Inhibition of the Sodium Calcium Exchanger Suppresses Alcohol Withdrawal-Induced Seizure Susceptibility
Abstract
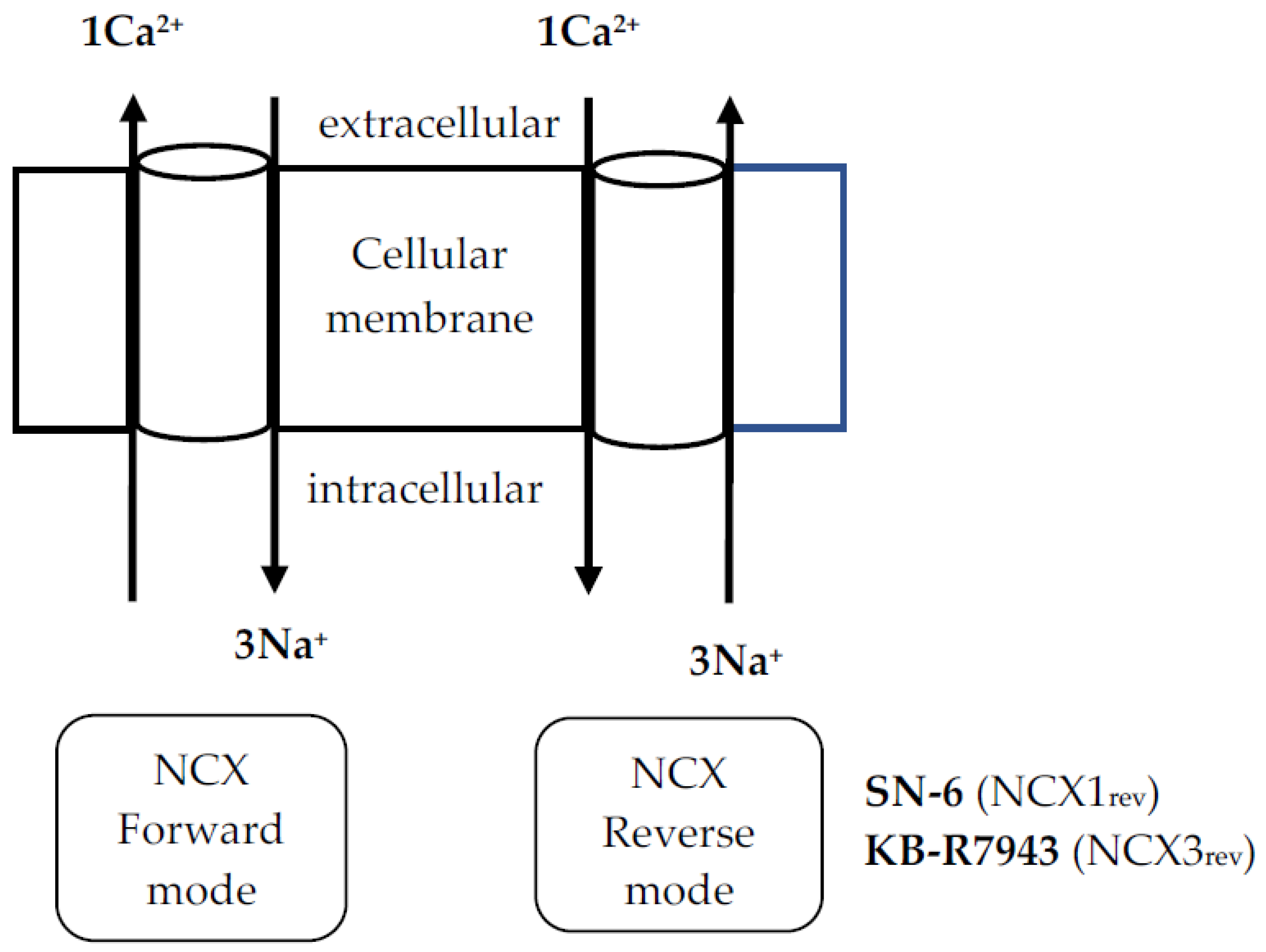
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethanol Administration
2.3. Acoustically Evoked Seizures and Pharmacological Treatment
2.4. Data Analysis
3. Results
3.1. Tremors
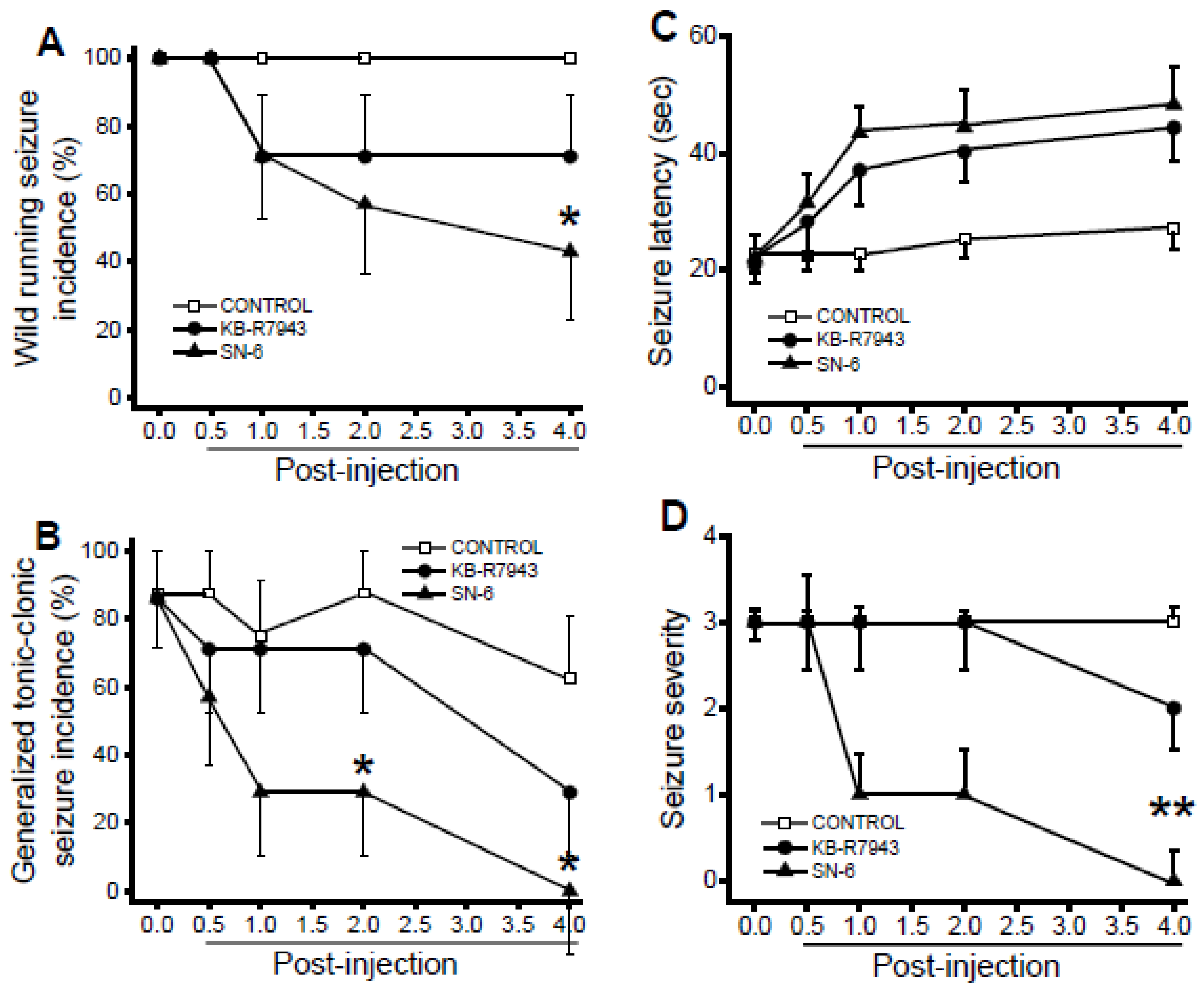
3.2. Acoustically Evoked Seizures
3.3. Effects of SN-6 and KB-R7943 at the Dose of 1 mg/kg on the Expression of Acoustically Evoked Seizures
3.4. Effects of SN-6 and KB-R7943 at the Dose of 3 mg/kg on the Expression of Acoustically Evoked Seizures
3.5. Effects of SN-6 and KB-R7943 at the dose of 10 mg/kg on the Expression of Acoustically Evoked Seizures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hillbom, M.; Pieninkeroinen, I.; Leone, M. Seizures in alcohol-dependent patients. CNS Drugs 2003, 17, 1013–1030. [Google Scholar] [CrossRef]
- Ripley, J.B. A 39-year-old man with fatal alcohol withdrawal seizures. J. Emerg. Nurs. 1990, 16, 67–69. [Google Scholar]
- Victor, M.; Brausch, C. The role of abstinence in the genesis of alcohol epilepsy. Epilepsia 1967, 8, 1–20. [Google Scholar] [CrossRef]
- Jesse, S.; Bråthen, G.; Ferrara, M.; Keindl, M.; Ben-Menachem, E.; Tanasescu, R.; Brodtkorb, E.; Hillbom, M.; Leone, M.A.; Ludolph, A.C. Alcohol withdrawal syndrome: Mechanisms, manifestations, and management. Acta Neurol. Scand. 2017, 135, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Long, B.; Koyfman, A. The emergency medicine management of severe alcohol withdrawal. Am. J. Emerg. Med. 2017, 35, 1005–1011. [Google Scholar] [CrossRef]
- McMicken, D.; Liss, J.L. Alcohol-related seizures. Emerg. Med. Clin. North Am. 2011, 29, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.J.; Doshi, M.R.; Holzhausen, J.M. Treatment of severe alcohol withdrawal. Ann. Pharmacol. Ther. 2016, 50, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Albowitz, B.; König, P.; Kuhnt, U. Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J. Neurophysiol. 1997, 77, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Lux, H.; Gutnick, M.J. Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp. Brain Res. 1977, 27, 237–243. [Google Scholar] [CrossRef]
