Determining the Optimal Administration Conditions under Which MIF Exerts Neuroprotective Effects by Inducing BDNF Expression and Inhibiting Apoptosis in an In Vitro Stroke Model
Abstract
:1. Introduction
2. Materials and Methods
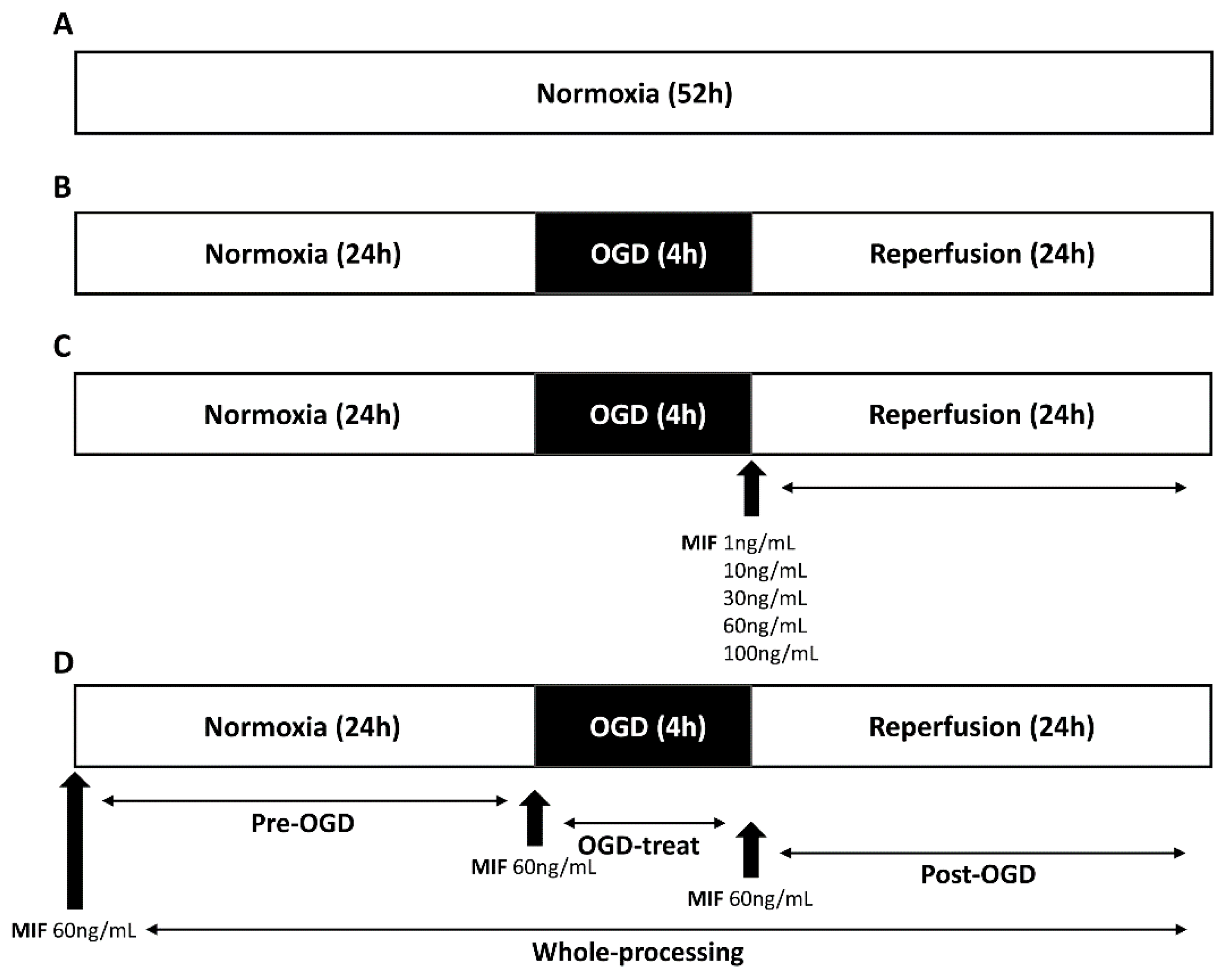
2.1. Cell Cultures
2.2. Oxygen and Glucose Deprivation/Reperfusion (OGD/R)
2.3. MIF Administration
2.3.1. MIF Administration at Different Concentrations
2.3.2. MIF Administration at Different Times
2.4. Water-Soluble Tetrazolium Salt-1 (WST-1) Assay
2.5. Western Blot Analysis
2.6. Immunocytochemistry
2.7. Statistical Analysis
3. Results
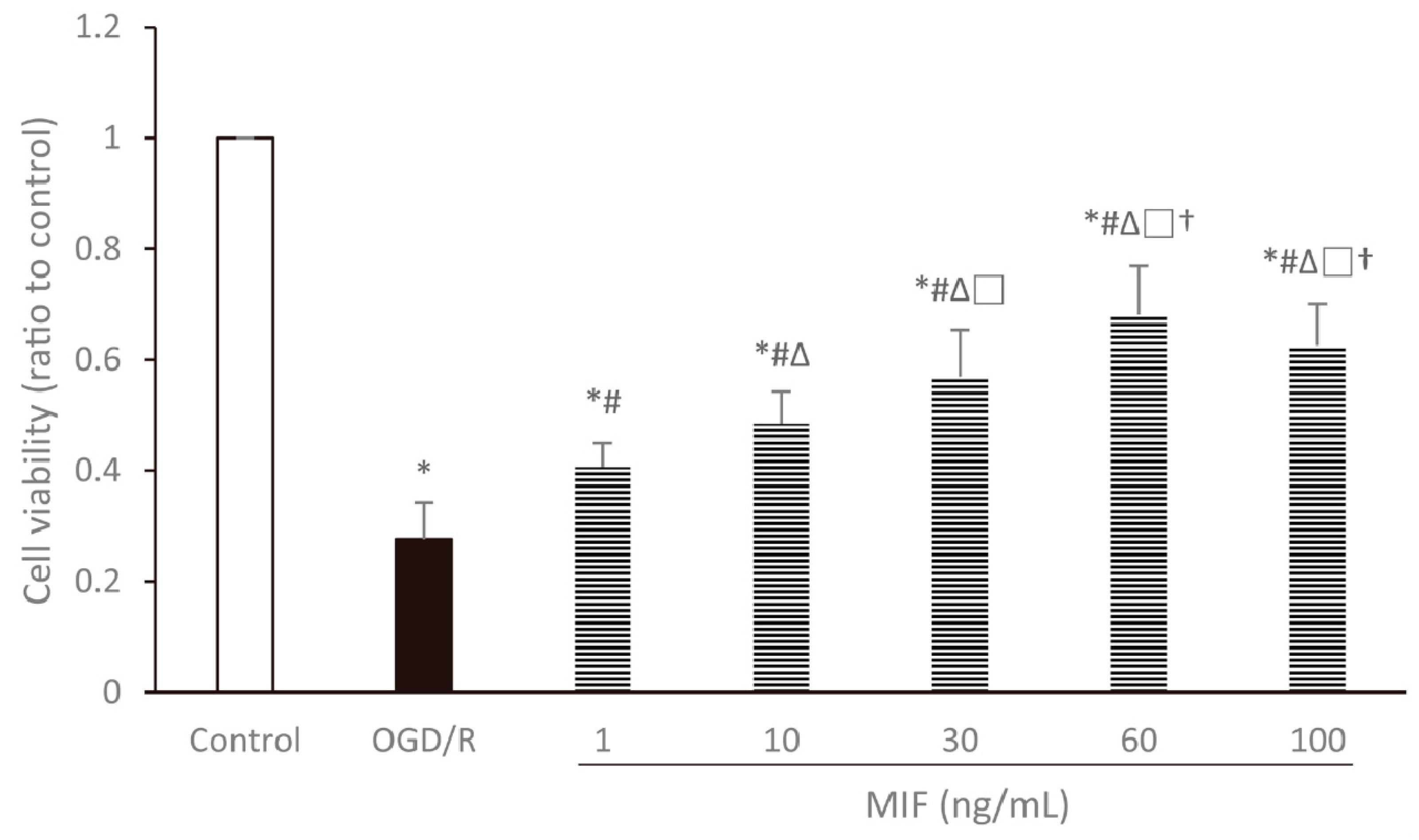
3.1. Optimal Concentration of MIF Administration
3.1.1. Cell Viability Is the Highest in the 60 ng/mL MIF Group
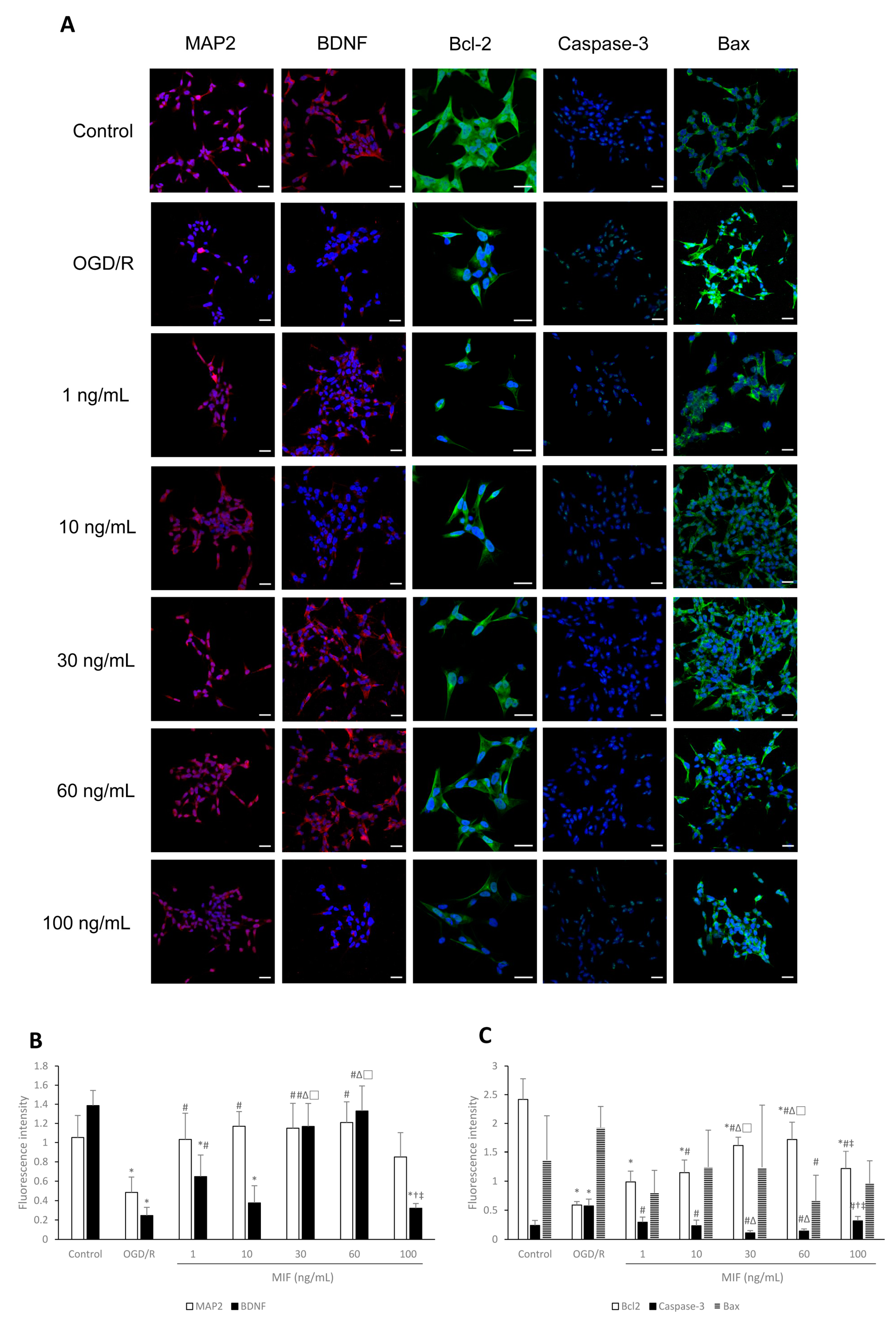
3.1.2. Bcl-2 Expression Is Significantly Increased in the 60 ng/mL MIF Group
3.1.3. Inducing BDNF Expression and Inhibiting Apoptosis Is Most Effective in the 60 ng/mL MIF Group
3.2. Optimal Time of MIF Administration
3.2.1. Cell Viability Is Increased at a Similar Degree in the MIF Groups Regardless of Administration Time
3.2.2. MAP2 Expression Is Significantly Increased in the OGD-Treat Group
3.2.3. Inhibiting Apoptosis Is Effective at a Similar Degree in the MIF Groups Regardless of Administration Time; However, BDNF Expression Is Significantly Increased in the Post-OGD Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults—United States, 2005. Morb. Mortal. Wkly. Rep. 2009, 58, 421–426. [Google Scholar]
- Kim, W.S.; Bae, H.J.; Lee, H.H.; Shin, H.I. Status of rehabilitation after ischemic stroke: A Korean nationwide study. Ann. Rehabil. Med. 2018, 42, 528–535. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Jung, K.H.; Mo, H.; Lee, C.H.; Kim, T.J.; Park, J.M.; Oh, M.; Lee, J.S.; Kim, B.J.; Kim, J.T.; et al. Characteristics and management of stroke in Korea: 2014-2018 data from Korean Stroke Registry. Int. J. Stroke 2020, 15, 619–626. [Google Scholar] [CrossRef]
