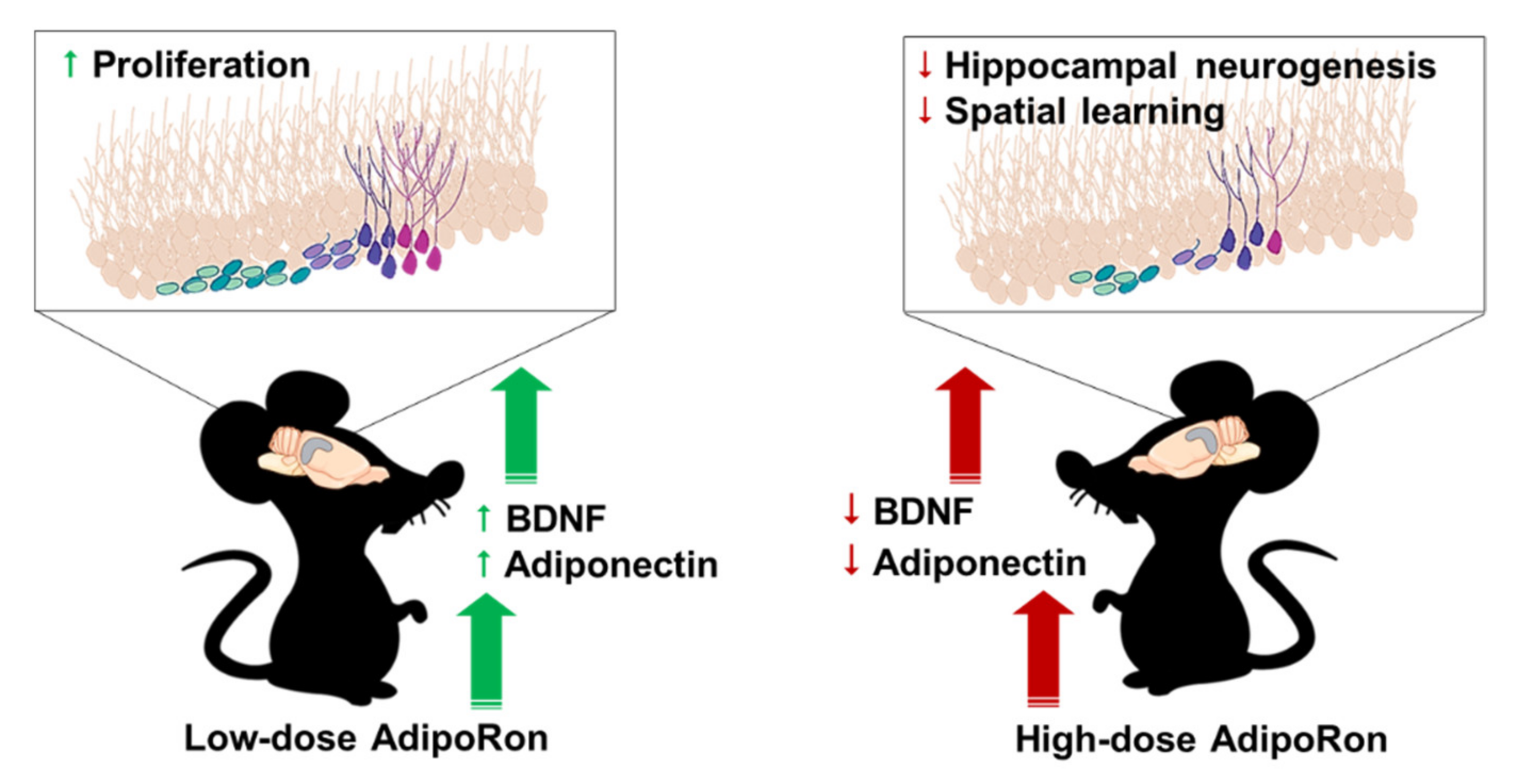
AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis
Abstract
:1. Introduction
2. Results
2.1. Chronic Treatment with 50 mg/kg AdipoRon Impaired Hippocampal-Dependent Spatial Memory
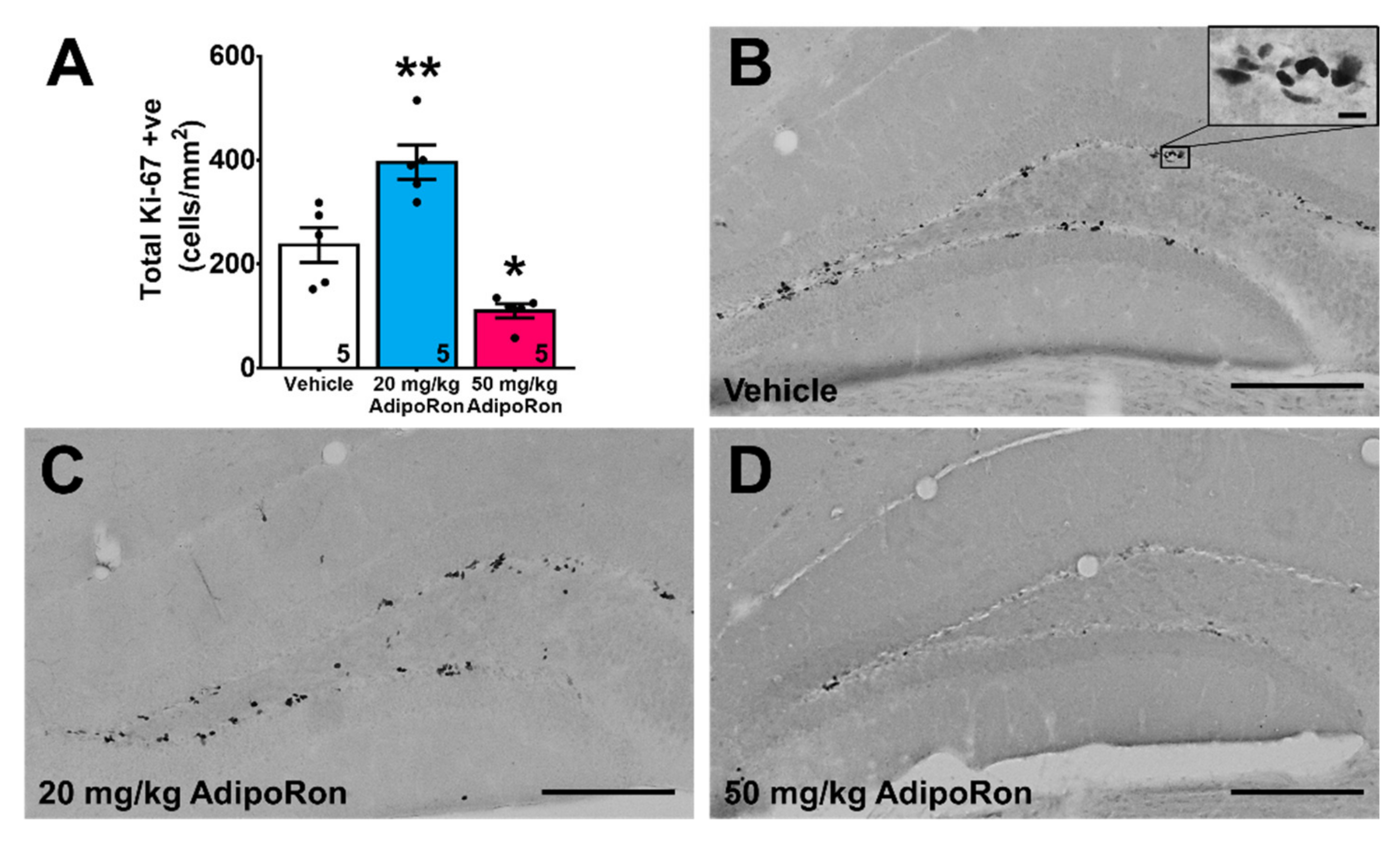
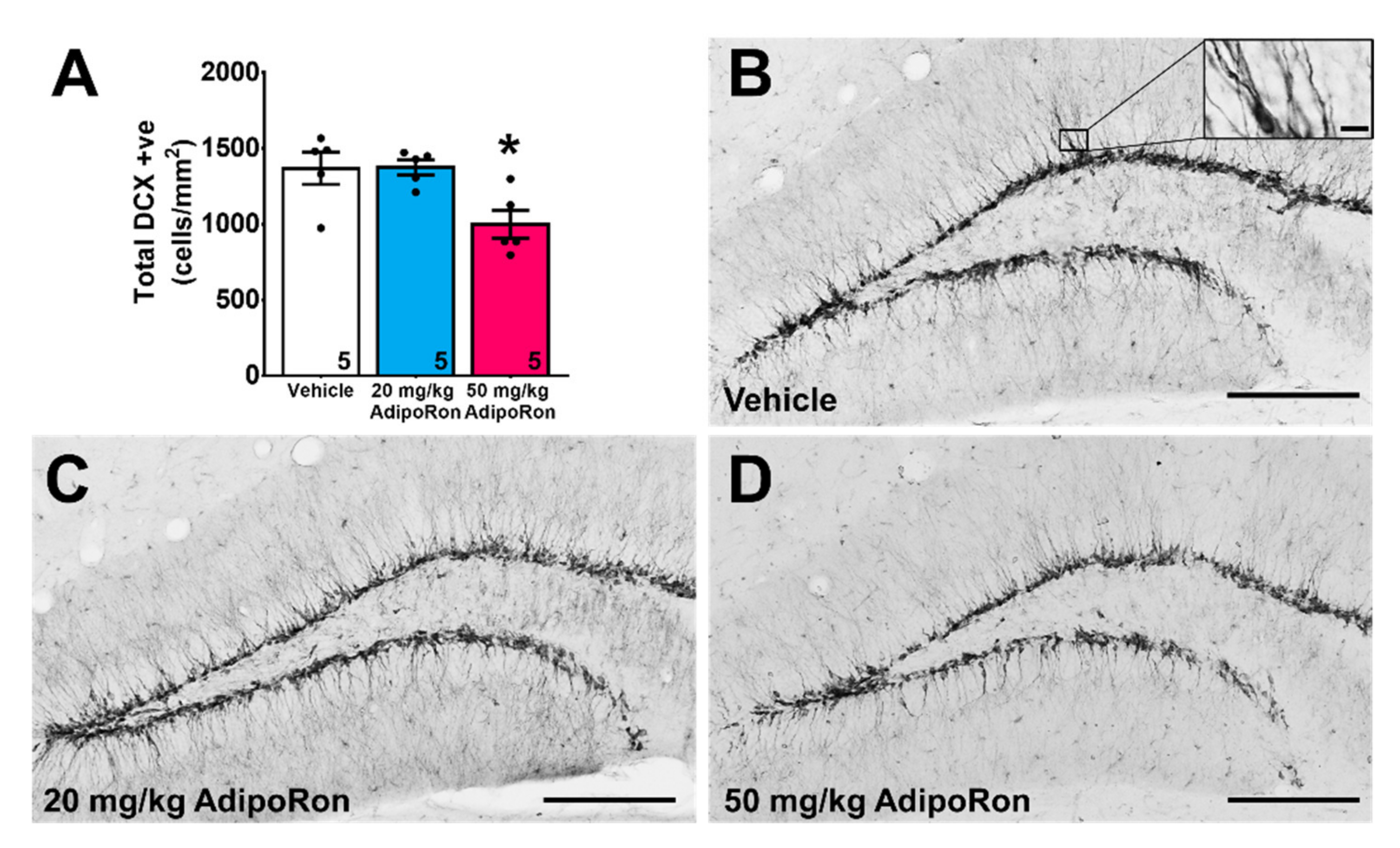
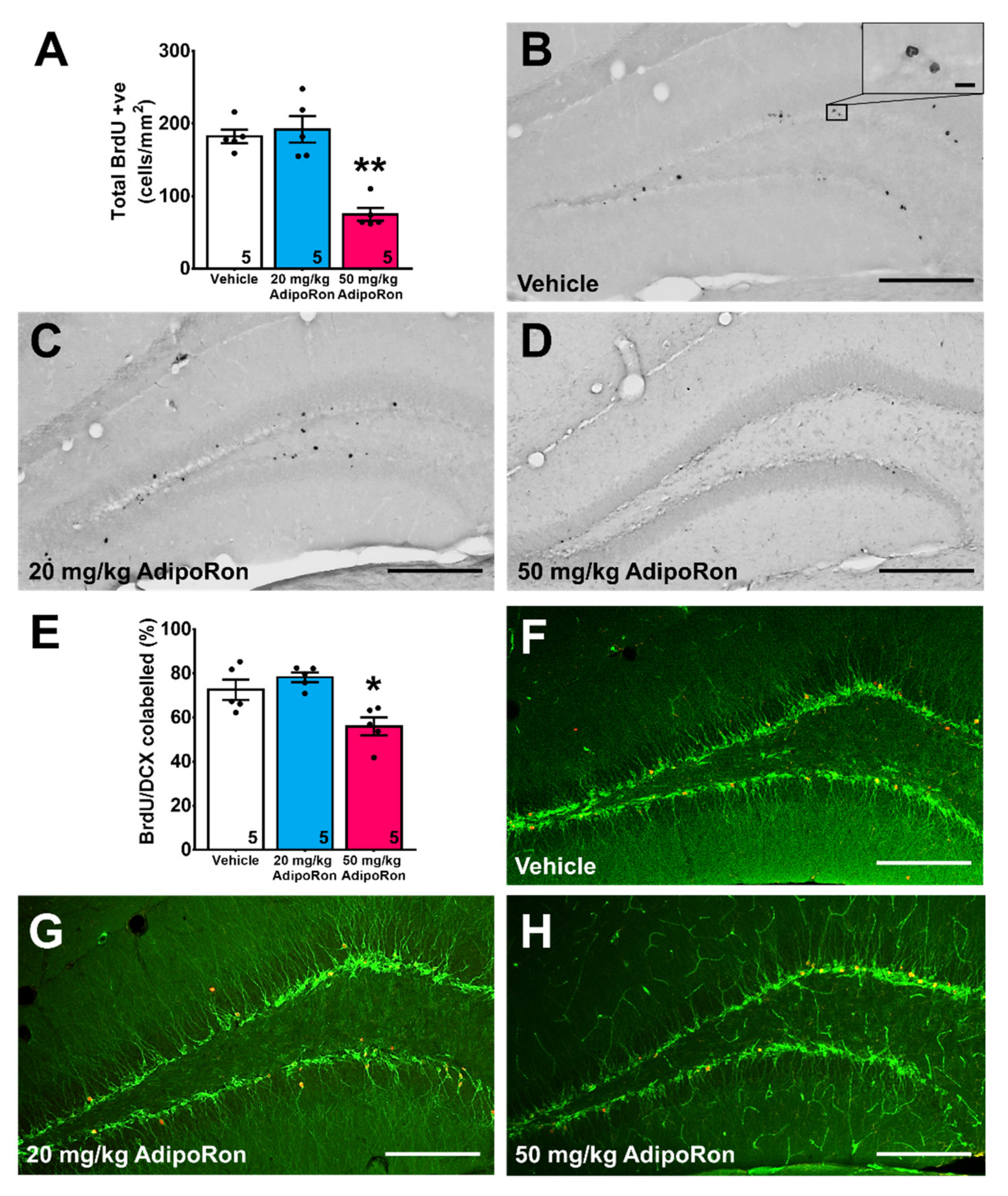
2.2. Treatment with 20 mg/kg AdipoRon Promoted Cell Proliferation, but 50 mg/kg AdipoRon Treatment Impaired Hippocampal Neurogenesis in the Dentate Gyrus
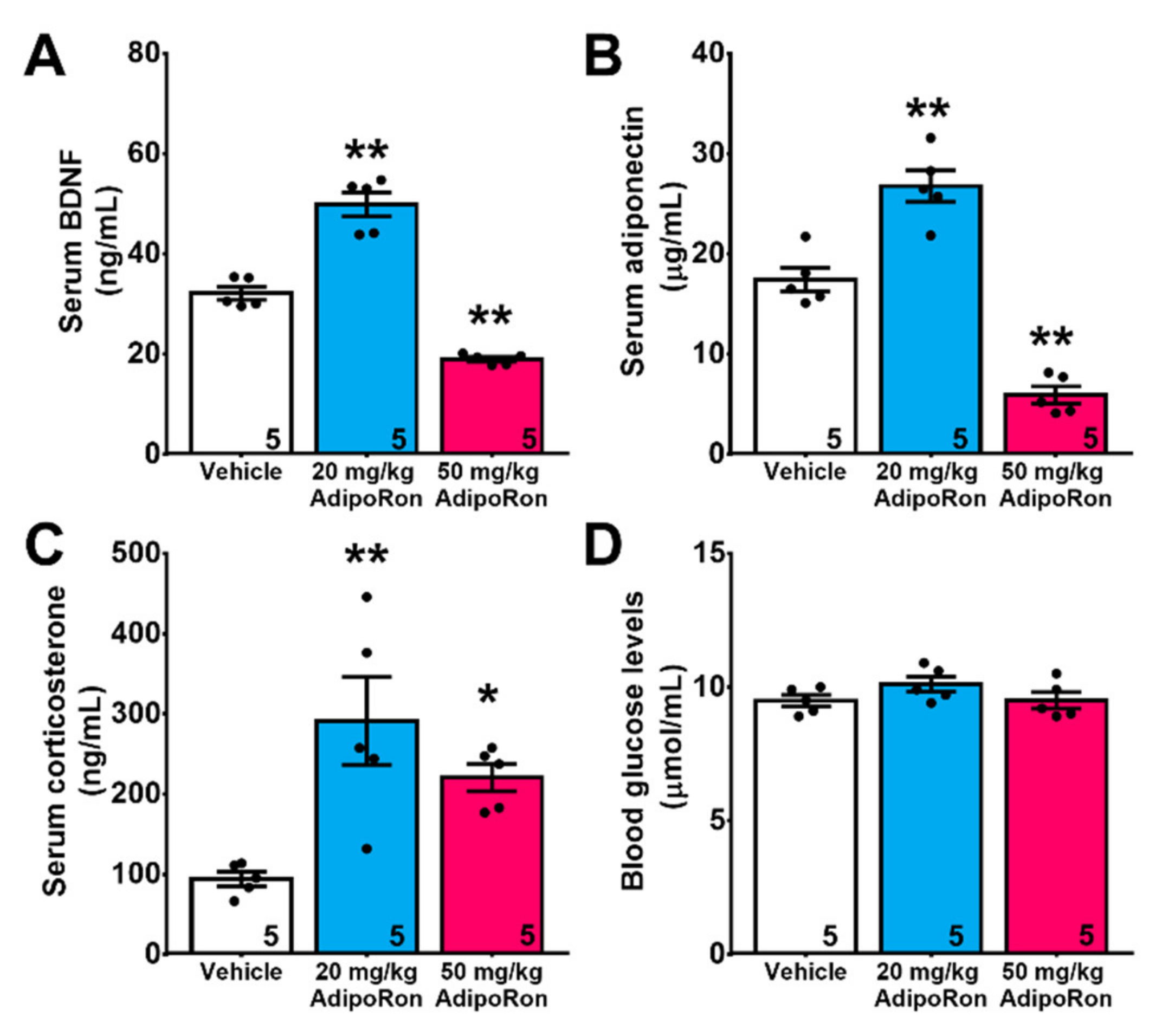
2.3. Chronic Treatment with AdipoRon Affects Serum Levels of BDNF, Adiponectin, and Corticosterone
3. Discussion
4. Materials and Methods
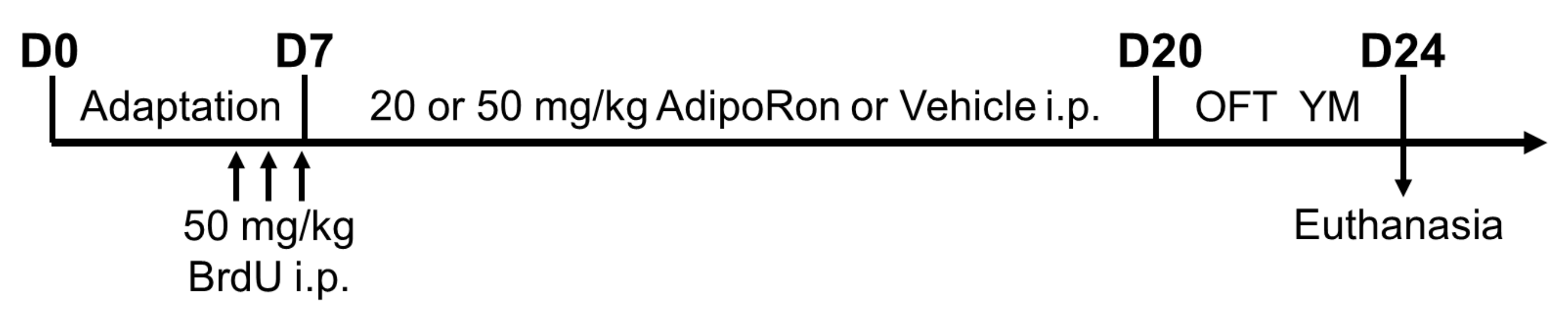
4.1. Animals
4.2. Drug Preparation and Treatment
4.3. Open Field Test
4.4. Y-Maze Task
4.5. Tissue Preparation
4.6. Immunohistochemistry and Immunofluorescent Staining
4.7. Quantification of BrdU, Ki67, and DCX Immunopositive Cells
4.8. Quantification of DCX/BrdU Co-Labeled Cells
4.9. Measurement of Serum Glucose Levels, Corticosterone, Adiponectin and Brain-Derived Neurotrophic Factor (BDNF) Levels
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Kusminski, C.M.; McTernan, P.G.; Schraw, T.; Kos, K.; O’Hare, J.P.; Ahima, R.; Kumar, S.; Scherer, P.E. Adiponectin complexes in human cerebrospinal fluid: Distinct complex distribution from serum. Diabetologia 2007, 50, 634–642. [Google Scholar] [CrossRef]
- Schön, M.; Kovaničová, Z.; Košutzká, Z.; Nemec, M.; Tomková, M.; Jacková, L.; Máderová, D.; Slobodová, L.; Valkovič, P.; Ukropec, J.; et al. Effects of running on adiponectin, insulin and cytokines in cerebrospinal fluid in healthy young individuals. Sci. Rep. 2019, 9, 1959. [Google Scholar] [CrossRef] [Green Version]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin Stimulates AMP-Activated Protein Kinase in the Hypothalamus and Increases Food Intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, S.Y.; Li, A.; Hoo, R.L.C.; Ching, Y.P.; Christie, B.R.; Lee, T.M.C.; Xu, A.; So, K.