Low Melting Temperature Sn-Bi Solder: Effect of Alloying and Nanoparticle Addition on the Microstructural, Thermal, Interfacial Bonding, and Mechanical Characteristics
Abstract
1. Introduction
2. Melting Temperature and Wetting Property of Sn-Bi Solders
2.1. Effect of Alloying Addition on the Melting Temperature of Sn-Bi Solder
2.2. Effect of Nanoparticles Addition on Melting Temperature of Sn-Bi Solder
2.3. Effect of Alloying Elements on Wetting and Spreading of Sn-Bi Solder
2.4. Effect of Nanoparticle Addition on Wetting and Spreading Property of Sn-Bi Solder
3. Microstructure and IMC
3.1. Effect of Alloying on the Sn-Bi Microstructure
3.2. Nanoparticles Effect on Sn-Bi Microstructure
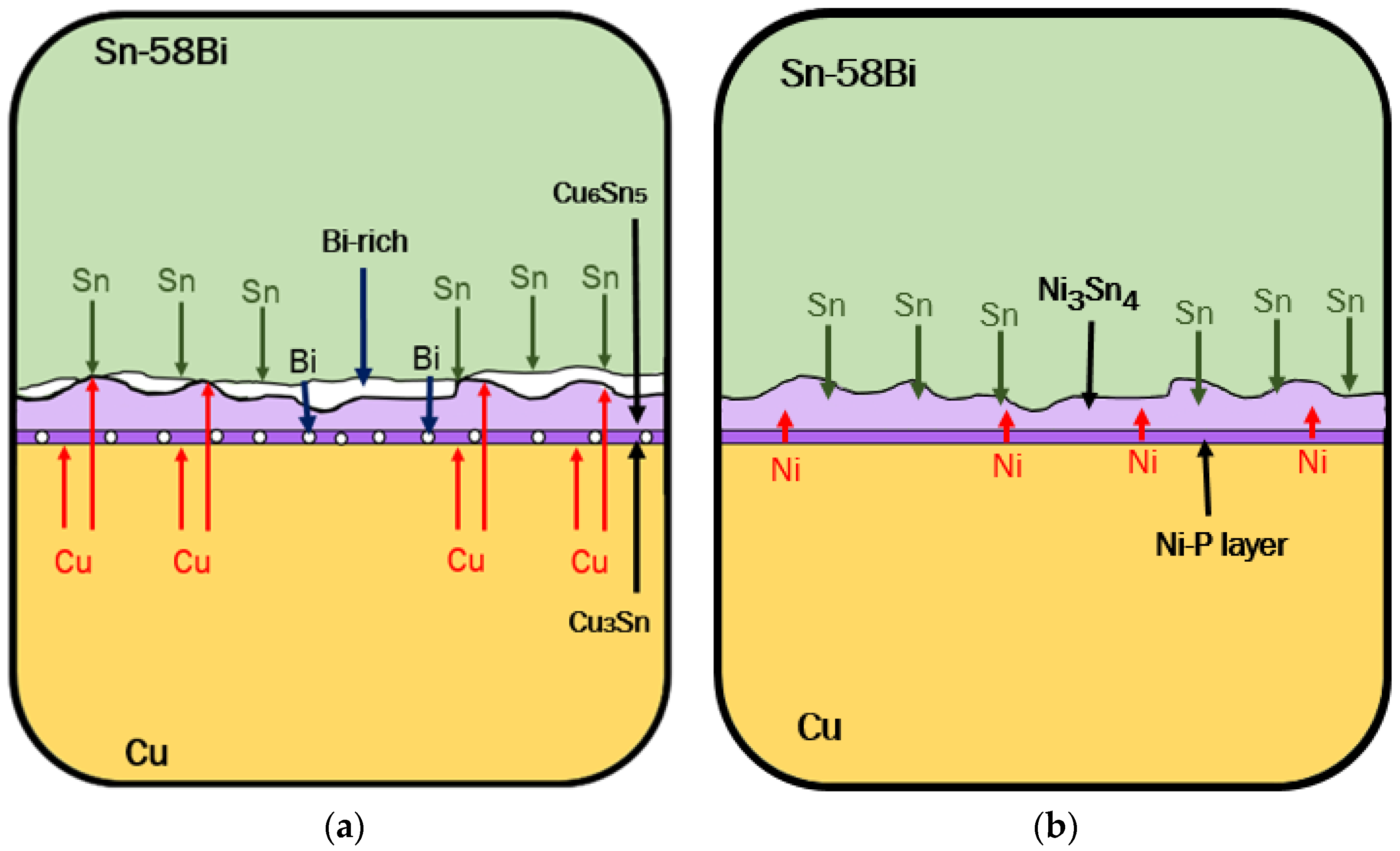
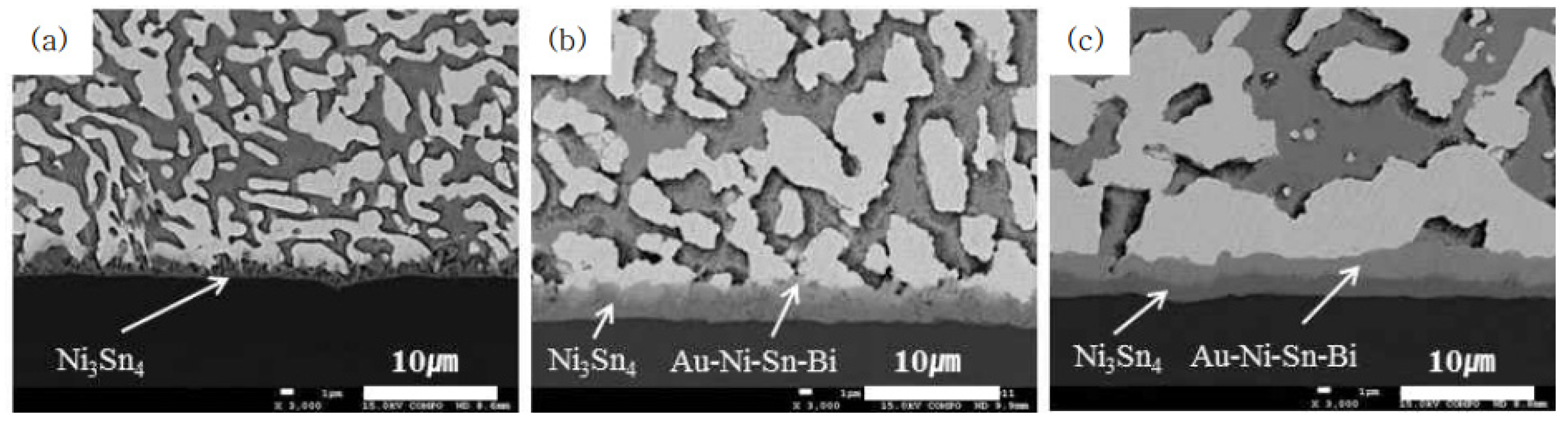
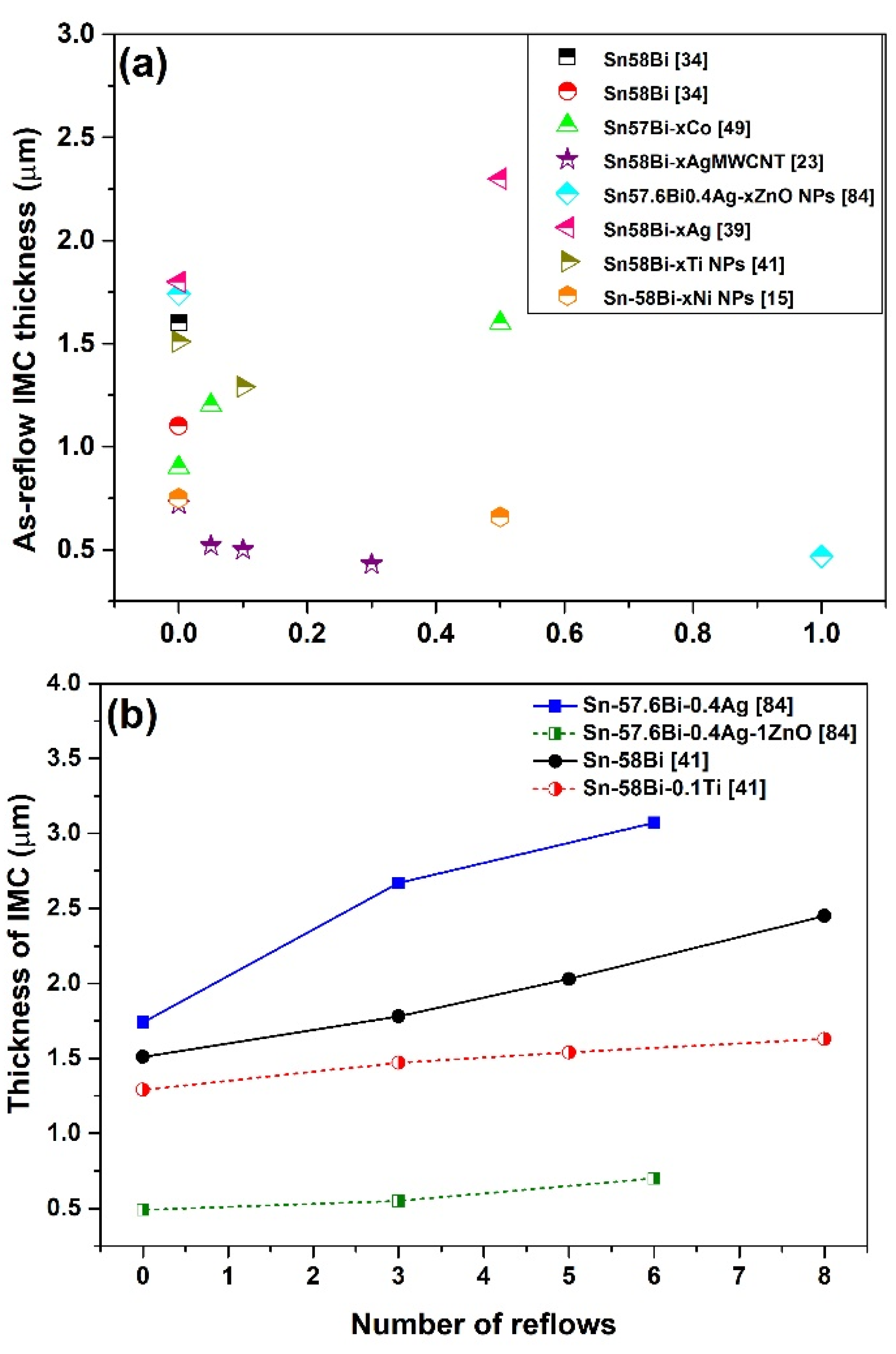
3.3. IMC at the Interface of Sn-Bi Solder
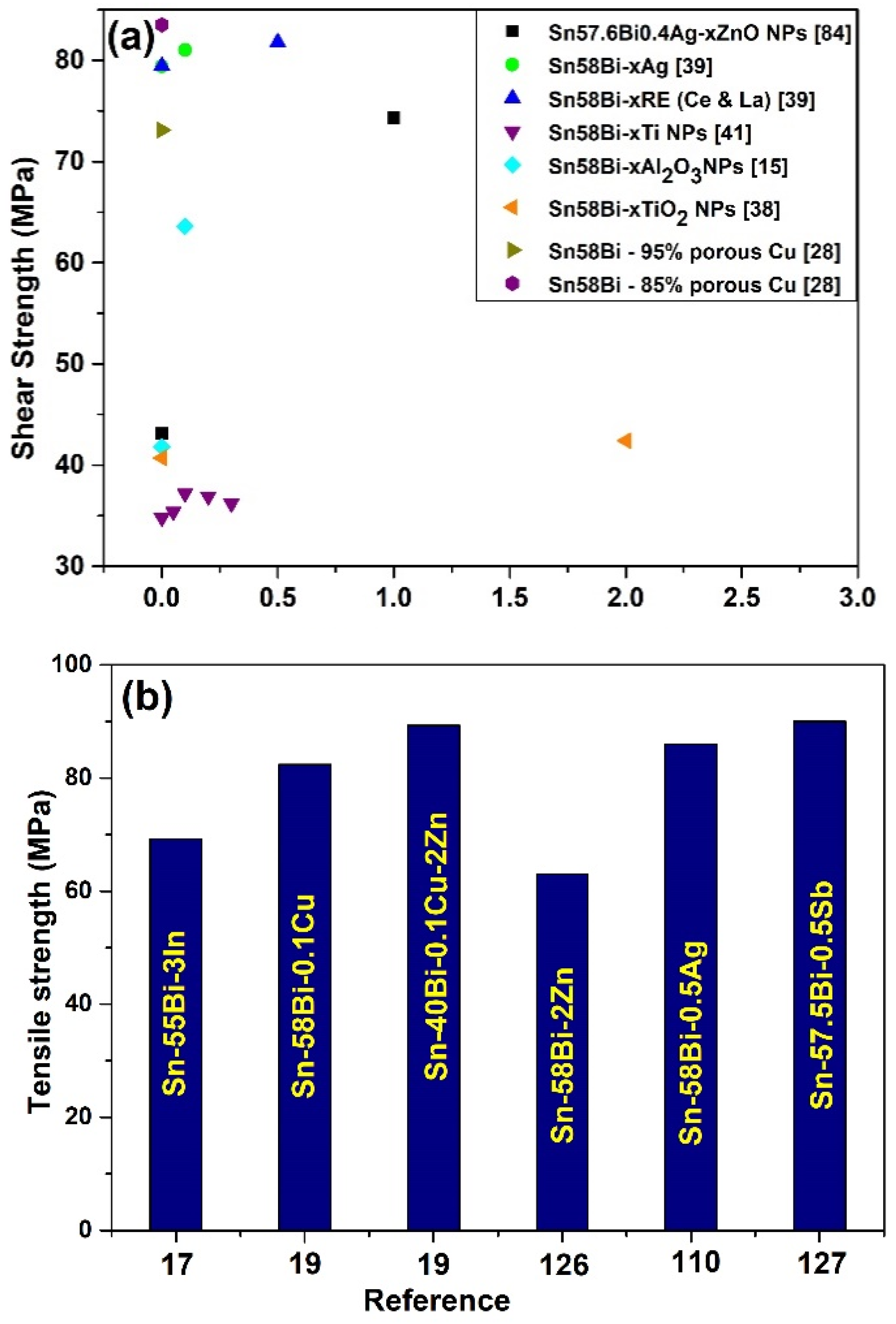
4. Mechanical Property of Sn-Bi Solder
Nanoparticle Addition on the Mechanical Property of the Sn-Bi Solder
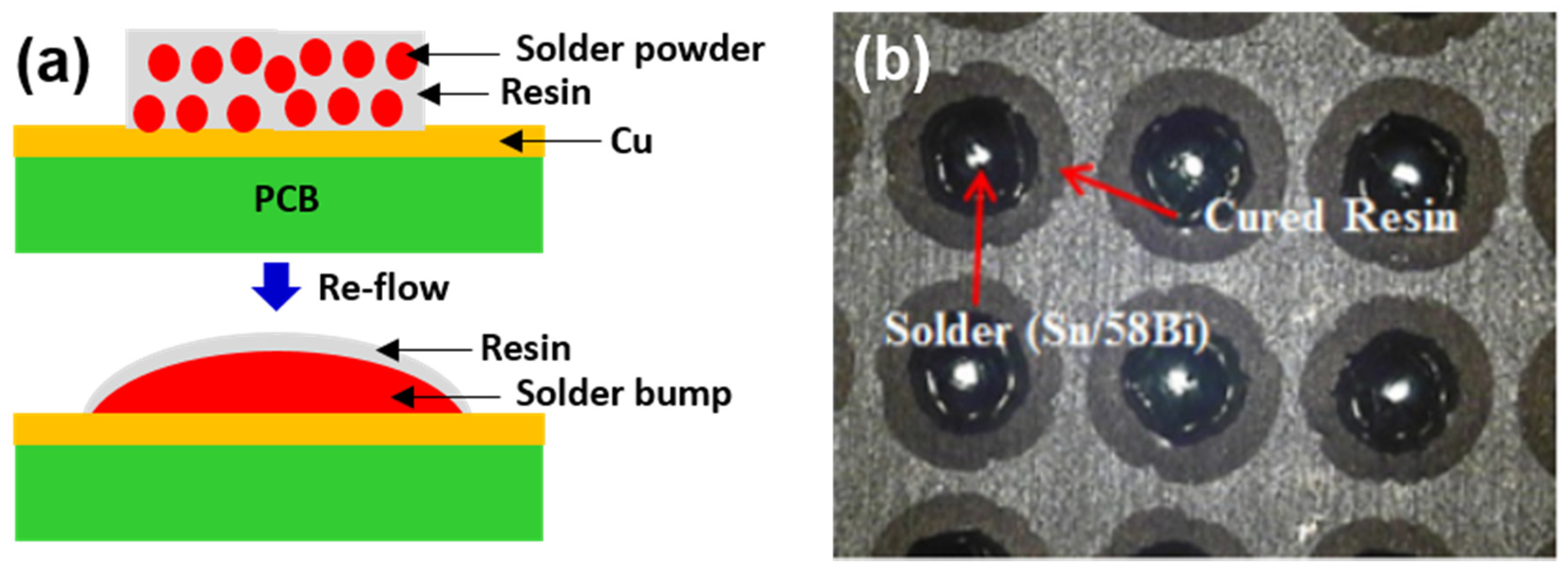
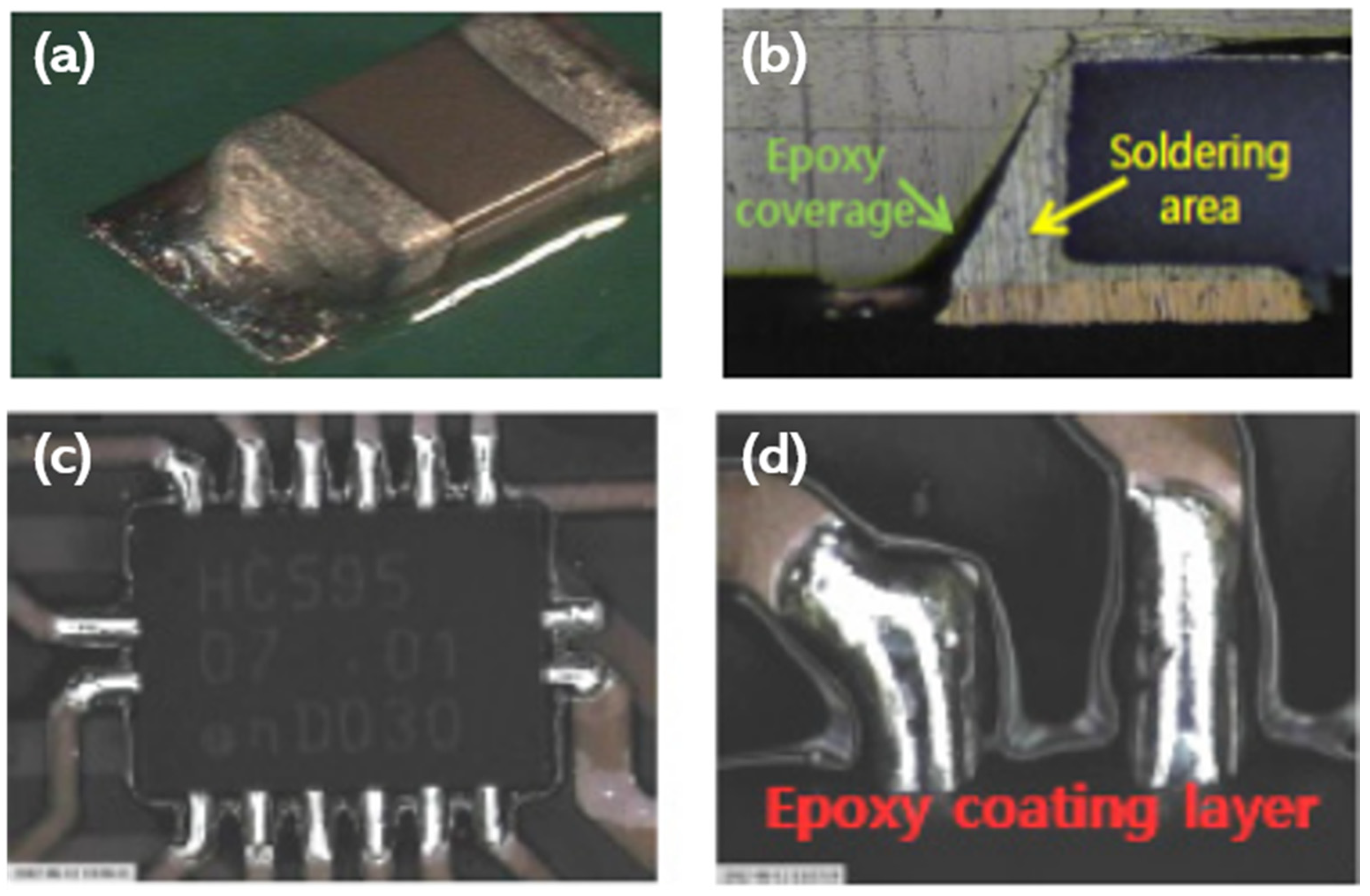
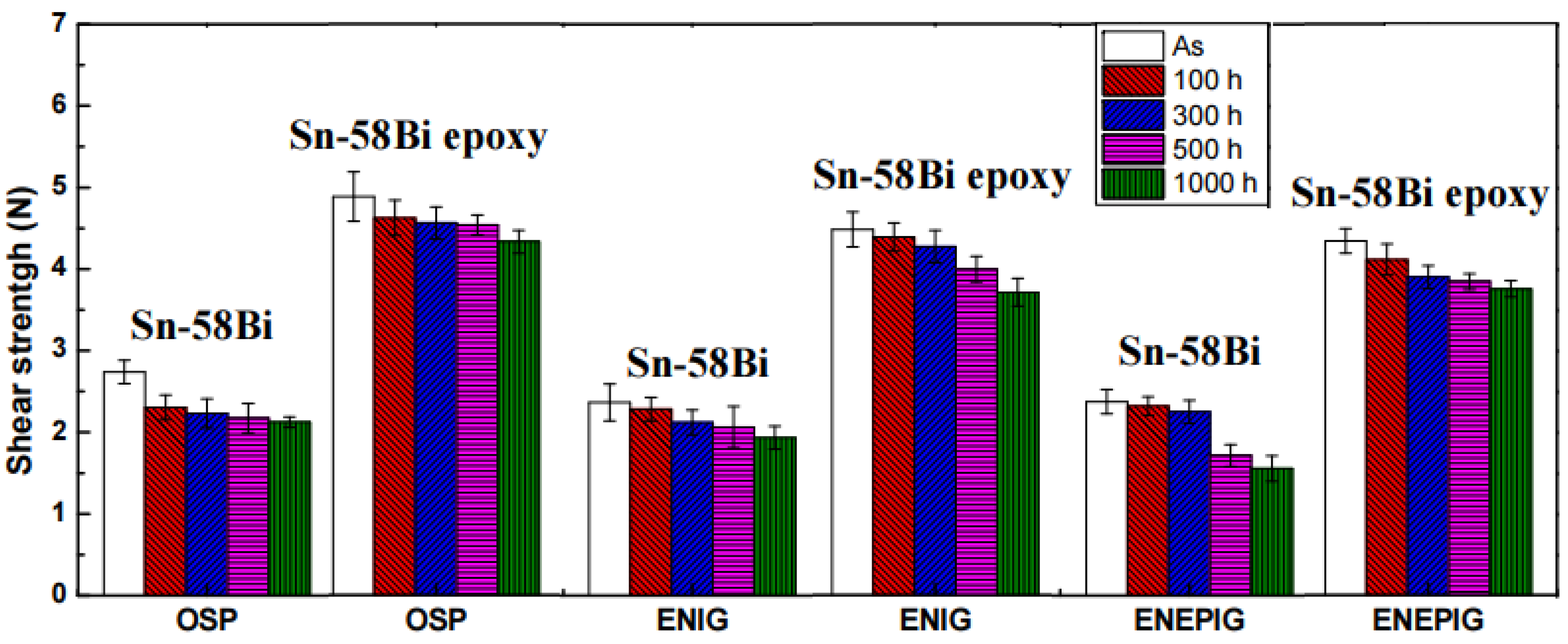
5. Epoxy Reinforced Sn-Bi Solder
- The curing temperature of epoxy is much lower than the reflow temperatures of solders (80–150 °C).
- Joining and curing occurs simultaneously.
- High reliability during thermal cycling is beneficial for automotive electronics.
- High surface insulation resistance (SIR) due to the presence of epoxy envelope protects the joint from environmental oxidation.
- No-clean technology. It provides a clean joint without residues and, hence, washing is not necessary after reflow.
- The epoxy film over the bumps resides at the necks of the solder bumps and prevents the short-circuiting and corrosion of joints after reflowing.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abtew, M.; Selvaduray, G. Lead-free solders in microelectronics. Mater. Sci. Eng. R 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Johnson, R.W.; Evans, J.L.; Jacobsen, P.; Thompson, J.R.; Christopher, M. The Changing Automotive Environment: High-Temperature Electronics. Ieee Trans. Electron. Packag. Manuf. 2004, 27, 164–176. [Google Scholar] [CrossRef]
- Nishida, H. 5G and packaging technology, recent progress/status and items to be considered. J. Jpn. Inst. Electron. Packag. 2019, 22, 596–606. [Google Scholar] [CrossRef]
- Gain, A.K.; Chan, Y.C.; Yung, K.C.; Sharif, A.; Ali, L. Effect of Nano Ni Additions on the Structure and Properties of Sn-9Zn and Sn-8Sn-3Bi Solder in Ball Grid Array Packages. In Proceedings of the 2nd Electronics System Integration Technology Conference, Greenwich, UK, 1–4 September 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1291–1294. [Google Scholar]
- Suganuma, K.; Kim, S.-J.; Kim, K.-S. High-Temperature Lead-Free Solders: Properties and Possibilities. Jom Min. Met. Mater. Soc. 2009, 61, 64–71. [Google Scholar] [CrossRef]
- Plevachuk, Y.; Hoyer, W.; Kaban, I.; Kohler, M.; Novakovic, R. Experimental study of density, surface tension, and contact angle of Sn–Sb-based alloys for high temperature soldering. J. Mater. Sci. 2010, 45, 2051–2056. [Google Scholar] [CrossRef]
- Kamal, M.; Gouda, E.S. Effect of zinc additions on structure and properties of Sn–Ag eutectic lead-free solder alloy. J. Mater. Sci. Mater. Electron. 2008, 19, 81–84. [Google Scholar] [CrossRef]
- Gain, A.K.; Fouzder, T.; Chan, Y.C.; Yung, W.K.C. Microstructure, kinetic analysis and hardness of Sn-Ag-Cu-1 wt% nano-ZrO2 composite solder on OSP-Cu pads. J. Alloys Compd. 2011, 509, 3319–3325. [Google Scholar] [CrossRef]
- Anderson, I.E. Development of Sn–Ag–Cu and Sn–Ag–Cu–X alloys for Pb-free electronic solder applications. J. Mater. Sci. Mater. Electron. 2007, 18, 55–76. [Google Scholar] [CrossRef]
- Liu, L.; Wu, P.; Zhou, W. Effects of Cu on the interfacial reactions between Sn–8Zn–3Bi–xCu solders and Cu substrate. Microelectron. Reliab. 2014, 54, 259–264. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Howes, P.D.; Mannan, S.H. On the development of low melting temperature Pb-free solders. Microelectron. Reliab. 2014, 54, 1253–1273. [Google Scholar] [CrossRef]