- Macrez, R.; Ali, C.; Toutirais, O.; Le Mauff, B.; Defer, G.; Dirnagl, U.; Vivien, D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011, 10, 471–480. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.W.; Lai, J.H.; Wu, J.C.; Tsai, Y.R.; Chen, Y.H.; Kang, S.J.; Chiang, Y.H.; Chang, C.F.; Chen, K.Y. Neuroprotective effects of emodin against ischemia/reperfusion injury through activating ERK-1/2 signaling pathway. Int. J. Mol. Sci. 2020, 21, 2899. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, X.; Yu, S.; Lin, X.; Wu, J.; Li, L.; Zhao, J.; Zhao, Y. Neuroprotection of tanshinone IIA against cerebral ischemia/reperfusion injury through inhibition of macrophage migration inhibitory factor in rats. PLoS ONE 2012, 7, e40165. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhang, L.; Sun, S.; Yi, Z.; Jiang, X.; Jia, D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int. J. Mol. Med. 2016, 38, 567–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabori, M.; Yenari, M.A. Inflammatory responses in brain ischemia. Curr. Med. Chem. 2015, 22, 1258–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zis, O.; Zhang, S.; Dorovini-Zis, K.; Wang, L.; Song, W. Hypoxia signaling regulates macrophage migration inhibitory factor (MIF) expression in stroke. Mol. Neurobiol. 2015, 51, 155–167. [Google Scholar] [CrossRef]
