immunization with recombinant Lactococcus lactis delivering a multi-epitope antigen CTB-UE attenuates Helicobacter pylori infection in mice. Pathog. Dis. 2014, 72, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Bermudez-Humaran, L.G.; Llull, D.; Solé, V.; Miraglio, N.; Langella, P.; Poquet, I. Lactococcus lactis, an Efficient Cell Factory for Recombinant Protein Production and Secretion. J. Mol. Microbiol. Biotechnol. 2008, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, P.H.; Leer, R.J.; Shaw, M.; den Bak-Glashouwer, M.J.; Tielen, F.D.; Smit, E.; Martinez, B.; Jore, J.; Conway, P.L. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 1998, 41, 155–167. [Google Scholar] [CrossRef]
- Seegers, J.F. Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends Biotechnol. 2002, 20, 508–515. [Google Scholar] [CrossRef]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef]
- Hirayama, K.; Rafter, J. The role of lactic acid bacteria in colon cancer prevention: Mechanistic considerations. Antonie Van Leeuwenhoek 1999, 76, 391–394. [Google Scholar]
- Bermúdez-Humarán, L.G.; Cortes-Perez, N.G.; Lefèvre, F.; Guimarães, V.; Rabot, S.; Alcocer-Gonzalez, J.M.; Gratadoux, J.J.; Rodriguez-Padilla, C.; Tamez-Guerra, R.S.; Corthier, G.; et al. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 2005, 175, 7297–7302. [Google Scholar] [CrossRef]
- Ng, D.T.W.; Sarkar, C.A. Engineering signal peptides for enhanced protein secretion from Lactococcus lactis. Appl. Environ. Microbiol. 2013, 79, 347–356. [Google Scholar] [CrossRef]
- Miyoshi, A.; Jamet, E.; Commissaire, J.; Renault, P.; Langella, P.; Azevedo, V. A xylose-inducible expression system for Lactococcus lactis. FEMS Microbiol. Lett. 2004, 239, 205–212. [Google Scholar] [CrossRef]
- Hoo, W.P.Y.; Siak, P.Y.; Alias, N.A.R.; Wong, J.J.; In, L.L.A. K-ras peptide mimotope induces a humoral immune response against G12V K-ras antigen in BALB/c mice. Asia Pac. J. Mol. Biol. Biotechnol. 2020, 22–35. [Google Scholar] [CrossRef]
- Holo, H.; Nes, I.F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp cremoris grown with glycine in osmotically-stabilized media. Appl. Environ. Microbiol. 1989, 55, 3119–3123. [Google Scholar] [CrossRef] [PubMed]
- Moodie, Z.; Price, L.; Gouttefangeas, C.; Mander, A.; Janetzki, S.; Löwer, M.; Welters, M.J.; Ottensmeier, C.; Van der Burg, S.H.; Britten, C.M. Response definition criteria for ELISPOT assays revisited. Cancer Immunol. Immunother. 2010, 59, 1489–1501. [Google Scholar] [CrossRef]
- Schägger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.R.; Reid, H.J.; Sharp, B.L. Tricine-SDS-PAGE. In Protein Electrophoresis; Methods in Molecular Biology (Methods and, Protocols); Kurien, B., Scofield, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 869. [Google Scholar]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Rochat, F.; Serrant, P.; Aeschlimann, J.M.; Schiffrin, E.J. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose. J. Dairy Sci. 1999, 82, 863–869. [Google Scholar] [CrossRef]
- Seaman, W.E. Natural killer cells and natural killer T cells. Arthritis Rheum. 2000, 43, 1204–1217. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Parra, M.D.; Martinez de Morentin, B.E.; Cobo, J.M.; Mateos, A.; Martinez, J.A. Daily ingestion of fermented milk containing Lactobacillus casei DN114001 improves innate-defense capacity in healthy middle-aged people. J. Physiol. Biochem. 2004, 60, 85–91. [Google Scholar] [CrossRef]