- Jung, J.P.; Shin, Y.E.; Lim, S.S. Pb-Free Micro Soldering, Seoul, South Korea, Samsung Books; 2005; 8p, ISBN 89-88197-43-7. [Google Scholar]
- Dongliang, M.; Ping, W. Improved microstructure and mechanical properties for Sn58Bi0.7Zn solder joint by addition of graphene nanosheets. J. Alloys Compd. 2016, 671, 127–136. [Google Scholar]
- McCluskey, P.; Munamarty, R.; Pecht, M. Popcorning in PBGA Packages During IR Reflow Soldering. Microelectron. Int. 1997, 14, 20–23. [Google Scholar] [CrossRef]
- Singh, A.; Durairaj, R. Study on Hardness and Shear Strength with Microstructure Properties of Sn52Bi/Cu + 1% Al2O3 Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 834, 1–5. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, D.; Oh, T.S. Chip Interconnection Process for Smart Fabrics Using Flip-chip Bonding of SnBi Solder. J. Microelectron. Packag. Soc. 2012, 19, 71–76. [Google Scholar] [CrossRef]
- Kim, M.S.; Ko, Y.H.; Yoo, S.; Lee, C.W. Mechanical Properties of Sn-Bi Bumps on Flexible Substrates. In Proceedings of the IEEE 63rd Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 971–975. [Google Scholar]
- Chen, X.; Xue, F.; Zhou, J.; Yao, Y. Effect of In on microstructure, thermodynamic characteristic and mechanical properties of Sn–Bi based lead-free solder. J. Alloys Compd. 2015, 633, 377–383. [Google Scholar] [CrossRef]
- Shen, J.; Pu, Y.; Yin, H.; Luo, D.; Chen, J. Effects of minor Cu and Zn additions on the thermal, microstructure and tensile properties of Sn–Bi-based solder alloys. J. Alloys Compd. 2014, 614, 63–70. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.K.; Chen, Y.X.; Song, Z.L. Influences of Ag and In alloying on Sn-Bi eutectic solder and SnBi/Cu solder joints. J. Mater. Sci. Mater. Electron. 2019, 30, 18524–18538. [Google Scholar] [CrossRef]
- Torres, A.; Hernández, L.; Domínguez, O. Effect of Antimony Additions on Corrosion and Mechanical Properties of Sn-Bi Eutectic Lead-Free Solder Alloy. Mater. Sci. Appl. 2012, 3, 355–362. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, C.-H.; Lin, S.-K.; AlHazaa, A.N.; Mokhtari, O.; Liu, X.; Nishikawa, H. Effects of Ti addition on the microstructure, mechanical properties and electrical resistivity of eutectic Sn58Bi alloy. Mater. Sci. Eng. A 2019, 744, 560–569. [Google Scholar] [CrossRef]
- Lee, C.-J.; Myung, W.-R.; Park, B.-G.; Jung, S.-B. Efect of Ag-decorated MWCNT on the mechanical and thermal property of Sn58Bi solder joints for FCLED package. J. Mater. Sci. Mater. Electron. 2020, 31, 10170–10176. [Google Scholar] [CrossRef]
- Shen, L.; Foo, A.Q.; Wang, S.; Chen, Z. Enhancing creep resistance of SnBi solder alloy with non-reactive nano fillers: A study using nanoindentation. J. Alloys Compd. 2017, 729, 498–506. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Wang, D.; Gao, H. Nano ZrO2 Particulate-reinforced Lead-Free Solder Composite. J. Mater. Sci. Technol. 2006, 22, 529–532. [Google Scholar]
- Mavoori, H.; Jin, S. New, creep-resistant, low melting point solders with ultrafine oxide dispersions. J. Electron. Mater. 1998, 27, 1216–1222. [Google Scholar] [CrossRef]
- Shen, L.; Wu, Y.; Wang, S.; Chen, Z. Creep behavior of Sn–Bi solder alloys at elevated temperatures studied by nanoindentation. J Mater Sci: Mater Electron 2017, 28, 4114–4124. [Google Scholar] [CrossRef]
- Yang, L.; Boqiao, R.; Min, Z.; Xianghua, Z.; Fenglian, S. Microstructure, mechanical, and thermal behaviors of SnBi/Cu solder joint enhanced by porous Cu. J. Mater. Sci. Mater. Electron. 2020, 31, 8258–8267. [Google Scholar]
- Lee, S.-M.; Yoon, J.-W.; Jung, S.-B. Board Level Drop Reliability of Epoxy-Containing Sn-58 mass% Bi Solder Joints with Various Surface Finishes. Mater. Trans. 2016, 57, 466–471. [Google Scholar] [CrossRef]
