Zoonotic Agents in Farmed Fish: A Systematic Review from the Interdisciplinary Perspective of the One Health Concept
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol and Guiding Question
2.2. Eligibility Criteria
2.3. Sources of Information
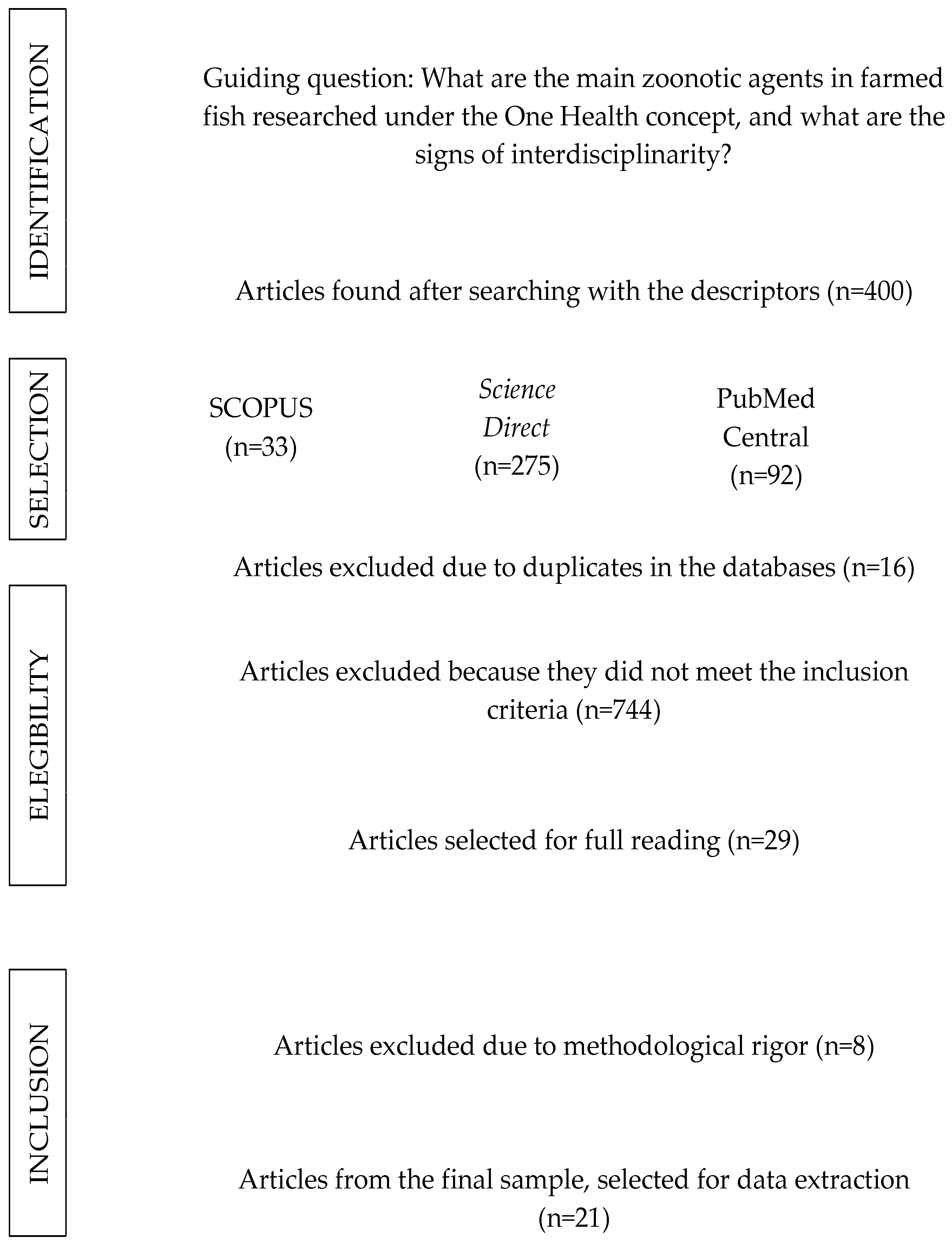
