Nitric Oxide Enhances Photosynthetic Nitrogen and Sulfur-Use Efficiency and Activity of Ascorbate-Glutathione Cycle to Reduce High Temperature Stress-Induced Oxidative Stress in Rice (Oryza sativa L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Determination of Photosynthetic-NUE and Photosynthetic-SUE
2.3. Measurement of Gas Exchange, Chlorophyll Content, and Rubisco Activity
2.4. PS II Activity
2.5. Estimation of Proline
2.6. Determination of TBARS and H2O2 Content
2.7. Determination of Leaf Nitrogen Content
2.8. Determination of Leaf Sulfur Content
2.9. Determination of Content of Cysteine and GSH
2.10. Assay of Antioxidant Enzymes
2.11. Activity of Nitrate Reductase
2.12. Determination of NO Generation
2.13. Determination of Growth Characteristics and Agronomic Traits
2.14. Scanning Electron Microscopy
2.15. Statistical Analysis
3. Results
3.1. Screening of Cultivars
3.2. Effect of NO on High Temperature Stress-Induced Oxidative Stress
3.3. Effect of NO Treatment on Proline Accumulation under High Temperature Stress
3.4. Effect of NO on NO Generation under High Temperature Stress
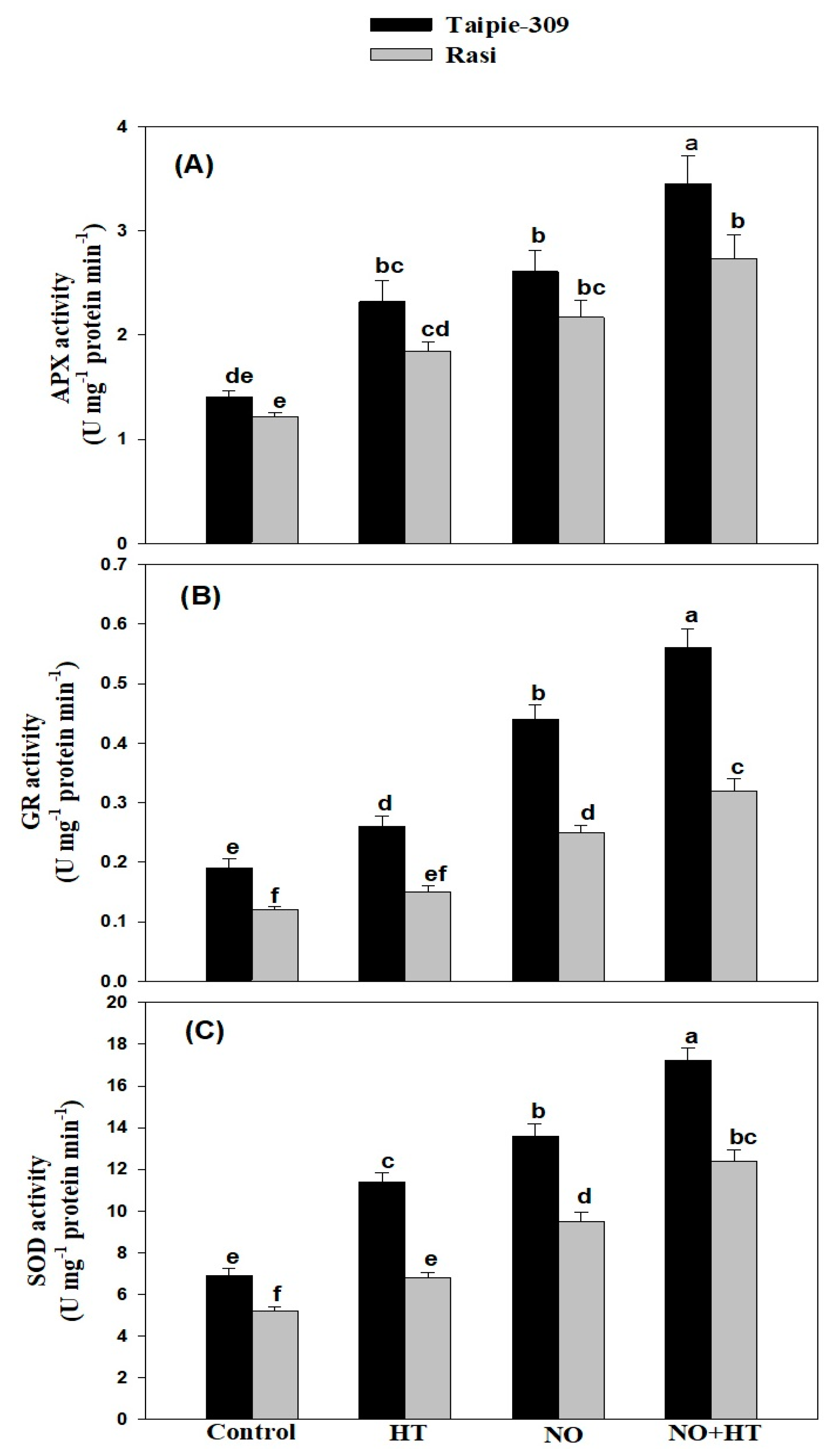
3.5. NO Modulates Activity of Enzymatic Antioxidants under High Temperature Stress
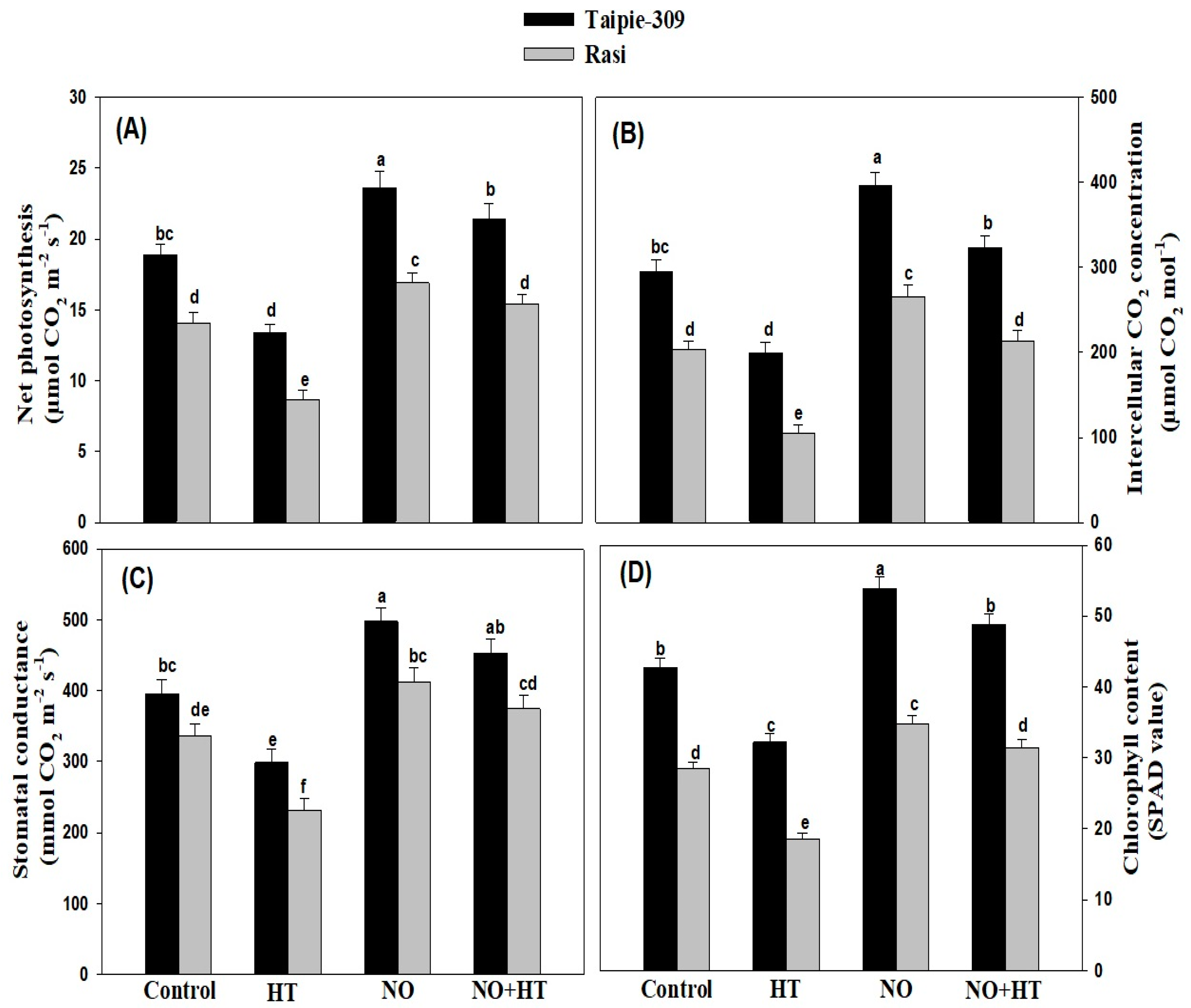
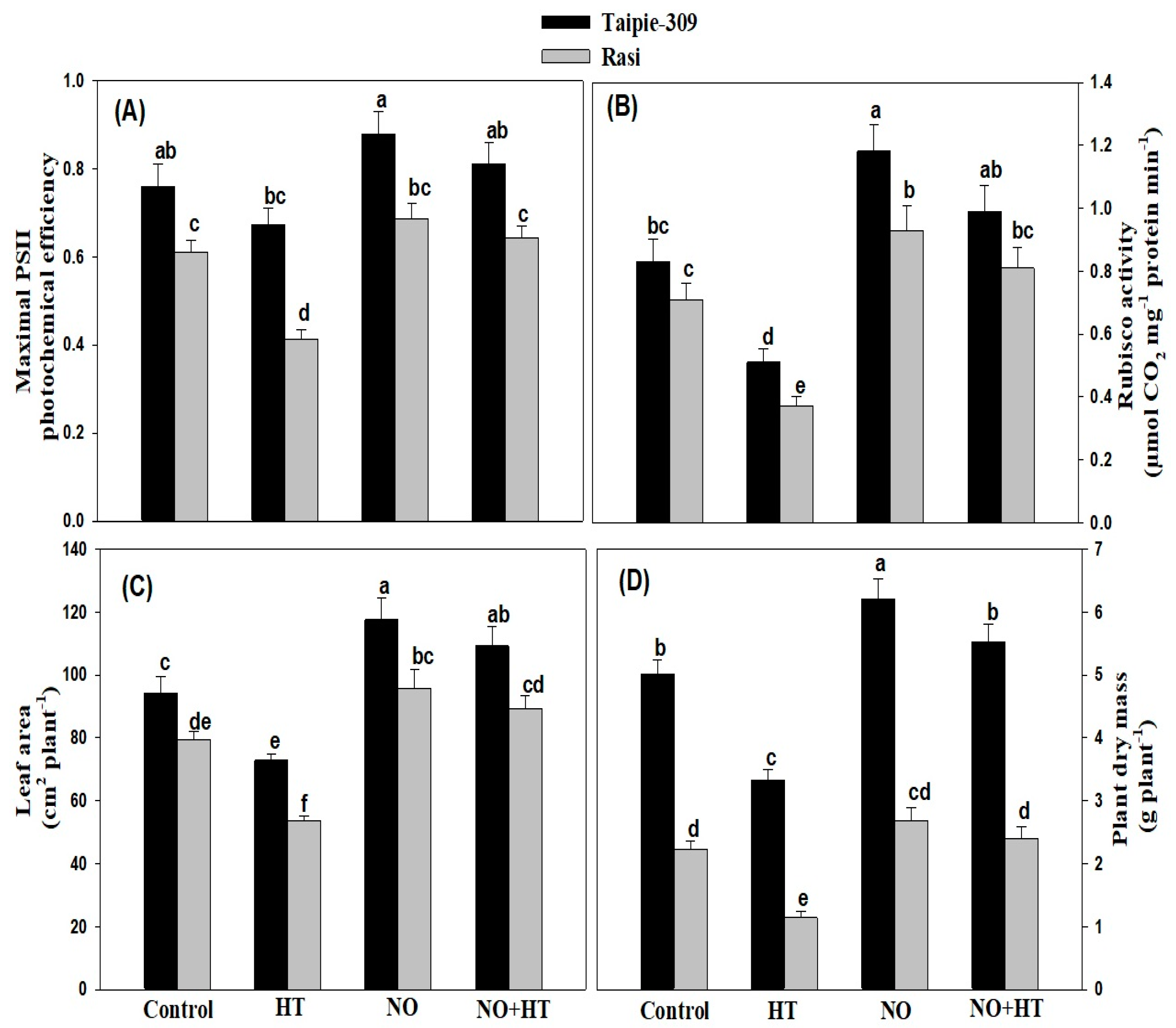