- Sharma, A.; Jung, D.H.; Cheon, J.S.; Jung, J.P. Epoxy Polymer Solder Pastes for Micro-Electronic Packaging Applications. J. Weld. Join. 2019, 37, 7–14. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Jeong, H.; Myung, W.-R.; Jung, S.-B. Microstructures and Drop Impact Test of SAC305, Sn58%Bi and Epoxy Sn58%Bi Solder Joint on the OSP Surface Finished PCB Substrate. J. Weld. Join. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Yim, B.-S.; Lee, J.I.; Kim, J.-M. Influence of Reductant Content within the Solderable Polymer Composite on the Coalescence and Wetting Behaviors of Low-Melting-Point Alloy Fillers. J. Weld. Join. 2019, 37, 76–81. [Google Scholar] [CrossRef]
- Moon, J.-T.; Eom, Y.-S.; Lee, J.-H. Epoxy solder paste and its applications. J. Weld. Join. 2015, 33, 32–39. [Google Scholar] [CrossRef]
- Woo-Ram, M.; Yon, G.K.; Kyung, Y.K.; Seung, B.J. Drop Reliability of Epoxy-Contained Sn-58 wt.% Bi Solder Joint with ENIG and ENEPIG Surface Finish under Temperature and Humidity Test. J. Electron. Mater. 2016, 45, 3651–3658. [Google Scholar]
- Arenas, M.F.; He, M.; Acoff, V.L. Effect of flux on the wetting characteristics of SnAg, SnCu, SnAgBi, and SnAgCu lead-free solders on copper substrates. J. Electron. Mater. 2006, 35, 1530–1536. [Google Scholar] [CrossRef]
- Zang, L.; Yuan, Z.; Zhao, H.; Zhang, X. Wettability of molten Sn–Bi–Cu solder on Cu substrate. Mater. Lett. 2009, 63, 2067–2069. [Google Scholar] [CrossRef]
- Yasuda, K.; Shohji, I.; Takemoto, T. Estimation of thermal fatigue resistance of Sn–Bi (–Ag) and Sn–Ag–Bi–Cu lead-free solders using strain rate sensitivity index. Mater. Sci. 2008, 580–582, 221–224. [Google Scholar] [CrossRef]
- Amares, S.; Bandar, T. Effect on Shear Strength and Hardness Properties of Tin Based Solder Alloy, Sn-50Bi, Sn-50Bi+2%TiO2 Nanoparticles. Adv. Mater. Res. 2020, 1159, 54–59. [Google Scholar] [CrossRef]
- Dong, W.; Shi, Y.; Xia, Z.; Lei, Y.; Guo, F. Effects of Trace Amounts of Rare Earth Additions on Microstructure and Properties of Sn-Bi-Based Solder Alloy. J. Electron. Mater. 2008, 37, 982–991. [Google Scholar] [CrossRef]
- Miao, H.W.; Duh, J.G. Microstructure evolution in Sn-Bi and Sn-Bi-Cu solder joints under thermal aging. Mater. Chem. Phys. 2001, 71, 255–271. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, M.L.; Wu, C.M.L.; Wang, L. Effect of Y2O3 particles on microstructure formation and shear properties of Sn–58Bi solder. J. Mater. Sci. Mater. Electron. 2010, 21, 1046–1054. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, L.; Liu, Z.-Q.; Sun, L.; Xiong, M.-Y.; Zhao, M.; Xu, K.-K. Infuences of doping Ti nanoparticles on microstructure and properties of Sn58Bi solder. J. Mater. Sci. Mater. Electron. 2019, 30, 17583–17590. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.D.; Qian, G.T.; Zhou, J.; Xue, F. Effect of Sb content on properties of Sn-Bi solders. Trans. Nonferrous Met. Soc. China 2014, 24, 184–191. [Google Scholar] [CrossRef]
- Ribas, M.; Kumar, A.; Kosuri, D.; Rangaraju, R.R.; Pritha, C.; Suresh, T.; Siuli, S. Low temperature soldering using Sn-Bi alloys. In Proceedings of the 2017 Surface Mount Technology Association International Conference (SMTA 2017), Rosemont, IL, USA, 17–21 September 2017; SMTA: Eden Prairie, MN, USA, 2017; pp. 201–206. [Google Scholar]
- Shalaby, R.M. Effect of silver and indium addition on mechanical properties and indentation creep behavior of rapidly solidified Bi-Sn based lead-free solder alloys. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2013, 560, 86–95. [Google Scholar] [CrossRef]
- Yim, B.-S.; Youn, H.J.; Lee, J.I.; Kim, J.-M. Influence of Carbon Nanotube Concentration on the Interconnection Properties of Solderable Isotropic and Anisotropic Conductive Adhesive. J. Weld. Join. 2020, 38, 152–157. [Google Scholar] [CrossRef]
- Ren, G.; Wilding, I.J.; Collins, M.N. Alloying influences on low melt temperature SnZn and SnBi solderalloys for electronic interconnections. J. Alloys Compd. 2016, 665, 251–260. [Google Scholar] [CrossRef]
- Dirasutisna, D.T.; Soegiono, B.; Kurniawan, B.; Yudi Masduki, M. Analysis of Thermal Properties of Solder Material Sn-Bi-Al using Differential Scanning Calorimetry (DSC). J. Adv. Res. Mater. Sci. 2016, 18, 1–9. [Google Scholar]