- Leyton-Jaimes, M.F.; Kahn, J.; Israelson, A. Macrophage migration inhibitory factor: A multifaceted cytokine implicated in multiple neurological diseases. Exp. Neurol. 2018, 301, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M.; King-Pospisil, K.; Metz, C.N.; Toborek, M. Macrophage migration inhibitory factor induces cell death and decreases neuronal nitric oxide expression in spinal cord neurons. Neuroscience 2006, 139, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Turtzo, L.C.; Li, J.; Persky, R.; Benashski, S.; Weston, G.; Bucala, R.; Venna, V.R.; McCullough, L.D. Deletion of macrophage migration inhibitory factor worsens stroke outcome in female mice. Neurobiol. Dis. 2013, 54, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zis, O.; Ly, P.T.; Wu, Y.; Zhang, S.; Zhang, M.; Cai, F.; Bucala, R.; Shyu, W.C.; Song, W. Down-regulation of MIF by NFκB under hypoxia accelerated neuronal loss during stroke. FASEB J. 2014, 28, 4394–4407. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zis, O.; Ma, G.; Shan, Z.; Zhang, X.; Wang, S.; Dai, C.; Zhao, J.; Lin, Q.; Lin, S.; et al. Upregulation of macrophage migration inhibitory factor gene expression in stroke. Stroke 2009, 40, 973–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.W.; Ma, P.J.; Wang, Y.Y.; Yang, M.; Su, L.L.; Wang, S.; Liu, Y.X.; Yuan, B.; Zhao, J.H. Serum level of macrophage migration inhibitory factor predicts severity and prognosis in patients with ischemic stroke. Cytokine 2019, 115, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Inacio, A.R.; Ruscher, K.; Leng, L.; Bucala, R.; Deierborg, T. Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1093–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Tsai, Y.H.; Tang, S.C.; Liou, H.C.; Kang, K.H.; Liou, H.H.; Jeng, J.S.; Fu, W.M. Cytokine MIF enhances blood-brain barrier permeability: Impact for therapy in ischemic stroke. Sci. Rep. 2018, 8, 743. [Google Scholar] [CrossRef]
- Chang, M.C.; Park, C.R.; Rhie, S.H.; Shim, W.H.; Kim, D.Y. Early treadmill exercise increases macrophage migration inhibitory factor expression after cerebral ischemia/reperfusion. Neural Regen. Res. 2019, 14, 1230–1236. [Google Scholar]