-F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cezaretto, A.; Suemoto, C.K.; Bensenor, I.; Lotufo, P.A.; de Almeida-Pititto, B.; Ferreira, S.R.G.; Aquino, E.M.L. Association of adiponectin with cognitive function precedes overt diabetes in the Brazilian Longitudinal Study of Adult Health: ELSA. Diabetol. Metab. Syndr. 2018, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Benavente, K.S.K.; Palmer, R.F.; Royall, D.R. Serum Adiponectin is Related to Dementia. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 75, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Jian, M.; Ma, O.K.; Bunting, M.; Kwan, J.S.; Zhou, G.J.; Senthilkumar, K.; Iyaswamy, A.; Chan, P.K.; Li, M.; et al. Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer’s disease mouse model. Mol. Psychiatry 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Teixeira, A.L.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Reduced serum levels of adiponectin in elderly patients with major depression. J. Psychiatr. Res. 2012, 46, 1081–1085. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, W.-H.; Tang, Y.-H.; Wu, L.-F.; Zeng, G.-R.; Wang, Y.-H.; Cheng, Z.; Jiang, D.-J. The correlation between adiponectin and FGF9 in depression disorder. Brain Res. 2020, 1729, 146596. [Google Scholar] [CrossRef]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pr. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2011, 165, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.-Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X.-Y. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, X.; Lu, X.-Y. Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jo, J.; Song, J. Adiponectin improves long-term potentiation in the 5XFAD mouse brain. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bloemer, J.; Pinky, P.D.; Smith, W.D.; Bhattacharya, D.; Chauhan, A.; Govindarajulu, M.; Hong, H.; Dhanasekaran, M.; Judd, R.; Amin, R.H.; et al. Adiponectin Knockout Mice Display Cognitive and Synaptic Deficits. Front. Endocrinol. 2019, 10, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wang, X.; Wang, B.; Garza, J.C.; Fang, X.; Wang, J.; Scherer, P.E.; Brenner, R.; Zhang, W.; Lu, X.-Y. Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Mol. Psychiatry 2016, 22, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, R.C.-L.; Cheng, O.-Y.; Jian, M.; Kwan, J.S.-C.; Ho, P.W.-L.; Cheng, K.K.-Y.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.-C.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.W.; Abid, N.B.; Jo, M.H.; Jo, M.G.; Yoon, G.H.; Kim, M.O. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci. Rep. 2017, 7, 12435. [Google Scholar] [CrossRef]
- Lee, T.H.-Y.; Cheng, K.K.Y.; Hoo, R.L.-C.; Siu, P.M.; Yau, S.-Y. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.-Y.; Yau, S.-Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Knittle, J.L.; Timmers, K.; Ginsberg-Fellner, F.; Brown, R.E.; Katz, D.P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Investig. 1979, 63, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nat. Cell Biol. 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Boitard, C.; Parkes, S.L.; Cavaroc, A.; Tantot, F.; Castanon, N.; Layé, S.; Tronel, S.; Pacheco-Lopez, G.; Coutureau, E.; Ferreira, G. Switching Adolescent High-Fat Diet to Adult Control Diet Restores Neurocognitive Alterations. Front. Behav. Neurosci. 2016, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, J.D.; Hoban, A.E.; Murphy, A.; O’Leary, O.F.; Cryan, J.F.; Nolan, Y.M. Differential effects of adolescent and adult-initiated exercise on cognition and hippocampal neurogenesis. Hippocampus 2019, 29, 352–365. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.D.; Hoban, A.E.; Cryan, J.F.; O’Leary, O.F.; Nolan, Y.M. Differential effects of adolescent and adult-initiated voluntary exercise on context and cued fear conditioning. Neuropharmacology 2019, 145, 49–58. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma Concentrations of a Novel, Adipose-Specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arter. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef]