- Huang, Y.C.; Chen, S.W. Effects of Co alloying and size on solidification and interfacial reactions in Sne57 wt.%Bie(Co)/Cu couples. J. Electron. Mater. 2011, 40, 62–70. [Google Scholar] [CrossRef]
- Šebo, P.; Švec, S.P.; Janičkovič, D.; Illeková, E.; Zemánková, M.; Plevachuk, Y.; Sidorov, V.; Švec, P. The influence of silver content on structure and properties of Sn-Bi-Ag solder and Cu/solder/Cu joints. Mater. Sci. Eng. 2013, 571, 184–192. [Google Scholar] [CrossRef]
- Lai, Z.M.; Ye, D. Microstructure and properties of Sn-10Bi-xCu solder alloy/joint. J. Electron. Mater. 2016, 45, 3702–3711. [Google Scholar] [CrossRef]
- Miao, H.W.; Duh, J.G.; Chiou, B.S. Thermal Cycling Test in Sn-Bi and Sn-Bi-Cu Solder Joints. J. Mater. Sci. Mater. Electron. 2000, 11, 609–618. [Google Scholar] [CrossRef]
- Xu, J.; He, H.; Zhang, F.; Zhao, Z.; Hu, Q. Study of Sn-Bi-Cu Lead-free Solder. In Proceedings of the 10th Electronics Packaging Technology Conference (EPTC), Singapore, 9–12 December 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1375–1380. [Google Scholar]
- Micro-Soldering and Joining Technology; Seoul, South Korea, MPC, Korea Welding & Joining Society, Ace Inc.: Seoul, Korea, 2018; pp. 5–10. ISBN 979-11-89547-00-4. (In Korean)
- Wang, K.; Wang, F.; Huang, Y.; Qi, K. Comprehensive Properties of a Novel Quaternary Sn-Bi-Sb-Ag Solder: Wettability, Interfacial Structure and Mechanical Properties. Metals 2019, 9, 791. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, C.S.; Jung, J.P. The analysis of the withdrawal force curve of the wetting curve using 63Sn-37Pb and 96.5Sn-3.5Ag eutectic solders. J. Electron. Mater. 1999, 28, 1256–1262. [Google Scholar] [CrossRef]
- Park, J.Y.; Jung, J.P.; Kang, C.S. The analysis of the withdrawal force curve of the wetting balance curve. Ieee Trans. Compon. Packag. Technol. 1999, 22, 372–377. [Google Scholar] [CrossRef]
- Park, J.Y.; Ha, J.S.; Kang, C.S.; Shin, K.S.; Kim, M.I.; Jung, J.P. Study on the soldering in partial melting state (1) analysis of surface tension and wettability. J. Electron. Mater. 2000, 29, 1145–1152. [Google Scholar] [CrossRef]
- Hong, S.-M.; Park, J.-Y.; Jung, J.-P.; Kang, C.-S. Fluxless Wetting Properties of One-Side-Coated under Bump Metallurgy and Top Surface Metallurgy. J. Electron. Mater. 2001, 30, 937–944. [Google Scholar] [CrossRef]
- Hong, S.-M.; Park, J.-Y.; Jung, J.-P.; Kang, C.-S. Fluxless Wetting Properties of Top Substrate Metallurgy in Different Pb-Free Solders. Mater. Trans. 2001, 42, 1423–1427. [Google Scholar] [CrossRef][Green Version]
- Hong, S.-M.; Park, J.-Y.; Kang, C.-S.; Jung, J.-P. Fluxless Wetting Properties of UBM-Coated Si-Wafer to Sn-3.5 wt%Ag Solder. Ieee Trans. Compon. Packag. Technol. 2003, 26, 255–261. [Google Scholar] [CrossRef]
- Shin, Y.E.; Lim, S.S.; Jung, J.-P. Soldering, Samsung Books; Samsung: Seoul, Korea, 2002; 13p, ISBN 89-88197-16-X. [Google Scholar]
- Takemoto, T.; Makoto, M. Effect of Excess Temperature above Liquidus of Lead-Free Solders on Wetting Time in a Wetting Balance Test. Mater. Trans. 2001, 42, 745–750. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Zhu, L.; Zhang, Y.C.; Liu, P.; Zhang, N.; Zhou, S.Y.; Jiang, L.C. Microstructure and reliability of Mo nanoparticle reinforced Sn–58Bi-based lead-free solder joints. Mater. Sci. Technol. 2018, 34, 992–1002. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, K.N. Structure and properties of lead-free solders bearing micro and nano particles. Mater. Sci. Eng. 2014, 82, 1–32. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Y.C. Recent advances in nanocomposite solders. Microelectron. Reliab. 2009, 49, 223–234. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Interfacial microstructure, wettability and material properties of nickel (Ni) nanoparticle doped tin-bismuth-silver (Sn-Bi-Ag) solder on copper (Cu) substrate. J. Mater. Sci. Mater. Electron. 2016, 27, 3982–3994. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kang, J.-W.; Yim, S.-H.; Jung, J.-P. Microstructure and Mechanical Properties of Sn-Bi Solder Reinforced with SnO2 Nanoparticles. In Proceedings of the 6th International Conference on Mathematics, Science, and Education (ICMSE 2019), Semarang, Indonesia, 9–10 October 2019. [Google Scholar]