3.6. NO Alleviates the Adverse Impacts of High Temperature Stress on Growth and Photosynthetic Traits
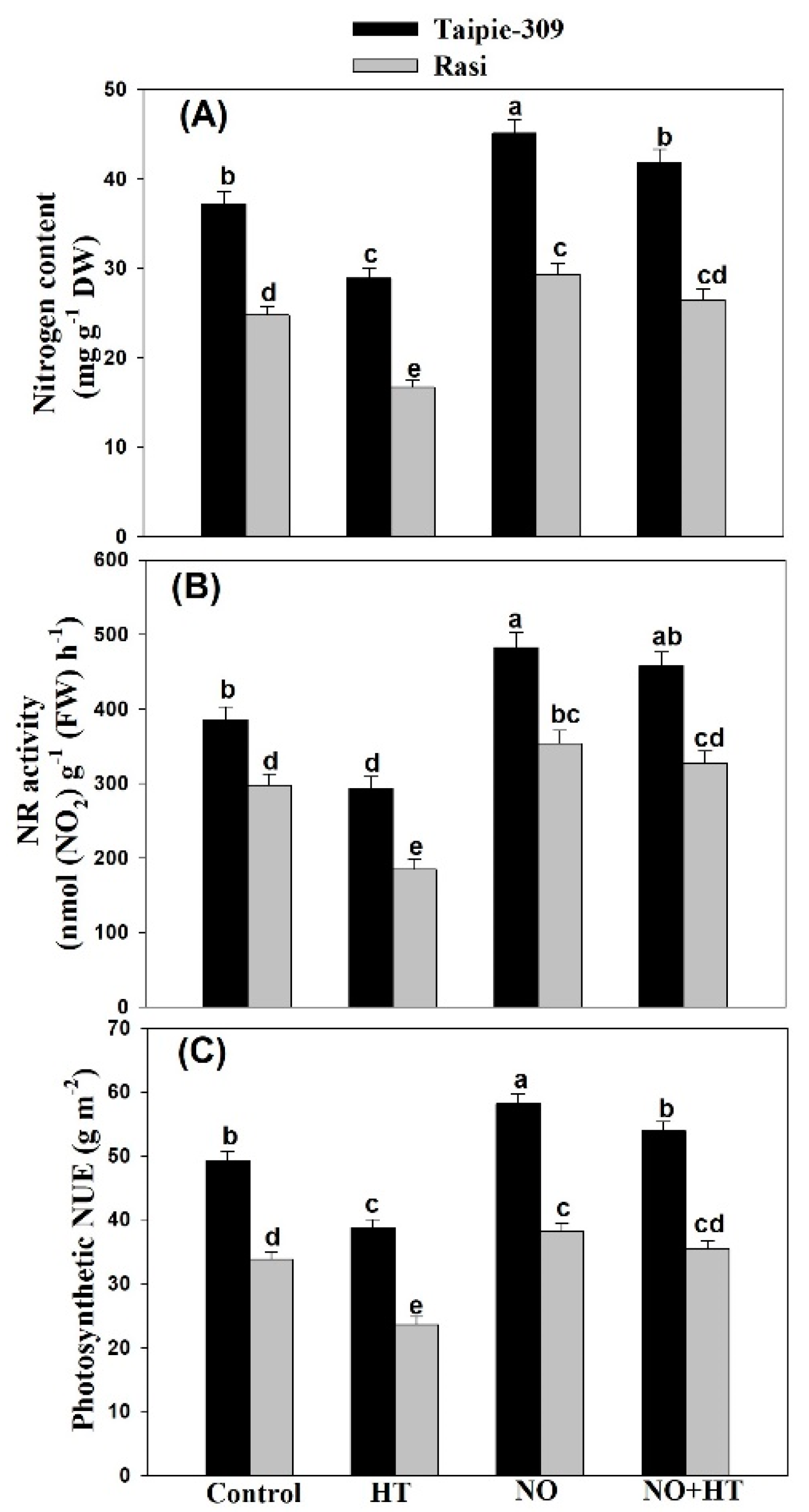
3.7. NO Increases Nitrogen Content, Nitrate Reductase Activity and Photosynthetic-NUE under High Temperature Stress
3.8. Effect of NO on Sulfur-Assimilation Capacity and Photosynthetic-SUE under High Temperature Stress
3.9. Effect of NO Application on Agronomic Traits under High Temperature Stress
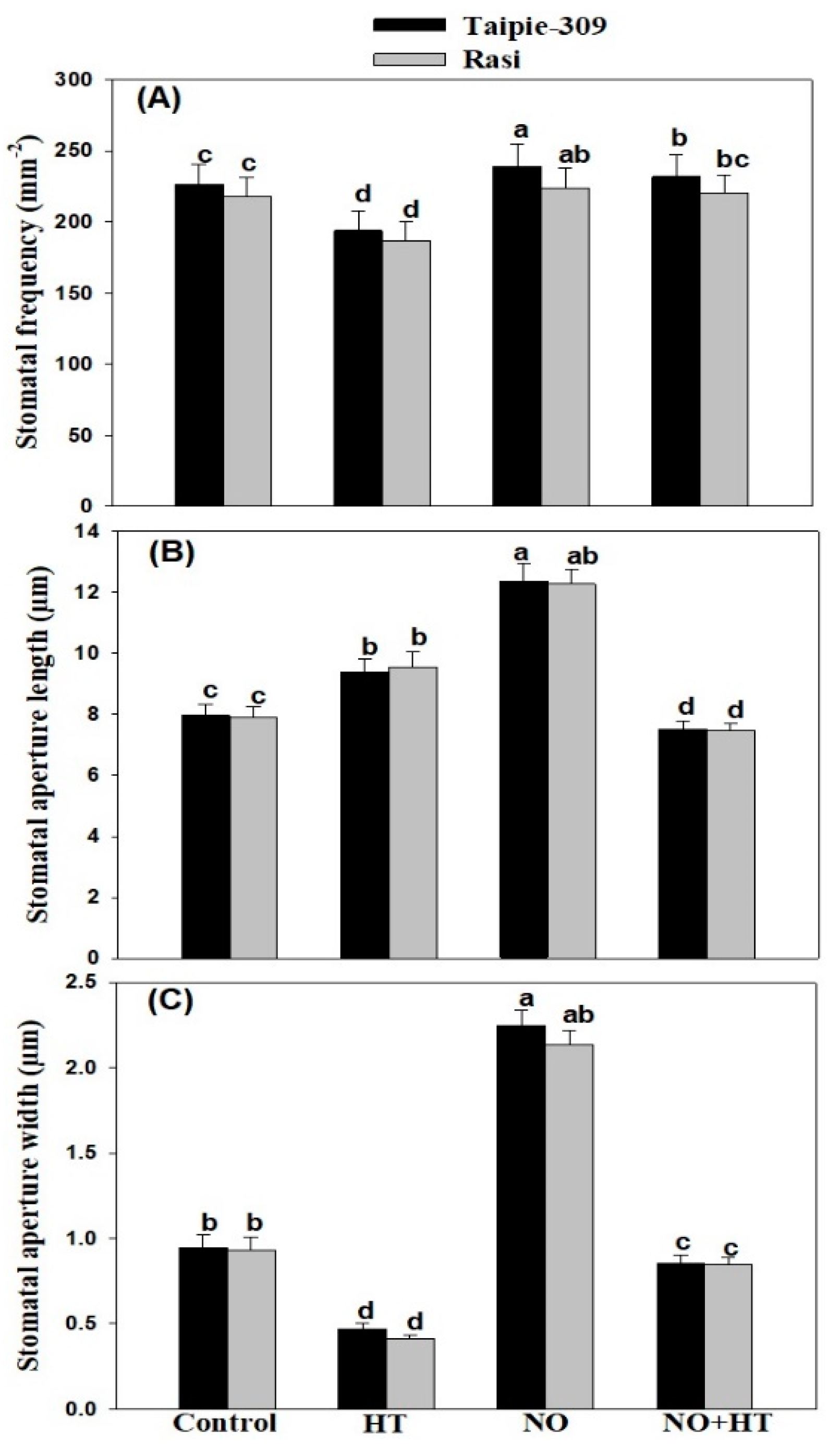
3.10. Effect of NO on Stomatal Response
3.11. Effect of NO Scavenger on Photosynthetic and Growth Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Crops/Regions/World List/Production Quantity (Pick Lists), Rice (Paddy); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014. [Google Scholar]
- FAOSTAT, Faostat.fao.org. 2011. Available online: http://www.fao.org/india/fao-in-india/india-at-a-glance/en/ (accessed on 10 October 2020).
- Food and Agriculture Organization of the United Nations (FAO). Statistics of the Food and Agriculture Organization of the United Nations; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015. [Google Scholar]
- Shahbandeh, M. Total Rice Consumption Worldwide from 2008/2009 to 2018/2019. 2019. Available online: https://www.statista.com/statistics/255977/total-global-rice-consumption/ (accessed on 10 October 2020).