- Fruebis, J.; Tsao, T.S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyamakasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.-I.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nat. Cell Biol. 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Yamashita, T.; Lakota, K.; Taniguchi, T.; Yoshizaki, A.; Sato, S.; Hong, W.; Zhou, X.; Sodin-Semrl, S.; Fang, F.; Asano, Y.; et al. An orally-active adiponectin receptor agonist mitigates cutaneous fibrosis, inflammation and microvascular pathology in a murine model of systemic sclerosis. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fairaq, A.; Shawky, N.M.; Osman, I.; Pichavaram, P.; Segar, L. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia. Pharmacol. Res. 2017, 119, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.-Z.; Zhang, L. Adiponectin receptor agonist AdipoRon relieves endotoxin-induced acute hepatitis in mice. Chin. Med. J. 2019, 132, 2438–2445. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Li, R.; Lau, W.B.; Yuan, Y.-X.; Liang, B.; Li, R.; Gao, E.-H.; Koch, W.J.; Ma, X.-L.; et al. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am. J. Physiol. Metab. 2015, 309, E275–E282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, S.; Debayle, D.; Béchade, C.; Maroteaux, L.; Gay, A.-S.; Bayer, P.; Heurteaux, C.; Guyon, A.; Chabry, J. Adiporon, an adiponectin receptor agonist acts as an antidepressant and metabolic regulator in a mouse model of depression. Transl. Psychiatry 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Yau, S.-Y.; Lee, T.H.-Y.; Li, A.; Xu, A.; So, K.-F. Adiponectin Mediates Running-Restored Hippocampal Neurogenesis in Streptozotocin-Induced Type 1 Diabetes in Mice. Front. Neurosci. 2018, 12, 679. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.-Y.; Sun, X.; Cheng, K.K.-Y.; Xu, A.; So, K.-F.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell. Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Saaltink, D.-J.; Vreugdenhil, E. Stress, glucocorticoid receptors, and adult neurogenesis: A balance between excitation and inhibition? Cell. Mol. Life Sci. 2014, 71, 2499–2515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, M.; Zhang, W.; Lu, X.Y. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J. Biol. Chem. 2011, 286, 44913–44920. [Google Scholar] [CrossRef] [Green Version]