- Ma, Y.; Li, X.; Yang, L.; Zhou, W.; Wang, M.; Zhu, W.; Wu, P. Effects of graphene nanosheets addition on microstructure and mechanical properties of SnBi solder alloys during solid-state aging. Mater. Sci. Eng. A 2017, 696, 437–444. [Google Scholar] [CrossRef]
- Vizdal, J.; Braga, H.; Kroupa, A.; Richter, K.; Soares, D.; Malheiros, L.; Ferreira, J. Thermodynamic assessment of the Bi-Sn-Zn system. Comput. Coupling Phase Diagr. Thermochem. 2007, 31, 438–448. [Google Scholar] [CrossRef]
- Lee, B.-J.; Oh, C.-S.; Shim, J.-H. Thermodynamic Assessments of the Sn-In and Sn-Bi Binary Systems. J. Electron. Mater. 1996, 25, 983–991. [Google Scholar] [CrossRef]
- Vianco, P.T.; Rejent, J.A. Properties of Ternary Sn-Ag-Bi Solder Alloys: Part I—Thermal Properties and microstructural Analysis. J. Electron. Mater. 1999, 28, 1127–1137. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Mokhtari, O.; Nishikawa, H. The Evaluation of Mechanical Properties of Sn58BiXTi Solder by Tensile Test. In Proceedings of the 18th International Conference on Electronic Packaging Technology (ICEPT), Harbin, China, 16–19 August 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 703–707. [Google Scholar]
- Sakuyama, S.; Akamatsu, T.; Uenishi, K.; Sato, T. Low Temperature Sn–Bi Soldering Technology for Low Stress Packaging. Ieice Trans. Electron. 2008, 11, 534–541. [Google Scholar]
- Mokhtari, O.; Nishikawa, H. Correlation between microstructure and mechanical properties of Sn-Bi-X solders. Mater. Sci. Eng. A 2016, 651, 831–839. [Google Scholar] [CrossRef]
- Zhou, S.; Mokhtari, O.; Rafique, M.G.; Shunmugasamy, V.C.; Mansoor, B.; Nishikawa, H. Improvement in the mechanical properties of eutectic Sn58Bi alloy by 0.5 and 1 wt% Zn addition before and after thermal aging. J. Alloys Compd. 2018, 765, 1243–1252. [Google Scholar] [CrossRef]
- He, M.; Acoff, V. Effect of Bi on the Interfacial Reaction between Sn-3.7Ag-xBi Solders and Cu. J. Electron. Mater. 2008, 37, 288–299. [Google Scholar] [CrossRef]
- Zhai, Q.J.; Guan, S.K.; Shang, Q.Y. Alloy Thermo-Mechanism: Theory and Application; Metallurgy Industry Press: Beijing, China, 1999. [Google Scholar]
- Zhang, L.; Tao, W.; Liu, J.; Zhang, Y.; Cheng, Z.; Andersson, C.; Gao, Y.; Zhai, Q. Manufacture, Microstructure and Microhardness Analysis of Sn-Bi Lead-Free Solder Reinforced with Sn-Ag-Cu Nano-Particles. In Proceedings of the 2008 International Conference on Electronic Packaging Technology & High Density Packaging, Shanghai, China, 28–31 July 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1–5. [Google Scholar]
- Lin, D.C.; Liu, S.; Guo, T.M.; Wang, G.-X.; Srivatsan, T.S.; Petraroli, M. An investigation of nanoparticles addition on solidification kinetics and microstructure development of tin-lead solder. Mater. Sci. Eng. A 2003, 360, 285–292. [Google Scholar] [CrossRef]
- Lin, D.C.; Wang, G.X.; Srivatsan, T.S.; Al-Hajri, M.; Petraroli, M. Influence of titanium dioxide nanopowder addition on microstructural development and hardness of tin–lead solder. Mater. Lett. 2003, 57, 3193–3198. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Mongkolwongrojn, M.; Ariga, T. Influence of indium addition on characteristics of Sn–0.3Ag–0.7Cu solder alloy. J. Alloy. Compd. 2009, 485, 225–230. [Google Scholar] [CrossRef]
- Wu, N.; Ismathullakhan, S.; Chan, Y.C. Effect of 1 wt% ZnO nanoparticles addition on the microstructure, IMC development, and mechanical properties of high Bi content Sn–57.6Bi–0.4Ag solder on Ni metalized Cu pads. J Mater Sci. Mater Electron 2014, 25, 2169–2176. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L.; Chan, Y.C. Microstructure, elastic modulus and shear strength of alumina (Al2O3) nanoparticles-doped tin–silver–copper (Sn–Ag–Cu) solders on copper (Cu) and gold/nickel (Au/Ni)-plated Cu substrates. J. Mater. Sci. Mater. Electron. 2015, 26, 7039. [Google Scholar] [CrossRef]