- Somjen, G.G. Stimulus-evoked and seizure-related responses of extracellular calcium activity in spinal cord compared to those in cerebral cortex. J. Neurophysiol. 1980, 44, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Peterson, D.W. Normal extracellular calcium levels block kindled seizures. Exp. Neurol. 1989, 106, 99–101. [Google Scholar] [CrossRef]
- Stringer, J.L.; Lothman, E.W. In vitro effects of extracellular calcium concentrations on hippocampal pyramidal cell responses. Exp. Neurol. 1988, 101, 132–146. [Google Scholar] [CrossRef]
- Little, H.J.; Dolin, J.J.; Hasley, M.J. Calcium channel antagonists decrease ethanol withdrawal syndrome. Life Sci. 1986, 39, 2059–2065. [Google Scholar] [CrossRef]
- N’Gouemo, P. Altered voltage-gated calcium channels in rat inferior colliculus neurons contribute to alcohol withdrawal seizures. Eur. Neuropsychopharmacol. 2015, 25, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.P.; Little, J.J. Effects of dihydropyridines on the components of the ethanol withdrawal syndrome: Possible evidence for involvement channels currents in rat inferior of potassium, as well as calcium? Alcohol Clin. Exp. Res. 1997, 21, 409–416. [Google Scholar]
- Brennan, C.H.; Crabbe, J.; Littleton, J.M. Genetic regulation of dihydropyridine-sensitive calcium channels in brain may determine susceptibility to physical dependence on alcohol. Neuropharmacology 1990, 20, 429–432. [Google Scholar] [CrossRef]
- N’Gouemo, P.; Allard, J.S.; Lovinger, D.M. Alcohol withdrawal‒induced seizure susceptibility is associated with an upregulation of CaV1.3 channels in the rat inferior colliculus. Int. J. Neuropsychopharmacol. 2015, 18, pyu123. [Google Scholar] [CrossRef] [PubMed]