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways. In The Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Guiteras, R. The Impact of Climate Change on Indian Agriculture. Manuscript; Department of Economics, University of Maryland: College Park, MD, USA, 2009. [Google Scholar]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Planchet, E.; Kaiser, W.M. Nitric oxide production in plants: Facts and fictions. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Delledonne, M. NO news is good news for plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef]
- Godber, B.L.; Doel, J.J.; Sapkota, G.P.; Blake, D.R.; Stevens, C.R.; Eisenthal, R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 2000, 275, 7757–7763. [Google Scholar] [CrossRef] [Green Version]
- Cooney, R.V.; Harwood, P.J.; Custer, L.J.; Franke, A.A. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ. Health Perspect. 1994, 102, 460–462. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Astier, J.; Rasul, S.; Wawer, I.; Dubreuil-Maurizi, C.; Jeandroz, S. Current view of nitric oxide-responsive genes in plants. Plant Sci. 2009, 177, 302–309. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Richter, A. Nitrogen oxides in the troposphere—What have we learned from satellite measurements? EPJ Web Conf. 2009, 1, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Ding, W.; Zhao, M.; Sun, B.; Zhang, L. Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci. 2006, 171, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Vital, R.G.; Müller, C.; da Silva, F.B.; Batista, P.F.; Merchant, A.; Fuentes, D. Nitric oxide increases the physiological and biochemical stability of soybean plants under high temperature. Agronomy 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, V.B.; Chumley, P.H.; Hogg, N.; Bloodsworth, A.; Darley-Usmar, V.M.; Freeman, B.A. Nitric oxide inhibition of lipid peroxidation: Kinetics of reaction with lipid peroxyl radicals and comparison with α-tocopherol. Biochemistry 1997, 36, 15216–15223. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020, 168, 490–510. [Google Scholar] [PubMed]
- Wang, J.; Yu, S.X.; Zhang, M.; Cui, X.M. Exogenous nitric oxide-mediated GSH-PC synthesis pathway in tomato under copper stress. Russian J. Plant Physiol. 2015, 62, 349–359. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakihama, Y.; Murakami, S.; Yamasaki, H. Involvement of nitric oxide in the mechanism for stomatal opening in Vicia faba leaves. Biol. Plant. 2003, 46, 117–119. [Google Scholar] [CrossRef]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1994, 19, 76. [Google Scholar] [CrossRef] [Green Version]
- Chesin, L.; Yien, C.H. Turbidimetric determination of available sulphates. Soil Sci. Soc. Am. J. 1951, 15, 149–151. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kuo, T.M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Daud, M.K.; Sun, Y.Q.; Dawood, M.; Hayat, Y.; Variatha, M.T.; Wu, Y.X. Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J. Hazard. Mater. 2009, 168, 614–625. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Rising temperature in the changing environment: A serious threat to plants. Clim. Chang. Environ. Sustain. 2013, 1, 25–36. [Google Scholar] [CrossRef]
- Chalanika De Silva, H.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Gupta, D.; Nayyar, H. Comparative response of maize and rice genotypes to heat stress: Status of oxidative stress and antioxidants. Acta Physiol. Plant. 2012, 34, 75–86. [Google Scholar] [CrossRef]
- Lindermayr, C. Crosstalk between reactive oxygen species and nitric oxide in plants: Key role of S-nitrosoglutathione reductase. Free Radical Biol. Med. 2018, 122, 110–115. [Google Scholar] [CrossRef]
- Laspina, N.V.; Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005, 169, 323–330. [Google Scholar] [CrossRef]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Bavita, A.; Shashi, B.; Navtej, S.B. Nitric oxide alleviates oxidative damage induced by high temperature stress in wheat. IJEB 2012, 50, 372–378. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (‘Triticum aestivum’ L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2012, 6, 1314. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, O.K.; Hegazy, A.E. Protective effect of nitric oxide on high temperature induced oxidative stress in wheat (Triticum aestivum) callus culture. Not. Sci. Biol. 2016, 8, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alakeel, K.A. Nitric oxide-mediated cross-talk of proline and heat shock proteins induce thermotolerance in Vicia faba L. Environ. Exp. Bot. 2019, 161, 290–302. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Hafeez, N.; Farid-ul-Haq, M.; Ahmad, A.; Sadiq, M.; Ashraf, M. Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol. Plant. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Fan, H.F.; Du, C.X.; Guo, S.R. Effect of nitric oxide on proline metabolism in cucumber seedlings under salinity stress. J. Am. Soc. Hortic. Sci. 2012, 137, 127–133. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, K. Nitric oxide improves thermotolerance in spring maize by inducing varied genotypic defense mechanisms. Acta Physiol. Plant. 2018, 40, 55. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, H.; Hong, J.; Han, Y.; Li, H.; Zhao, W. Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World J. Agric. Sci. 2008, 4, 307–313. [Google Scholar]
- Jday, A.; Rejeb, K.B.; Slama, I.; Saadallah, K.; Bordenave, M.; Planchais, S.; Abdelly, C. Effects of exogenous nitric oxide on growth, proline accumulation and antioxidant capacity in Cakile maritima seedlings subjected to water deficit stress. Funct. Plant Biol. 2016, 43, 939–948. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.W.; Jiang, H.; Hu, J.; Zhang, X.Q.; Guo, L.B.; Zeng, D.L.; Qian, Q. Characterization of physiological response and identification of associated genes under heat stress in rice seedlings. Plant Physiol. Biochem. 2012, 61, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Rai, N.; Rai, S.P. Investigating the impact of high temperature on growth and yield of Lablab purpureus L. inbred lines using integrated phenotypical, physiological, biochemical and molecular approaches. Indian J. Plant Physiol. 2018, 23, 209–226. [Google Scholar] [CrossRef]
- Rai, K.K.; Rai, N.; Aamir, M.; Tripathi, D.; Rai, S.P. Interactive role of salicylic acid and nitric oxide on transcriptional reprogramming for high temperature tolerance in Lablab purpureus L.: Structural and functional insights using computational approaches. J. Biotechnol. 2020, 309, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.N. Effect of temperature on chlorophyll degradation of senescing rice leaves. J. Sci. Res. 1981, 3, 9–10. [Google Scholar]
- Wu, X.X.; Ding, H.D.; Chen, J.L.; Zhang, H.J.; Zhu, W.M. Attenuation of salt-induced changes in photosynthesis by exogenous nitric oxide in tomato (Lycopersicon esculentum Mill. L.) seedlings. Afr. J. Biotechnol. 2010, 9, 7837–7846. [Google Scholar]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitric Oxide Pre-Treatment Advances Seed Germination and Alleviates Copper-Induced Photosynthetic Inhibition in Indian Mustard. Plants 2020, 9, 776. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Hou, J.; Fan, Z. Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Kong, J.; Dong, Y.; Xu, L.; Liu, S.; Bai, X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot. Stud. 2014, 55, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Chen, W.; Xu, L.; Kong, J.; Liu, S.; He, Z. Nitric oxide can induce tolerance to oxidative stress of peanut seedlings under cadmium toxicity. Plant Growth Regul. 2016, 79, 19–28. [Google Scholar] [CrossRef]
- Yang, Q.; He, H.; Li, H.; Tian, H.; Zhang, J.; Zhai, L.; Peng, X. NOA1 functions in a temperature-dependent manner to regulate chlorophyll biosynthesis and Rubisco formation in rice. PLoS ONE 2011, 6, e20015. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Y.; Chen, S.; Jiang, J.; Chen, F.; Fang, W.; Liu, Z. The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed chrysanthemum. Biol. Plant. 2011, 55, 737. [Google Scholar] [CrossRef]
- Chen, K.; Chen, L.; Fan, J.; Fu, J. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 2013, 116, 21–31. [Google Scholar] [CrossRef]
- Song, L.; Yue, L.; Zhao, H.; Hou, M. Protection effect of nitric oxide on photosynthesis in rice under heat stress. Acta Physiol. Plant. 2013, 35, 3323–3333. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Weston, D.J.; Bauerle, W.L. Inhibition and acclimation of C3 photosynthesis to moderate heat: A perspective from thermally contrasting genotypes of Acer rubrum (red maple). Tree Physiol. 2007, 27, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- von Caemmerer, S.; Evans, J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2015, 38, 629–637. [Google Scholar] [CrossRef]
- Kostaki, K.I.; Coupel-Ledru, A.; Bonnell, V.C.; Gustavsson, M.; Sun, P.; Mclaughlin, F.J.; Franklin, K.A. Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiol. 2020, 182, 1404–1419. [Google Scholar] [CrossRef] [Green Version]