- Islam, O.; Loo, T.X.; Heese, K. Brain-Derived Neurotrophic Factor (BDNF) has Proliferative Effects on Neural Stem Cells through the Truncated TRK-B Receptor, MAP Kinase, AKT, and STAT-3 Signaling Pathways. Curr. Neurovascular Res. 2009, 6, 42–53. [Google Scholar] [CrossRef]
- Lang, U.E.; Hellweg, R.; Seifert, F.; Schubert, F.; Gallinat, J. Correlation Between Serum Brain-Derived Neurotrophic Factor Level and An In Vivo Marker of Cortical Integrity. Biol. Psychiatry 2007, 62, 530–535. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Bergami, M.; Rimondini, R.; Santi, S.; Blum, R.; Götz, M.; Canossa, M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 15570–15575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, J.; Wang, J.-G.; Liu, C.-L.; Yan, H. AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nat. Cell Biol. 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Šišková, Z.; Justus, D.; Kaneko, H.; Friedrichs, D.; Henneberg, N.; Beutel, T.; Pitsch, J.; Schoch, S.; Becker, A.; von der Kammer, H.; et al. Dendritic Structural Degeneration Is Functionally Linked to Cellular Hyperexcitability in a Mouse Model of Alzheimer’s Disease. Neuron 2014, 84, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Lee, C.L.; Smith, K.L.; Swann, J.W. Spine Loss and Other Persistent Alterations of Hippocampal Pyramidal Cell Dendrites in a Model of Early-Onset Epilepsy. J. Neurosci. 1998, 18, 8356–8368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musto, A.E.; Rosencrans, R.F.; Walker, C.P.; Bhattacharjee, S.; Raulji, C.M.; Belayev, L.; Fang, Z.; Gordon, W.C.; Bazan, N.G. Dysfunctional epileptic neuronal circuits and dysmorphic dendritic spines are mitigated by platelet-activating factor receptor antagonism. Sci Rep. 2016, 6, 30298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musto, A.E.; Gjorstrup, P.; Bazan, N.G. The omega-3 fatty acid-derived neuroprotectin D1 limits hippocampal hyperexcitability and seizure susceptibility in kindling epileptogenesis. Epilepsia 2011, 52, 1601–1608. [Google Scholar] [CrossRef]