- N’Gouemo, P.; Morad, M. Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ colliculus neurons. Neuropharmacology 2003, 45, 429–437. [Google Scholar] [CrossRef]
- Newton, J.; Suman, S.; Akinfiresoye, L.R.; Datta, K.; Lovinger, D.M.; N’Gouemo, P. Alcohol withdrawal upregulates mRNA encoding for CaV2.1-a1 subunit in the rat inferior colliculus. Alcohol 2018, 66, 21–26. [Google Scholar] [CrossRef]
- Whittington, M.A.; Lambert, J.D.; Little, H.J. Increases in synaptic activation of calcium currents as a mechanism for generation of ethanol withdrawal seizures. Alcohol Alcohol. 1993, 2, 391–394. [Google Scholar]
- Blaustein, M.P.; Lederer, W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, L.; Pignataro, G.; Di Renzo, G.F. Pharmacology of brain Na+/Ca2+ exchanger: From molecular biology to therapeutic perspectives. Pharmacol. Rev. 2004, 56, 633–654. [Google Scholar] [CrossRef]
- Annunziato, L.; Pignataro, G.; Boscia, F.; Sirabella, R.; Formisano, L.; Saggese, M.; Cuomo, O.; Gala, R.; Secondo, A.; Viggiano, D.; et al. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann. N. Y. Acad. Sci. 2007, 1099, 413–426. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ruknudin, A.; Bambrick, L.L.; Lederer, W.J.; Schulze, D.H. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKA. J. Neurosci. 1998, 18, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T. Forefront of Na+/Ca2+ exchanger studies: Molecular pharmacology of Na+/Ca2+ exchange inhibitors. J. Pharmacol. Sci. 2004, 96, 27–32. [Google Scholar] [CrossRef]
- Papa, M.; Canitano, A.; Boscia, F.; Castaldo, P.; Sellitti, S.; Porzig, H.; Taglialatela, M.; Annunziato, L. Differential expression of the Na+-Ca2+ exchanger transcripts and proteins in rat brain regions. J. Comp. Neurol. 2003, 461, 31–48. [Google Scholar] [CrossRef]
- Thurneysen, T.; Nicoll, D.A.; Philipson, K.D.; Porzig, H. Sodium/calcium exchanger subtypes NCX1, NCX2 and NCX3 show cell-specific expression in rat hippocampal cultures. Mol. Brain Res. 2002, 197, 145–156. [Google Scholar] [CrossRef]