- Seifert, S.; Bub, A.; Franz, C.; Watzl, B. Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J. Nutr. 2011, 141, 978–984. [Google Scholar] [CrossRef]
- Xin, K.Q.; Hoshino, Y.; Toda, Y.; Igimi, S.; Kojima, Y.; Jounai, N.; Ohba, K.; Kushiro, A.; Kiwaki, M.; Hamajima, K.; et al. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 2003, 102, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Asensi, G.F.; de Sales, N.F.; Dutra, F.F.; Feijó, D.F.; Bozza, M.T.; Ulrich, R.G.; Miyoshi, A.; de Morais, K.; de Carvalho Azevedo, V.A.; Silva, J.T.; et al. Oral immunization with Lactococcus lactis secreting attenuated recombinant staphylococcal enterotoxin B induces a protective immune response in a murine model. Microb. Cell Fact. 2013, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Mestecky, J.; Lamm, M.E.; Strober, W.; McGhee, J.R.; Bienenstock, J. Mucosal Immunology, 4th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Shakya, A.K.; Chowdhury, M.Y.; Tao, W.; Gill, H.S. Mucosal vaccine delivery: Current state and a pediatric perspective. J. Control Release 2016, 240, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Humarán, L.G. Lactococcus lactis as a live vector for mucosal delivery of therapeutic proteins. Hum. Vaccin. 2009, 5, 264–267. [Google Scholar] [CrossRef]
- Adachi, K.; Kawana, K.; Yokoyama, T.; Fujii, T.; Tomio, A.; Miura, S.; Tomio, K.; Kojima, S.; Oda, K.; Sewaki, T.; et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 2010, 28, 2810–2817. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Azizpour, M.; Hosseini, S.D.; Jafari, P.; Akbary, N. Lactococcus lactis: A new strategy for vaccination. Avicenna J. Med. Biotechnol. 2017, 9, 163–168. [Google Scholar]
- Hahn, H.P.; von Specht, B.U. Secretory delivery of recombinant proteins in attenuated Salmonella strains: Potential and limitations of Type I protein transporters. FEMS Immunol. Med. Microbiol. 2003, 37, 87–98. [Google Scholar] [CrossRef]
- Van Wely, K.H.; Swaving, J.; Freudl, R.; Driessen, A.J.M. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 2001, 25, 437–454. [Google Scholar] [CrossRef]
- Mathiesen, G.; Sveen, A.; Piard, J.C.; Axelsson, L.; Eijsink, V.G. Heterologous protein secretion by Lactobacillus plantarum using homologous Signal peptides. J. Appl. Microbiol. 2008, 105, 215–216. [Google Scholar] [CrossRef]
- Van Asseldonk, M.; de Vos, W.M.; Simons, G. Functional analysis of the Lactococcus lactis usp45 secretion signal in the secretion of a homologous proteinase and a heterologous α-amylase. Mol. Gen. Genet. 1993, 240, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Hoekman, A.J.; Clier, F.; Juillard, V.; Boot, H.J.; Piard, J.C. Ability of Lactococcus lactis to export viral capsid antigens: A crucial step for development of live vaccines. Appl. Environ. Microbiol. 2003, 69, 7281–7288. [Google Scholar] [CrossRef] [PubMed]
- Le Loir, Y.; Azevedo, V.; Oliveira, S.C.; Freitas, D.A.; Miyoshi, A.; Bermúdez-Humarán, L.G.; Nouaille, S.; Ribeiro, L.A.; Leclercq, S.; Gabriel, J.E.; et al. Protein secretion in Lactococcus lactis: An efficient way to increase the overall heterologous protein production. Microb. Cell Fact. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Hazebrouck, S.; Pothelune, L.; Azevedo, V.; Corthier, G.; Wal, J.M.; Langella, P. Efficient Production and Secretion of bovine β-lactoglobulin by Lactobacillus casei. Microb. Cell Fact. 2007, 6, 12. [Google Scholar] [CrossRef]