- Palop., J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Minkeviciene, R.; Rheims, S.; Dobszay, M.B.; Zilberter, M.; Hartikainen, J.; Fulop, L.; Penke, B.; Zilberter, Y.; Harkany, T.; Pitkanen, A.; et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 2009, 29, 3453–3462. [Google Scholar] [CrossRef]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef] [Green Version]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, T.; Arai, Y. Age-related production of new granule cells in the adult dentate gyrus. NeuroReport 1995, 6, 2479–2482. [Google Scholar] [CrossRef]
- Hodes, G.E.; Yang, L.; van Kooy, J.; Santollo, J.; Shors, T.J. Prozac during puberty: Distinctive effects on neurogenesis as a function of age and sex. Neuroscience 2009, 163, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Lu, W.Y.; Liu, K.Y. Adiponectin receptor agonist AdipoRon suppresses adipogenesis in C3H10T1/2 cells through the adenosine monophosphateactivated protein kinase signaling pathway. Mol. Med. Rep. 2017, 16, 7163–7169. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilisation and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Domise, M.; Sauvé, F.; Didier, S.; Caillerez, R.; Bégard, S.; Carrier, S.; Colin, M.; Marinangeli, C.; Buée, L.; Vingtdeux, V. Neuronal AMP-activated protein kinase hyper-activation induces synaptic loss by an autophagy-mediated process. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-B.; Wu, Y.-T.; Wu, T.-P.; Wei, Y.-H. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Arsikin, K.; Kravic-Stevovic, T.; Jovanovic, M.; Ristic, B.; Tovilovic, G.; Zogovic, N.; Bumbasirevic, V.; Trajkovic, V.; Harhaji-Trajkovic, L. Autophagy-dependent and -independent involvement of AMP-activated protein kinase in 6-hydroxydopamine toxicity to SH-SY5Y neuroblastoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1826–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autry, A.E.; Adachi, M.; Nosyreva, E.D.; Na, E.S.; Los, M.F.; Cheng, P.-F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nat. Cell Biol. 2011, 475, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.L.; Castrén, E. Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different but Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Garthe, A.; Behr, J.; Kempermann, G. Adult-Generated Hippocampal Neurons Allow the Flexible Use of Spatially Precise Learning Strategies. PLoS ONE 2009, 4, e5464. [Google Scholar] [CrossRef] [Green Version]