- Fouzder, T.; Chan, Y.C.; Chan, D.K. Influence of cerium oxide (CeO2) nanoparticles on the microstructure and hardness of tin–silver–copper (Sn–Ag–Cu) solders on silver (Ag) surface-finished copper (Cu) substrates. J. Mater. Sci. Mater. Electron. 2014, 25, 5375–5387. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Microstructure, mechanical and electrical performances of zirconia nanoparticles-doped tin-silver-copper solder alloys. J. Mater. Sci. Mater. Electron. 2016, 27, 7524–7533. [Google Scholar] [CrossRef]
- He, P.; An, J.; Ma, X.; Chen, S.; Qian, Y.Y.; Lin, T.S. Investigation preparation method and soldering behaviors of Sn-58Bi lead-free solder with carbon nanotubes. Trans. China Weld. Inst. 2011, 32, 9–12. [Google Scholar]
- Yang, L.; Zhou, W.; Liang, Y.; Cui, W.; Wu, P. Improved microstructure and mechanical properties for Sn58Bi solder alloy by addition of Ni-coated carbon nanotubes. Mater. Sci. Eng. A. 2015, 642, 7–15. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Zhou, W.; Wu, P. Effects of nanoscale Cu6Sn5 particles addition on microstructure and properties of SnBi solder alloys. Mater. Sci. Eng. A 2017, 684, 328–334. [Google Scholar] [CrossRef]
- Kang, J. Microstructure and mechanical proerpties of Sn-Bi solder reinforced with SnO2 nanoparticles. Master’s Thesis, University of Seoul, Seoul, Korea, 2019. [Google Scholar]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.M. A numerical method to determine interdiffusion coefficients of Cu6Sn5 and Cu3Sn intermetallic compounds. Intermetallics 2013, 40, 50–59. [Google Scholar] [CrossRef][Green Version]
- Onishi, M.; Fujibuchi, H. Reaction-diffusion in the Cu-Sn system. Trans. Jpn. Inst. Met. 1975, 16, 539–547. [Google Scholar] [CrossRef][Green Version]
- Wu, C.M.L.; Yua, D.Q.; Law, C.M.T.; Wang, L. Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. R 2004, 44, 1–44. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, C.B.; Jung, S.B. Interfacial Reactions Between Sn-58 mass % Bi Eutectic Solder and (Cu, Electroless Ni–P/Cu) Substrate. Mater. Trans. 2002, 43, 1821–1826. [Google Scholar] [CrossRef]
- Wang, F. Interfacial Reaction and Mechanical Properties of Sn-Bi solder joint. Materials 2017, 10, 920. [Google Scholar] [CrossRef]
- Liu, P.L.; Shang, J.K. Interfacial segregation of bismuth in copper/tin-bismuth solder interconnect. Scr. Mater. 2001, 44. [Google Scholar] [CrossRef]
- Liu, P.L.; Shang, J.K. Interfacial embrittlement by bismuth segregation in copper/tin-bismuth Pb-free solder interconnect. J. Mater. Res. 2001, 16, 1651–1659. [Google Scholar] [CrossRef]
- Hu, F.Q.; Zhang, Q.K.; Jiang, J.J.; Song, Z.L. Influences of Ag addition to Sn-58Bi solder on SnBi/Cu interfacial Reaction. Mater. Lett. 2020, 214, 142–145. [Google Scholar] [CrossRef]
- Shen, L.; Tan, Z.Y.; Chen, Z. Nano indentation study on the creep resistance ofSnBi solder alloy with reactive nano-metallic fillers. Mater. Sci. Eng., A 2013, 561, 232–238. [Google Scholar] [CrossRef]
- Lin, S.K.; Nguyen, T.L.; Wu, S.C.; Wang, Y.H. Effective suppression of interfacial intermetallic compound growth between Sn–58 wt.% Bi solders and Cu substrates by minor Ga addition. J.Alloys Compd. 2014, 586, 319–327. [Google Scholar] [CrossRef]
- Wang, F.J.; Zhou, L.L.; Wang, X.J.; He, J.P. Microstructural evolution and joint strength of Sn-58Bi/Cu joints through minor Zn alloying substrate during isothermal aging. J. Alloys Compd. 2016, 688, 639–648. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.W.; Li, Y.L.; Zhang, R.H. Effect of alloying Cu substrate on microstructure and coarsening behavior of Cu 6 Sn 5 grains of soldered joints. J. Mater. Sci. Mater. Electron. 2015, 26, 2782–2794. [Google Scholar] [CrossRef]
- Pang, X.Y.; Liu, Z.Q.; Wang, S.Q.; Shang, J.K. Effects of Bi segregation on the tensile properties of Cu/Cu3Sn (1 0 0) interface. Microelectron. Reliab. 2011, 51, 2330–2335. [Google Scholar] [CrossRef]
- Shin, H.P.; Ahn, B.W.; Ahn, J.H.; Lee, J.G.; Kim, K.S.; Kim, D.H.; Jung, S.B. Interfacial Reaction and Joint Strength of the Sn-58% Bi Solder Paste with ENIG Surface Finished Substrate. J. Weld. Join. 2012, 30, 458–463. [Google Scholar]