- Bae, S.H.; Yoo, M.R.; Kim, Y.Y.; Hong, I.K.; Kim, M.H.; Lee, S.H.; Kim, D.Y. Brain-derived neurotrophic factor mediates macrophage migration inhibitory factor to protect neurons against oxygen-glucose deprivation. Neural Regen. Res. 2020, 15, 1483–1489. [Google Scholar]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.Y.; Kim, S.H.; Yang, Y.R.; Song, P.; Yu, H.S.; Park, H.G.; Hwang, O.; Lee-Kwon, W.; Seo, J.K.; Hwang, D.; et al. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc. Natl. Acad. Sci. USA 2012, 109, 13094–13099. [Google Scholar] [CrossRef] [Green Version]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.P.; Choi, D.W. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993, 13, 3510–3524. [Google Scholar] [CrossRef]
- Ohira, K.; Hayashi, M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr. Neuropharmacol. 2009, 7, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Kim, W.S.; Lim, J.Y.; Shin, J.H.; Park, H.K.; Tan, S.A.; Park, K.U.; Paik, N.J. Effect of the presence of brain-derived neurotrophic factor val(66)met polymorphism on the recovery in patients with acute subcortical stroke. Ann. Rehabil. Med. 2013, 37, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kong, Y.; Ren, H.; Li, M.; Wei, C.J.; Shi, E.; Jin, W.N.; Hao, J.; Vandenbark, A.A.; Offner, H. Upregulation of CD74 and its potential association with disease severity in subjects with ischemic stroke. Neurochem. Int. 2017, 107, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Weber, C.; Bernhagen, J. Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2014, 102, 321–328. [Google Scholar] [CrossRef]