- Baradaran, A.; Sieo, C.C.; Foo, H.L.; Illias, R.M.; Yusoff, K.; Rahim, R.A. Cloning and in silico characterization of two Signal peptides from Pediococcus pentosaceus and their function for the secretion of heterologous protein in Lactococcus lactis. Biotechnol. Lett. 2013, 35, 235–238. [Google Scholar] [CrossRef]
- Joan, S.S.; Pui-Fong, J.; Song, A.A.; Chang, L.Y.; Yusoff, K.; AbuBakar, S.; Rahim, R.A. Oral vaccine of Lactococcus lactis harboring pandemic H1N1 2009 haemagglutinin1 and nisP anchor fusion protein elevates anti-HA1 sIgA levels in mice. Biotechnol. Lett. 2016, 38, 793–799. [Google Scholar] [CrossRef]
- Tayeb, I.; Jamel, B.; Essaid, L.; Nour-Eddine, K. Survival of Lactobacillus plantarum bj0021 and Pediococcus acidilactici in the digestive tract of rabbit. Int. J. Probiotics Prebiotics 2007, 2, 49–54. [Google Scholar]
- Klijn, N.; Weerkamp, A.H.; de Vos, W.M. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 1995, 61, 2771–2774. [Google Scholar] [CrossRef]
- Kim, W.S.; Ren, J.; Dunn, N.W. Differentiation of Lactococcus lactis subspecies lactis and subspecies cremoris strains by their adaptive response to stresses. FEMS Microbiol. Lett. 1999, 171, 57–65. [Google Scholar] [CrossRef]
- Tan, E.W.; Tan, K.Y.; Phang, L.V.; Kumar, P.V.; In, L.L.A. Enhanced gastrointestinal survivability of recombinant Lactococcus lactis using a double coated mucoadhesive film approach. PLoS ONE 2019, 14, e0219912. [Google Scholar] [CrossRef]
- Speck, M.L. Acidophilus food products. Dev. Ind. Microbiol. 1978, 19, 95–101. [Google Scholar]
- Kim, H.S. Characterization of lactobacilli and bifidobacteria as applied to dietary adjuncts. Cult. Dairy Prod. J. 1988, 23, 6–9. [Google Scholar]
- Kang, S.H.; Hong, S.J.; Lee, Y.K.; Cho, S. Oral Vaccine Delivery for Intestinal Immunity-Biological Basis, Barriers, Delivery System, and M Cell Targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.T.; Machiels, J.P.; Emens, L.A.; Ercolini, A.M.; Okoye, F.I.; Lei, R.Y.; Weintraub, D.; Jaffee, E.M. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001, 61, 880–883. [Google Scholar] [PubMed]
- Reilly, R.T.; Emens, L.A.; Jaffee, E.M. Humoral and cellular immune responses: Independent forces or collaborators in the fight against cancer? Curr. Opin. Investig. Drugs 2001, 2, 133–135. [Google Scholar]
- Emens, L.A. Cancer vaccines: On the threshold of success. Expert Opin. Emerg. Drugs 2008, 13, 295–308. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siak, P.Y.; Wong, K.Y.; Song, A.A.-L.; Rahim, R.A.; In, L.L.A. K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice. Vaccines 2021, 9, 195. https://doi.org/10.3390/vaccines9030195
Siak PY, Wong KY, Song AA-L, Rahim RA, In LLA. K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice. Vaccines. 2021; 9(3):195. https://doi.org/10.3390/vaccines9030195
Chicago/Turabian StyleSiak, Pui Yan, Kuan Yee Wong, Adelene Ai-Lian Song, Raha Abdul Rahim, and Lionel Lian Aun In. 2021. "K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice" Vaccines 9, no. 3: 195. https://doi.org/10.3390/vaccines9030195
APA StyleSiak, P. Y., Wong, K. Y., Song, A. A.-L., Rahim, R. A., & In, L. L. A. (2021). K-Ras Peptide Mimotope Induces Antigen Specific Th1 and B-Cell Immune Responses against G12A-Mutated K-Ras Antigen in Balb/c Mice. Vaccines, 9(3), 195. https://doi.org/10.3390/vaccines9030195