- Han, D.Y.; Jing, Y.H.; Nai, M.L.S.; Xu, L.Y.; Tan, C.M.; Wei, J. Interfacial reaction and shear strength of Ni-coated carbon nanotubes reinforced Sn–Ag–Cu solder joints during thermal cycling. Intermetallics 2012, 31, 72–78. [Google Scholar] [CrossRef]
- Shimokawa, H.; Soga, T.; Serizawa, K.; Katayama, K.; Shohji, I. Evaluation on Mechanical Properties of Sn-Bi-Ag Solder and Reliability of the Solder Joint. Trans. Jpn. Inst. Electron. Packag. 2015, 8, 46–54. [Google Scholar] [CrossRef]
- Mokhtari, O.; Nishikawa, H. Effects of In and Ni addition on microstructure of Sn-58Bi solder joints. J. Electron. Mater. 2014, 43, 4158–4170. [Google Scholar] [CrossRef]
- Sakuyama, S.; Akamatsu, T.; Uenishi, K.; Sato, T. Effects of a Third Element on Microstructure and Mechanical Properties of Eutectic Sn-Bi Solder. Trans. Jpn. Inst. Electron. Packag. 2009, 2, 98–103. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Ibrahiem, A.A. Influence of rotating magnetic field on solidification microstructure and tensile properties of Sn-Bi lead-free solder. Microelectron. Reliab. 2018, 81, 352–361. [Google Scholar] [CrossRef]
- Takao, H.; Yamada, A.; Hasegawa, H. Mechanical properties and solder joint reliability of low-melting Sn–Bi–Cu lead free solder alloy. J. Jpn. Inst. Electron. Packag. 2004, 39, 49–56. [Google Scholar]
- Hu, X.; Li, K.; Min, Z. Microstructure evolution and mechanical properties of Sn0.7Cu0.7Bi lead-free solders produced by directional solidification. J. Alloys Compd. 2013, 566, 239–245. [Google Scholar] [CrossRef]
- EI-Daly, A.A.; Swilem, Y.; Makled, M.H. Thermal and mechanical properties of Sn–Zn–Bi lead-free solder alloys. J. Alloys Compd. 2009, 484, 134–142. [Google Scholar] [CrossRef]
- Lee, C.W.; Shin, Y.; Yoo, S. Effect of SiC Nanoparticles Dispersion on the Microstructure and Mechanical Properties of Electroplated Sn-Bi Solder Alloy. J. Nano Res. 2010, 11, 113–118. [Google Scholar] [CrossRef]
- Choi, K.S.; Joo, J.; Jang, K.-S.; Choi, G.M.; Yun, H.G.; Moon, S.H.; Eom, Y.S. Laser-assisted bonding, its bonding materials and their applications. J. Weld. Join. 2020, 38, 138–144. [Google Scholar] [CrossRef]
- Bismarck, L.S.; Marcella, G.C.X.; Amauri, G.; José, E.S. Cu and Ag additions affecting the solidification microstructure and tensile properties of Sn-Bi lead-free solder alloys. Mater. Sci. Eng. A 2017, 705, 325–334. [Google Scholar]
- Li, J.F.; Mannan, S.H.; Clode, M.P.; Whalley, D.C.; Hutt, D.A. Interfacial reactions between molten Sn–Bi–X solders and Cu substrates for liquid solder interconnects. Acta Mater. 2006, 54, 2907–2922. [Google Scholar] [CrossRef]
- Kim, Y. The Improvement of Bonding Strength with an Addition of Epoxy and the Effects of Various Surface Finishes on Sn-58% Bi Epoxy Solder. In Proceedings of the 12th International Symposium on Microelectronics and Packaging (ISMP), Busan, South Korea, 15–16 October 2014. [Google Scholar]
- Efzan Mhd Noor, E.; Singh, A.; Tze Chuan, Y. A review influence of nano particles reinforced on solder alloy. Solder. Surface Mount Technol. 2013, 25, 229–241. [Google Scholar] [CrossRef]
- Li, Y.; Chan, Y.C. Effect of silver (Ag) nanoparticle size on the microstructure and mechanical properties of Sn58Bi–Ag composite solders. J. Alloys Compd. 2015, 645, 566–576. [Google Scholar] [CrossRef]
- Ramakrishnan, N. An analytical study on strengthening of particulate reinforced metal matrix composites. Acta Mater. 1996, 44, 69–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Dai, L.H.; Ling, Z.; Bai, Y.L. Size-dependent inelastic behavior of particle-reinforced metal-matrix composites. Compos. Sci. Technol. 2001, 61, 1057–1063. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, L.; Lee, C.; Gupta, M. Properties and deformation behavior of Mg-Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Ma, D.L.; Wu, P. Effects of Zn addition on mechanical properties of eutectic Sn-58Bi solder during liquid-state aging. Trans. Nonferrous. Met. Soc. China 2015, 24, 1225–1233. [Google Scholar] [CrossRef]