- Rossello, X.; Burke, N.; Stoppe, C.; Bernhagen, J.; Davidson, S.M.; Yellon, D.M. Exogenous administration of recombinant mif at physiological concentrations failed to attenuate infarct size in a Langendorff perfused isolated mouse heart model. Cardiovasc. Drugs Ther. 2016, 30, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Barhwal, K.K.; Biswal, S.N.; Srivastava, A.K.; Bhardwaj, P.; Kumar, A.; Chaurasia, O.P.; Hota, S.K. Hypoxia-mediated alteration in cholesterol oxidation and raft dynamics regulates BDNF signalling and neurodegeneration in hippocampus. J. Neurochem. 2019, 148, 238–251. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Kenessey, A.; Powell, S.R.; Sison, C.P.; Miller, E.J.; Ojamaa, K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid. Redox Signal. 2011, 14, 1191–1202. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, C.; Kim, M.H.; Kim, Y.Y.; Kim, J.A.; Ko, E.J.; Lee, S.H.; Kim, D.Y. Determining the Optimal Administration Conditions under Which MIF Exerts Neuroprotective Effects by Inducing BDNF Expression and Inhibiting Apoptosis in an In Vitro Stroke Model. Brain Sci. 2021, 11, 280. https://doi.org/10.3390/brainsci11020280
Jung C, Kim MH, Kim YY, Kim JA, Ko EJ, Lee SH, Kim DY. Determining the Optimal Administration Conditions under Which MIF Exerts Neuroprotective Effects by Inducing BDNF Expression and Inhibiting Apoptosis in an In Vitro Stroke Model. Brain Sciences. 2021; 11(2):280. https://doi.org/10.3390/brainsci11020280
Chicago/Turabian StyleJung, Chul, Mi Hee Kim, Ye Yeong Kim, Ji Ae Kim, Eun Jae Ko, Seung Hak Lee, and Dae Yul Kim. 2021. "Determining the Optimal Administration Conditions under Which MIF Exerts Neuroprotective Effects by Inducing BDNF Expression and Inhibiting Apoptosis in an In Vitro Stroke Model" Brain Sciences 11, no. 2: 280. https://doi.org/10.3390/brainsci11020280