- Yu, L.; Colvin, R.A. Regional differences in expression of transcripts for Na+/Ca2+ exchanger isoforms in rat brain. Brain Res. Mol. Brain Res. 1997, 50, 285–292. [Google Scholar] [CrossRef]
- Giladi, M.; Shor, R.; Lisnyansky, M.; Khananshvili, D. Structure-functional basis of ion transport in sodium-calcium exchanger (NCX) protein. Int. J. Mol. Sci. 2016, 17, 1949. [Google Scholar] [CrossRef]
- Saito, R.; Kaneko, E.; Tanaka, Y.; Honda, K.; Matsuda, T.; Baba, A.; Komuro, I.; Kita, S.; Iwamoto, T.; Takano, Y. Involvement of Na+/Ca2+ exchanger in pentylenetetrazol-induced convulsion by use of Na+/Ca2+ exchanger knockout mice. Biol. Pharm. Bull. 2009, 32, 1928–1930. [Google Scholar] [CrossRef]
- Martinez, Y.; N’Gouemo, P. Blockade of the sodium calcium exchanger exhibits anticonvulsant activity in a pilocarpine model of acute seizures in rats. Brain Res. 2010, 1366, 211–216. [Google Scholar] [CrossRef]
- N’Gouemo, P. Probing the role of the sodium/calcium exchanger in pentylenetetrazole-induced generalized seizures in rats. Brain Res. Bull. 2013, 90, 52–57. [Google Scholar] [CrossRef]
- Quansah, H.; N’Gouemo, P. Amiloride and SN-6 suppress audiogenic seizure susceptibility in genetically epilepsy-prone rats. CNS Neurosci. Ther. 2014, 20, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Li, Y.; Xia, Z.; Liu, Y.; Yu, H.; Xu, G.; Wu, X.; Zhao, R.; Zhang, G. Chronic ethanol exposure reduces the expression of NCX3 in the hippocampus of male C57BL/6 mice. Neuroreport 2019, 30, 397–403. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watano, T.; Shigekawa, M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 1996, 271, 22391–22397. [Google Scholar] [CrossRef] [PubMed]
- Faingold, C.L.; N’Gouemo, P.; Riaz, A. Ethanol and neurotransmitter interaction-from molecular to integration effects. Prog. Neurobiol. 1998, 55, 509–535. [Google Scholar] [CrossRef]