- Paschke, L.; Zemleduch, T.; Rucinski, M.; Ziolkowska, A.; Szyszka, M.; Malendowicz, L.K. Adiponectin and adiponectin receptor system in the rat adrenal gland: Ontogenetic and physiologic regulation, and its involvement in regulating adrenocortical growth and steroidogenesis. Peptides 2010, 31, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Resch, J.M.; Douglass, A.M.; Madara, J.C.; Rabin-Court, A.; Kucukdereli, H.; Wu, C.; Song, J.D.; Lowell, B.B.; Shulman, G.I. Leptin’s hunger-suppressing effects are mediated by the hypothalamic-pituitary-adrenocortical axis in rodents. Proc. Natl. Acad. Sci. USA 2019, 116, 13670–13679. [Google Scholar] [CrossRef] [Green Version]
- Hare, B.D.; Beierle, J.A.; Toufexis, D.J.; Hammack, S.E.; Falls, W.A. Exercise-Associated Changes in the Corticosterone Response to Acute Restraint Stress: Evidence for Increased Adrenal Sensitivity and Reduced Corticosterone Response Duration. Neuropsychopharmacology 2014, 39, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nat. Cell Biol. 1995, 373, 109. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.N.; Burhans, M.S.; Clark, J.P.; Howell, P.R.; Polewski, M.A.; de Muth, T.M.; Eliceiri, K.W.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 2017, 16, 497–507. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; McLennan, S.V.; Twigg, S.M. Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: Possible role of muscle adiponectin. Physiol. Rep. 2018, 6, e13599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging 2005, 26, 88–93. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hua, L.; Feng, B.; Jiang, X.; Li, J.; Jiang, D.; Huang, X.; Zhu, Y.; Li, Z.; Yan, L.; et al. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. EBioMedicine 2019, 41, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costall, B.; Jones, B.; Kelly, M.; Naylor, R.; Tomkins, D. Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989, 32, 777–785. [Google Scholar] [CrossRef]