- Krapp, A.; David, L.C.; Chardin, C.; Girin, T.; Marmagne, A.; Leprince, A.S.; Daniele-Vedele, F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 789–798. [Google Scholar] [CrossRef]
- Onwueme, I.C.; Laude, H.M.; Huffaker, R.C. Nitrate Reductase Activity in Relation to Heat Stress in Barley Seedlings 1. Crop Sci. 1971, 11, 195–200. [Google Scholar] [CrossRef]
- Hasan, S.; Sehar, Z.; Khan, N.A. Gibberellic Acid and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in mungbean (Vigna radiata L.) involves nitric oxide. J. Plant Growth Regul. 2020, 39, 1605–1615. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Y.; Lin, X.; Wang, Y.U.E.; Tang, C. Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ. 2008, 31, 195–204. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Mehmood, K. Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef]
- Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D. Nitric oxide production in tobacco leaf cells: A generalized stress response? Plant Cell Environ. 2003, 26, 1851–1862. [Google Scholar] [CrossRef]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Yuan, M. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef]
- Chaudhary, T.N.; Ghildyal, B.P. Influence of Submerged Soil Temperature Regimes on Growth, Yield, and Nutrient Composition of Rice Plant 1. Agron. J. 1970, 62, 281–285. [Google Scholar] [CrossRef]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Produc. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Chauhan, B.S.; Khan, F.; Alharby, H. Responses of rapid viscoanalyzer profile and other rice grain qualities to exogenously applied plant growth regulators under high day and high night temperatures. PLoS ONE 2016, 11, e0159590. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, Y.; Tang, L.; Chen, J.; Sun, T.; Cao, W.; Tian, Y. Differential effects of temperature and duration of heat stress during anthesis and grain filling stages in rice. Environ. Exp. Bot. 2016, 132, 28–41. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Alharby, H. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Ming-Hsuan, C.; Tarpley, L. Effects of high night temperature and abscisic acid (ABA) on rice (Oryza sativa L.) physiology. In Proceedings of the Rice Technical Working Group Meeting Proceedings, New Orleans, LA, USA, 18–21 February 2014; p. 113. [Google Scholar]
- Fahad, S.; Hussain, S.; Saud, S.; Khan, F.; Hassan, S.; Nasim, W.; Huang, J. Exogenously applied plant growth regulators affect heat-stressed rice pollens. J. Agron. Crop Sci. 2016, 202, 139–150. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]


| Cultivars | Nitrogen Content (mg g−1 DW) | Sulfur Content (mg g−1 DW) | Photosynthetic- NUE (g m−2) | Photosynthetic- SUE (g m−2) | Plant Dry Mass (g Plant−1) | Leaf Area (cm2 plant−1) |
|---|---|---|---|---|---|---|
| BPT-5204 | 34.5 ± 1.27 ab | 6.5 ± 0.30 ab | 46.3 ± 1.44 ab | 17.1 ± 1.23 abc | 4.44 ± 0.18 b | 91.5 ± 4.95 ab |
| Taipie-309 | 37.2 ± 1.41 a | 6.8 ± 0.35 a | 49.2 ± 1.50 a | 19.3 ± 1.32 a | 5.02 ± 0.20 a | 94.2 ± 5.35 a |
| Pusa-44 | 28.6 ± 1.15 cd | 5.8 ± 0.20 bcde | 38.6 ± 1.34 d | 14.7 ± 1.09 bcde | 3.91 ± 0.17 cd | 83.6 ± 3.70 ab |
| Panvel | 27.4 ± 1.11 cd | 5.6 ± 0.17 cdef | 38.1 ± 1.30 de | 14.4 ± 1.06 bcde | 4.21 ± 0.18 bc | 82.7 ± 3.65 ab |
| Rasi | 24.8 ± 0.95 d | 4.9 ± 0.1 f | 33.8 ± 1.21 e | 12.1 ± 0.85 e | 2.23 ± 0.13 g | 79.3 ± 2.70 b |
| MTU-1010 | 29.3 ± 1.20 c | 6.0 ± 0.26 bcd | 40.9 ± 1.31 cd | 15.2 ± 1.17 bcde | 3.67 ± 0.16 de | 86.8 ± 4.27 ab |
| CR-DHAN-310 | 35.7 ± 1.33 ab | 6.5 ± 0.30 ab | 47.5 ± 1.47 ab | 17.8 ± 1.28 ab | 4.68 ± 0.19 ab | 89.7 ± 4.79 ab |
| Nidhi | 26.8 ± 1.08 cd | 5.3 ± 0.15 def | 37.4 ± 1.27 de | 13.9 ± 0.98 cde | 3.11 ± 0.14 f | 81.8 ± 3.27 ab |
| CR-Dhan-311 | 26.1 ± 1.05 cd | 5.1 ± 0.11 ef | 36.8 ± 1.24 de | 13.2 ± 0.95 de | 3.03 ± 0.13 f | 81.2 ± 3.21 ab |
| Nagina-22 | 32.9 ± 1.25 d | 6.2 ± 0.28 abc | 43.7 ± 1.37 bc | 16.7 ± 1.21 abcd | 3.26 ± 0.15 ef | 87.4 ± 4.45 ab |
| Cultivars | Net Photosynthesis (µmol CO2 m−2 s−1) | Intercellular CO2 Concentration (µmol CO2 mol−1) | Stomatal Conductance (mmol CO2 m−2 s−1) | Chlorophyll Content (SPAD Value) | Maximal PSII Photochemical Efficiency |
|---|---|---|---|---|---|
| BPT-5204 | 17.7 ± 0.81 abc | 275.2 ± 13.06 ab | 383.8 ± 19.13 abc | 40.9 ± 1.25 ab | 0.711 ± 0.041 ab |
| Taipie-309 | 18.9 ± 0.88 a | 294.8 ± 13.72 a | 395.5 ± 19.28 a | 42.7 ± 1.36 a | 0.76 ± 0.049 a |