- Faingold, C.L. The Majchrowicz binge alcohol protocol: An intubation technique to study alcohol dependence in rats. Curr. Protoc. Neurosci. 2008, 44, 9.28.1–9.28.12. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, E. Induction of physical dependence on alcohol and the associated metabolic and behavioral changes in rats. Psychopharmacologia 1975, 43, 245–254. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.); Institute for Laboratory Animal Research (U.S.); National Academies Press (U.S.). Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Chakravarty, D.N.; Faingold, C.L. Comparison of neuronal response patterns in the external and central nuclei of inferior colliculus during ethanol administration and ethanol withdrawal. Brain Res. 1998, 783, 102–108. [Google Scholar] [CrossRef]
- Faingold, C.L.; Riaz, A. Ethanol withdrawal induces increased firing in the inferior colliculus neurons associated with audiogenic seizure susceptibility. Exp. Neurol. 1995, 132, 91–98. [Google Scholar] [CrossRef]
- Nicoll, D.A.; Quednau, B.D.; Qui, Z.; Xia, Y.R.; Lusis, A.J.; Philipson, K.D. Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3. J. Biol. Chem. 1996, 271, 24914–24921. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newton, J.; Akinfiresoye, L.R.; N’Gouemo, P. Inhibition of the Sodium Calcium Exchanger Suppresses Alcohol Withdrawal-Induced Seizure Susceptibility. Brain Sci. 2021, 11, 279. https://doi.org/10.3390/brainsci11020279
Newton J, Akinfiresoye LR, N’Gouemo P. Inhibition of the Sodium Calcium Exchanger Suppresses Alcohol Withdrawal-Induced Seizure Susceptibility. Brain Sciences. 2021; 11(2):279. https://doi.org/10.3390/brainsci11020279
Chicago/Turabian StyleNewton, Jamila, Luli Rebecca Akinfiresoye, and Prosper N’Gouemo. 2021. "Inhibition of the Sodium Calcium Exchanger Suppresses Alcohol Withdrawal-Induced Seizure Susceptibility" Brain Sciences 11, no. 2: 279. https://doi.org/10.3390/brainsci11020279
APA StyleNewton, J., Akinfiresoye, L. R., & N’Gouemo, P. (2021). Inhibition of the Sodium Calcium Exchanger Suppresses Alcohol Withdrawal-Induced Seizure Susceptibility. Brain Sciences, 11(2), 279. https://doi.org/10.3390/brainsci11020279