- Borta, A.; Schwarting, R.K. Inhibitory avoidance, pain reactivity, and plus-maze behavior in Wistar rats with high versus low rearing activity. Physiol. Behav. 2005, 84, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bouwknecht, J.A.; Paylor, R. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav. Pharmacol. 2008, 19, 385–402. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.-L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 2008, 29, 493–498. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.-Y.; Lee, T.H.-Y.; Formolo, D.A.; Lee, W.-L.; Li, L.C.-K.; Siu, P.M.; Chan, C.C.H. Effects of Maternal Voluntary Wheel Running During Pregnancy on Adult Hippocampal Neurogenesis, Temporal Order Memory, and Depression-Like Behavior in Adult Female and Male Offspring. Front. Neurosci. 2019, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.-Y.; Lau, B.W.-M.; Tong, J.-B.; Wong, R.; Ching, Y.-P.; Qiu, G.; Tang, S.-W.; Lee, T.M.; So, K.-F. Hippocampal Neurogenesis and Dendritic Plasticity Support Running-Improved Spatial Learning and Depression-Like Behaviour in Stressed Rats. PLoS ONE 2011, 6, e24263. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.; Christie, B.R.; van Praag, H.; Lin, K.; Siu, P.M.-F.; Xu, A.; So, K.-F.; Yau, S.-y. AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis. Int. J. Mol. Sci. 2021, 22, 2068. https://doi.org/10.3390/ijms22042068
Lee TH, Christie BR, van Praag H, Lin K, Siu PM-F, Xu A, So K-F, Yau S-y. AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis. International Journal of Molecular Sciences. 2021; 22(4):2068. https://doi.org/10.3390/ijms22042068
Chicago/Turabian StyleLee, Thomas H., Brian R. Christie, Henriette van Praag, Kangguang Lin, Parco Ming-Fai Siu, Aimin Xu, Kwok-Fai So, and Suk-yu Yau. 2021. "AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis" International Journal of Molecular Sciences 22, no. 4: 2068. https://doi.org/10.3390/ijms22042068