- Kubota, Y.; Shohji, I.; Tsuchida, T.; Nakamura, K. Tensile Properties of Low-Melting Point Sn-Bi-Sb solder. In Proceedings of the 16th IEEE Electronics Packaging Technology Conference, (EPTC ’14), Singapore, 3–5 December 2014. [Google Scholar]
- Li, J.F.; Mannan, S.H.; Clode, M.P. Interactions between Liquid Sn-Bi Based Solders and Contactmetals for High Temperature Applications. In Proceedings of the 55th Electronic Components and Technology Conference (ECTC ’05), Lake Buena Vista, FL, USA, 31 May 2005; pp. 441–448. [Google Scholar]
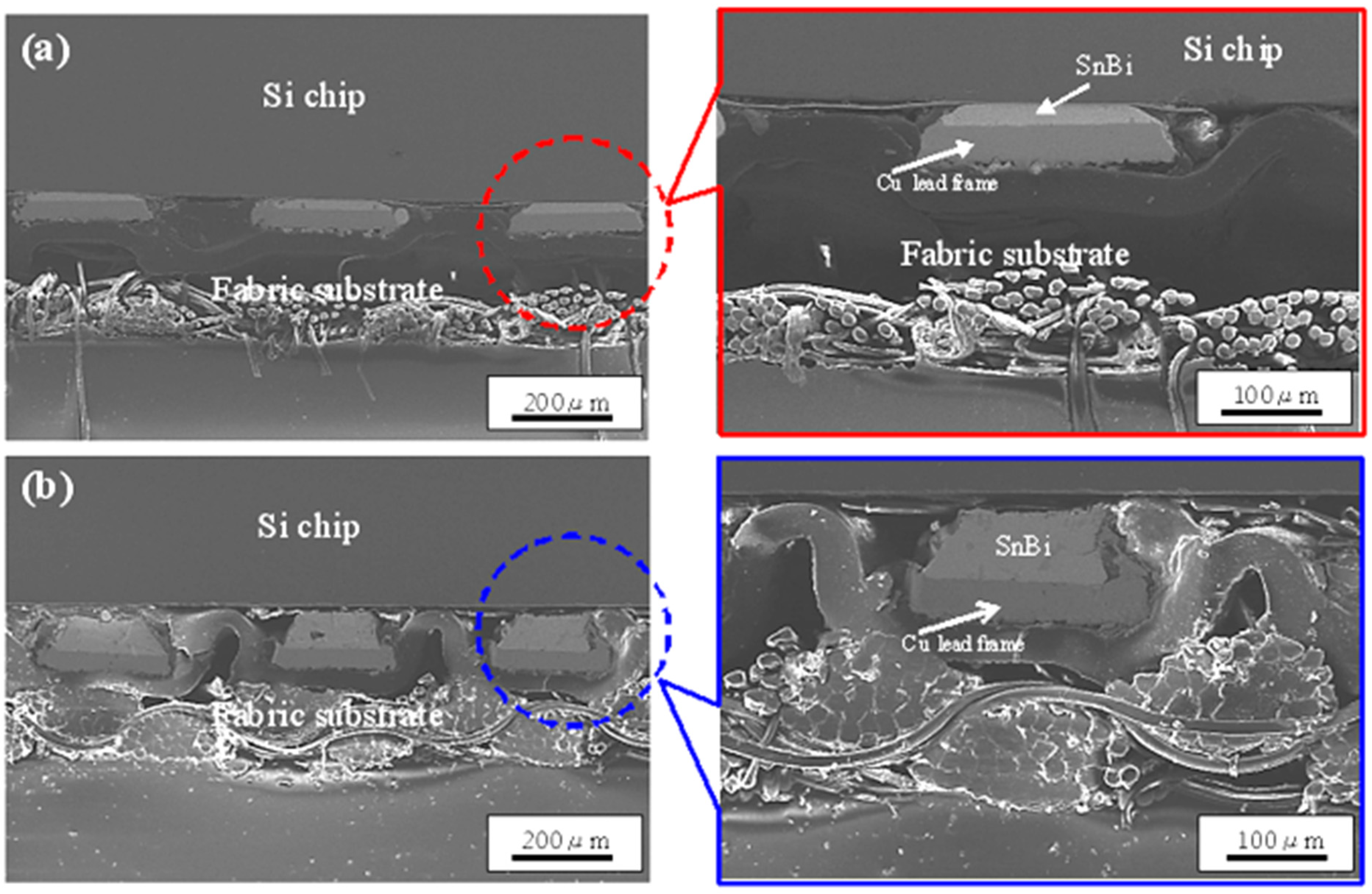
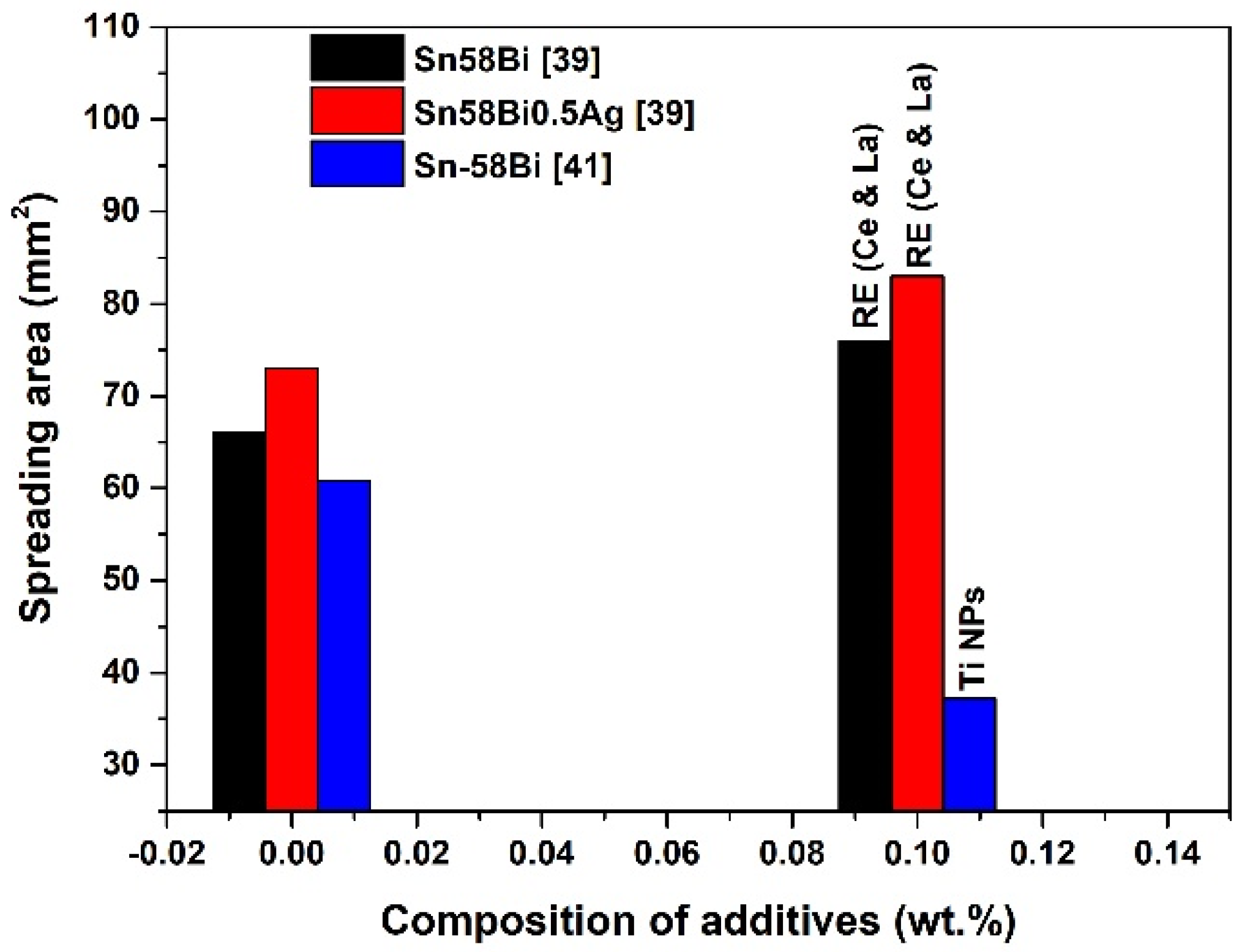
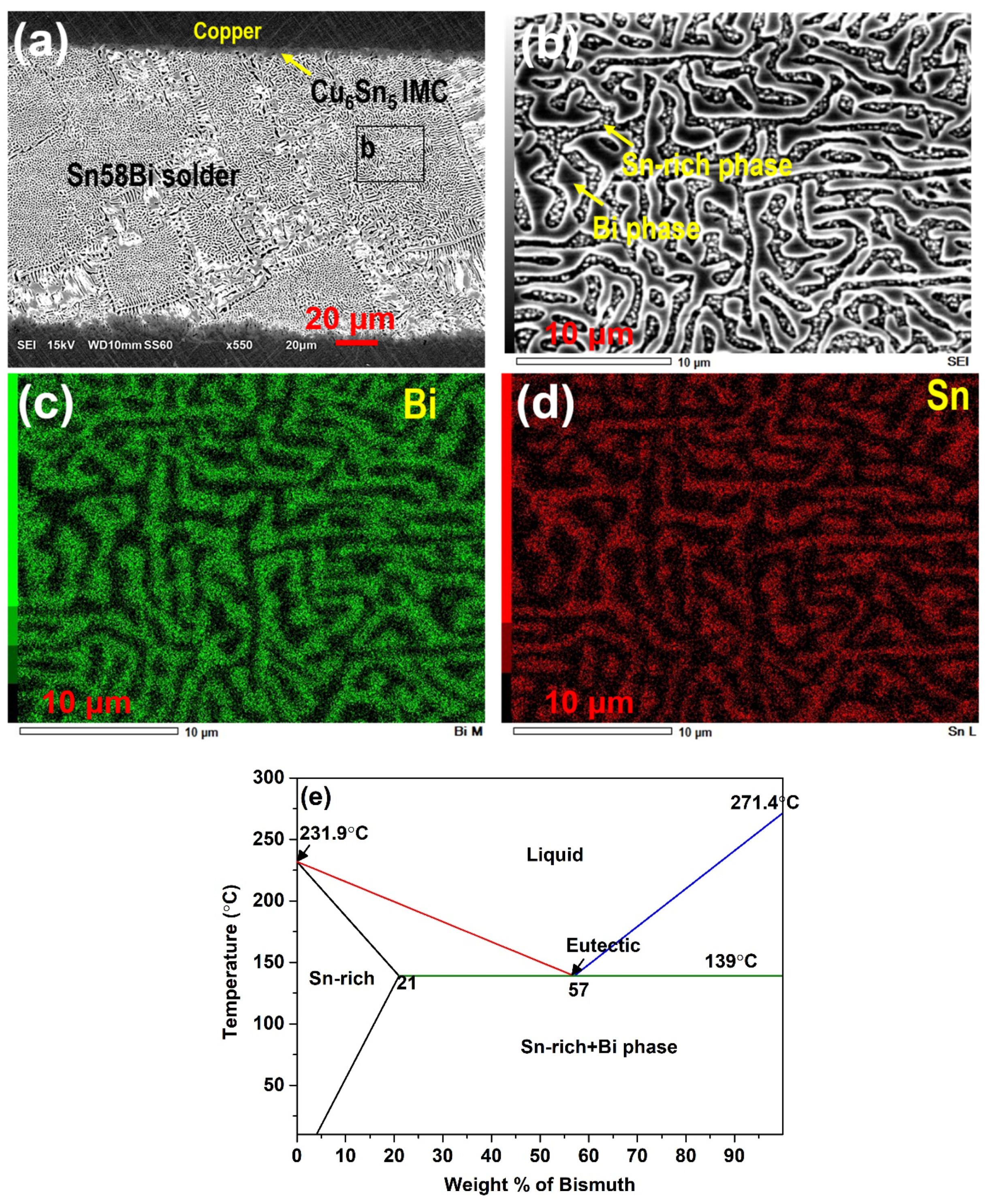
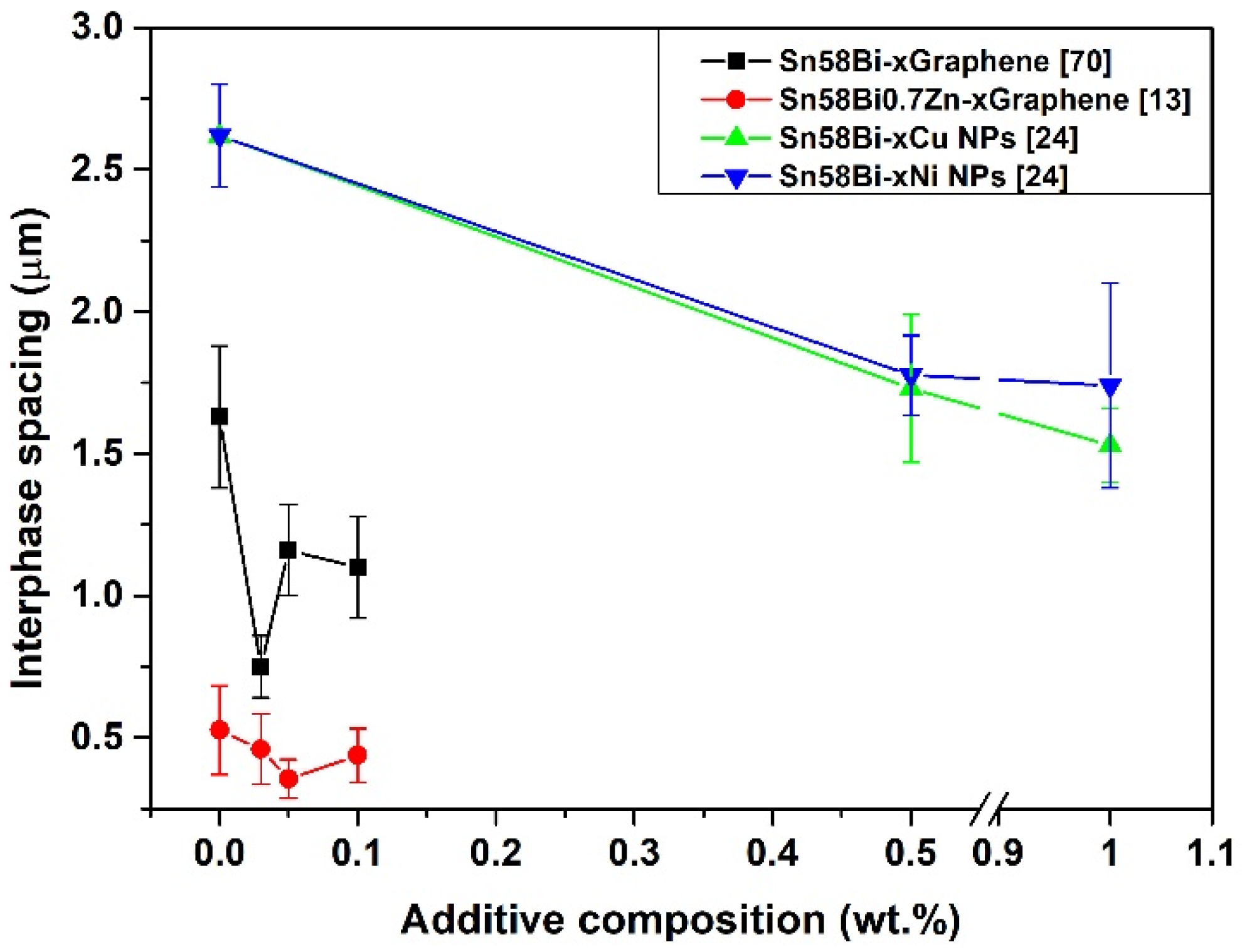
| Solder Composition | Solidus (°C) | Liquidus (°C) | Solidification Interval (°C) | Reference |
|---|---|---|---|---|
| Sn-58Bi | 130.2 | 139 | 8.8 | [19] |
| Sn–58Bi | 136.1 | 139.1 | 3 | [14] |
| Sn–58Bi | 136.1 | 139.1 | 3.0 | [40] |
| Sn-58Bi | 139.0 | 143.1 | 4.1 | [22] |
| Sn-58Bi | 139.3 | 147.6 | 8.3 | [15] |
| Sn-58Bi | 139.5 | 147.6 | 8.1 | [21] |
| Sn-58Bi | 139.4 | 148.0 | 8.6 | [16] |
| Sn-58Bi | 139.6 | 147.4 | 7.8 | [47] |
| Sn-58Bi | 140.3 | 146.0 | 5.7 | [49] |
| Sn-55Bi | 138 | 144 | 6 | [46] |
| Sn-50Bi | 138 | 155 | 17 | [46] |
| Sn-45Bi | 138 | 168 | 30 | [46] |
| Sn-40Bi | 138 | 178 | 40 | [46] |
| Sn-35Bi | 138 | 186 | 48 | [46] |
| Sn-58Bi-0.5Ti | 138.9 | 142.7 | 3.8 | [22] |
| Sn-58Bi-1Ti | 139.1 | 143.4 | 4.3 | [22] |
| Sn-58Bi-0.1Ag | 136.2 | 139.7 | 3.5 | [14] |
| Sn-57.6Bi-0.4Ag | 137 | 142 | 5 | [46] |
| Sn-58Bi-0.5Ag | 135.7 | 138.2 | 2.5 | [14] |
| Sn-58Bi-0.5Ag-0.1RE (RE = Ce and La) | 136.6 | 139.1 | 2.5 | [14] |
| Sn-58Bi-1Ag | 137 | 142 | 5 | [46] |
| Sn-38Bi-1Ag | 137 | 176 | 39 | [46] |
| Sn-58Bi-1Ag-1In | 133 | 137 | 4 | [46] |
| Sn-58Bi-1Ag-3In | 125 | 133 | 8 | [46] |
| Sn-58Bi-2Ag | 139.1 | 145.4 | 6.3 | [47] |
| Sn-58Bi-2Ag-2In | 131.2 | 136.0 | 4.8 | [47] |
| Sn-58Bi-2.0In | 129.8 | 135.0 | 5.2 | [47] |
| Sn-58Bi-3.0In | 119.9 | 140.5 | 20.6 | [15] |
| Sn-58Bi-4.0Ag | 138.1 | 145.5 | 7.4 | [15] |
| Sn-58Bi-3In4Ag | 116.9 | 138.1 | 21.2 | [15] |
| Sn-58Bi-0.05Co | 140.3 | 147.0 | 6.7 | [49] |
| Sn-58Bi-0.5Co | 140.1 | 145.0 | 4.9 | [49] |
| Sn-58Bi-0.1RE (RE = Ce and La) | 136.2 | 139.7 | 3.5 | [39] |
| Sn–40Bi–0.1Cu | 125.1 | 132.2 | 7.1 | [19] |
| Sn–40Bi–2Zn–0.1Cu. | 127.7 | 136.3 | 8.6 | [19] |
| Sn-56.2Bi-3.1Sb | 139.5 | 147.6 | 8.1 | [21] |
| Sn-53.2Bi-5.8Sb | 139.5 | 149.2 | 9.7 | [21] |
| Sn-52Bi-1.8Sb | 140.6 | 152.0 | 11.4 | [16] |
| Sn-44Bi-1.8Sb | 141.9 | 180.5 | 38.6 | [16] |
| Sn-48Bi-1.0Sb | 140.6 | 168.7 | 28.1 | [16] |
| Sn-48Bi-1.4Sb | 141.2 | 170.4 | 29.2 | [16] |
| Sn-48Bi-1.8Sb | 140.9 | 172.7 | 31.8 | [16] |
| Sn-48Bi-2.0Sb | 142.3 | 169.7 | 27.4 | [16] |
| Sn-48Bi-2.4Sb | 142.8 | 169.3 | 26.5 | [16] |
| Sn-48Bi-2.8Sb | 143.6 | 168.4 | 24.8 | [16] |
| Sn-20Bi-10In | 143 | 193 | 50 | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Rajendran, S.H.; Jung, J.P. Low Melting Temperature Sn-Bi Solder: Effect of Alloying and Nanoparticle Addition on the Microstructural, Thermal, Interfacial Bonding, and Mechanical Characteristics. Metals 2021, 11, 364. https://doi.org/10.3390/met11020364
Kang H, Rajendran SH, Jung JP. Low Melting Temperature Sn-Bi Solder: Effect of Alloying and Nanoparticle Addition on the Microstructural, Thermal, Interfacial Bonding, and Mechanical Characteristics. Metals. 2021; 11(2):364. https://doi.org/10.3390/met11020364
Chicago/Turabian StyleKang, Hyejun, Sri Harini Rajendran, and Jae Pil Jung. 2021. "Low Melting Temperature Sn-Bi Solder: Effect of Alloying and Nanoparticle Addition on the Microstructural, Thermal, Interfacial Bonding, and Mechanical Characteristics" Metals 11, no. 2: 364. https://doi.org/10.3390/met11020364
APA StyleKang, H., Rajendran, S. H., & Jung, J. P. (2021). Low Melting Temperature Sn-Bi Solder: Effect of Alloying and Nanoparticle Addition on the Microstructural, Thermal, Interfacial Bonding, and Mechanical Characteristics. Metals, 11(2), 364. https://doi.org/10.3390/met11020364