| Pusa-44 | 16.1 ± 0.65 bcdef | 249.4 ± 11.97 bcd | 366.7 ± 18.34 abcde | 34.7 ± 1.20 cd | 0.688 ± 0.035 ab |
| Panvel | 15.7 ± 0.51 cdef | 232.6 ± 11.50 cde | 360.4 ± 18.25 bcde | 33.2 ± 1.13 de | 0.678 ± 0.034 ab |
| Rasi | 14.1 ± 0.37 f | 203.1 ± 10.25 e | 336.5 ± 17.29 e | 28.5 ± 0.9 f | 0.609 ± 0.029 b |
| MTU-1010 | 16.8 ± 0.66 abcde | 255.7 ± 12.48 abcd | 371.8 ± 18.53 abcde | 35.6 ± 1.16 cd | 0.701 ± 0.036 ab |
| CR-Dhan-310 | 18.0 ± 0.85 ab | 280.3 ± 13.32 ab | 389.2 ± 19.16 ab | 38.2 ± 1.21 bc | 0.736 ± 0.046 ab |
| Nidhi | 15.2 ± 0.47 def | 230.2 ± 10.95 cde | 353.1 ± 18.15 cde | 31.9 ± 1.10 def | 0.632 ± 0.031 ab |
| CR-Dhan-311 | 14.9 ± 0.41 ef | 217.4 ± 10.47 de | 348.6 ± 18.12 de | 30.4 ± 1.05 ef | 0.644 ± 0.031 ab |
| Nagina-22 | 17.3 ± 0.70 abcd | 266.0 ± 12.91 abc | 377.3 ± 18.90 abcd | 37.9 ± 1.20 bc | 0.705 ± 0.037 ab |
| Cultivars | Number of Tillers/Plant | Number of Panicles/Plant | Panicles Length (cm) | Number of Grains/Panicle |
|---|---|---|---|---|
| BPT-5204 | 15 ± 0.60 c | 15 ± 0.63 ab | 22.0 ± 0.87 b | 153 ± 11.0 abc |
| Taipie-309 | 20 ± 0.66 a | 16 ± 0.65 a | 25.0 ± 0.90 a | 180 ± 12.0 a |
| Pusa-44 | 10 ± 0.58 d | 10 ± 0.56 de | 19.3 ± 0.78 c | 157 ± 11.2 ab |
| Panvel | 8 ± 0.47 ef | 11 ± 0.58 cd | 19.1 ± 0.72 c | 142 ± 10.4 bc |
| Rasi | 7 ± 0.41 f | 6 ± 0.49 f | 13.9 ± 0.65 f | 98 ± 9.33 d |
| MTU-1010 | 9 ± 0.51 de | 14 ± 0.61 b | 20.3 ± 0.83 bc | 148 ± 10.6 abc |
| CR-Dhan-310 | 18 ± 0.65 b | 15 ± 0.62 ab | 22.2 ± 0.89 b | 165 ± 11.6 ab |
| Nidhi | 8 ± 0.46 ef | 9 ± 0.55 e | 15.7 ± 0.66 ef | 103 ± 9.41 d |
| CR-Dhan-311 | 9 ± 0.52 de | 9 ± 0.55 e | 16.5 ± 0.68 de | 121 ± 9.93 cd |
| Nagina-22 | 9 ± 0.52 de | 12 ± 0.60 c | 18.3 ± 0.70 cd | 130 ± 10.1 bcd |
| Cultivar | Treatments | Number of Tillers per Plant | Number of Panicles per Plant | Panicles Length (cm) | Number of Grains per Panicle |
|---|---|---|---|---|---|
| Taipie-309 | Control | 20.0 ± 0.66 c | 16.0 ± 0.43 b | 25.0 ± 0.90 ab | 180.0 ± 12.0 a |
| HT | 15.9 ± 0.60 d | 12.4 ± 0.40 c | 22.8 ± 0.86 b | 142.5 ± 11.3 b | |
| NO | 23.5 ± 0.69 a | 17.9 ± 0.60 a | 26.7 ± 0.97 a | 199.9 ± 15.5 a | |
| NO + HT | 21.7 ± 0.65 b | 16.9 ± 0.50 ab | 25.6 ± 0.92 a | 189.7 ± 13.5 ab | |
| Rasi | Control | 7.0 ± 0.41 e | 6.0 ± 0.32 d | 13.9 ± 0.66 cd | 98.0 ± 9.33 cd |
| HT | 4.3 ± 0.30 f | 3.5 ± 0.23 e | 11.5 ± 0.55 d | 71.6 ± 7.40 d | |
| NO | 7.9 ± 0.51 e | 6.5 ± 0.40 d | 14.4 ± 0.72 c | 109.8 ± 10.9 bc | |
| NO + HT | 7.4 ± 0.45 e | 6.2 ± 0.35 d | 14.1 ± 0.70 c | 101.3 ± 10.2 cd |
| Cultivar | Treatments | H2O2 Content | Photosynthetic-NUE | Photosynthetic-SUE | Net Photosynthesis | Plant Dry Mass |
|---|---|---|---|---|---|---|
| Taipie-309 | Control | 47.6 ± 2.6 h | 49.2 ± 1.50 ab | 19.3 ± 1.21 b | 18.9 ± 0.76 b | 5.02 ± 0.22 ab |
| HT | 104.9 ± 4.74 cd | 38.7 ± 1.34 c | 12.8 ± 0.95 cd | 13.4 ± 0.71 cde | 3.32 ± 0.185 c | |
| NO + HT | 56.7 ± 3.66 fg | 52.3 ± 1.50 a | 22.2 ± 1.23 a | 20.7 ± 1.27 a | 5.53 ± 0.27 a | |
| NO + HT + cPTIO | 108.6 ± 4.89 c | 32.6 ± 1.12 ef | 9.9 ± 0.78 f | 10.72 ± 0.67 f | 2.54 ± 0.12 d | |
| Rasi | Control | 59.8 ± 2.9 f | 33.8 ± 1.21 e | 12.1 ± 0.93 cde | 14.1 ± 0.68 cd | 2.23 ± 0.127 ef |
| HT | 141.2 ± 5.15 ab | 23.6 ± 1.93 g | 6.8 ± 0.64 g | 8.7 ± 0.60 g | 1.14 ± 0.97 g | |
| NO + HT | 73.7 ± 4.18 e | 35.2 ± 1.85 cd | 13.5 ± 0.94 c | 15.1 ± 0.71 c | 2.39 ± 0.19 de | |
| NO + HT + cPTIO | 148.6 ± 5.26 a | 18.4 ± 1.67 h | 5.44 ± 0.53 h | 6.78 ± 0.56 h | 0.89 ± 0.45 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric Oxide Enhances Photosynthetic Nitrogen and Sulfur-Use Efficiency and Activity of Ascorbate-Glutathione Cycle to Reduce High Temperature Stress-Induced Oxidative Stress in Rice (Oryza sativa L.) Plants. Biomolecules 2021, 11, 305. https://doi.org/10.3390/biom11020305
Gautam H, Sehar Z, Rehman MT, Hussain A, AlAjmi MF, Khan NA. Nitric Oxide Enhances Photosynthetic Nitrogen and Sulfur-Use Efficiency and Activity of Ascorbate-Glutathione Cycle to Reduce High Temperature Stress-Induced Oxidative Stress in Rice (Oryza sativa L.) Plants. Biomolecules. 2021; 11(2):305. https://doi.org/10.3390/biom11020305
Chicago/Turabian StyleGautam, Harsha, Zebus Sehar, Md Tabish Rehman, Afzal Hussain, Mohamed F. AlAjmi, and Nafees A. Khan. 2021. "Nitric Oxide Enhances Photosynthetic Nitrogen and Sulfur-Use Efficiency and Activity of Ascorbate-Glutathione Cycle to Reduce High Temperature Stress-Induced Oxidative Stress in Rice (Oryza sativa L.) Plants" Biomolecules 11, no. 2: 305. https://doi.org/10.3390/biom11020305