2.4. Search Strategy and Selection of Studies
2.5. Data Collection Process
2.6. Assessment of Methodological Quality and Risk of Bias
2.7. Interdisciplinarity
3. Results
3.1. Publication Identification
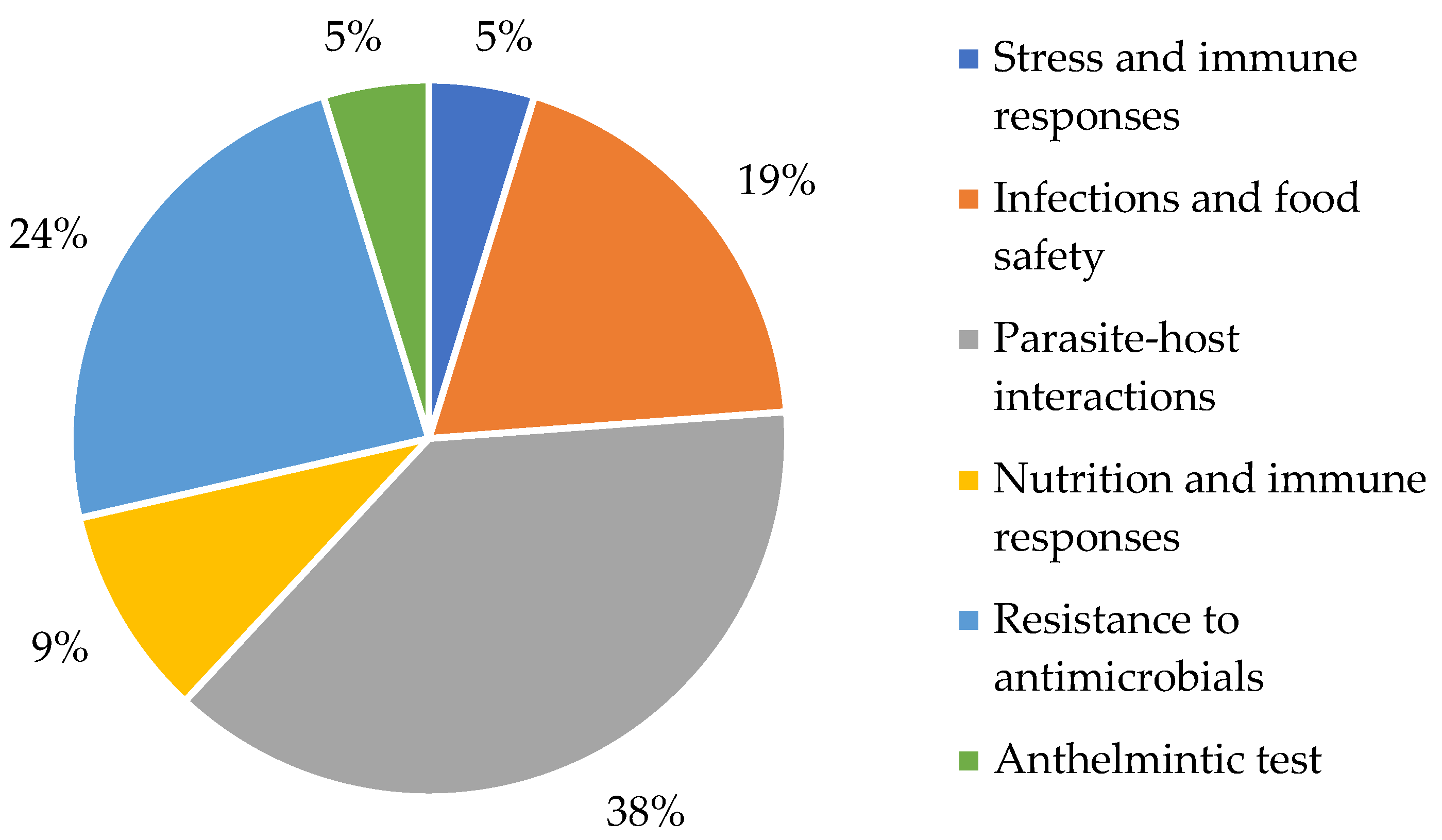
3.2. Methodological Aspects
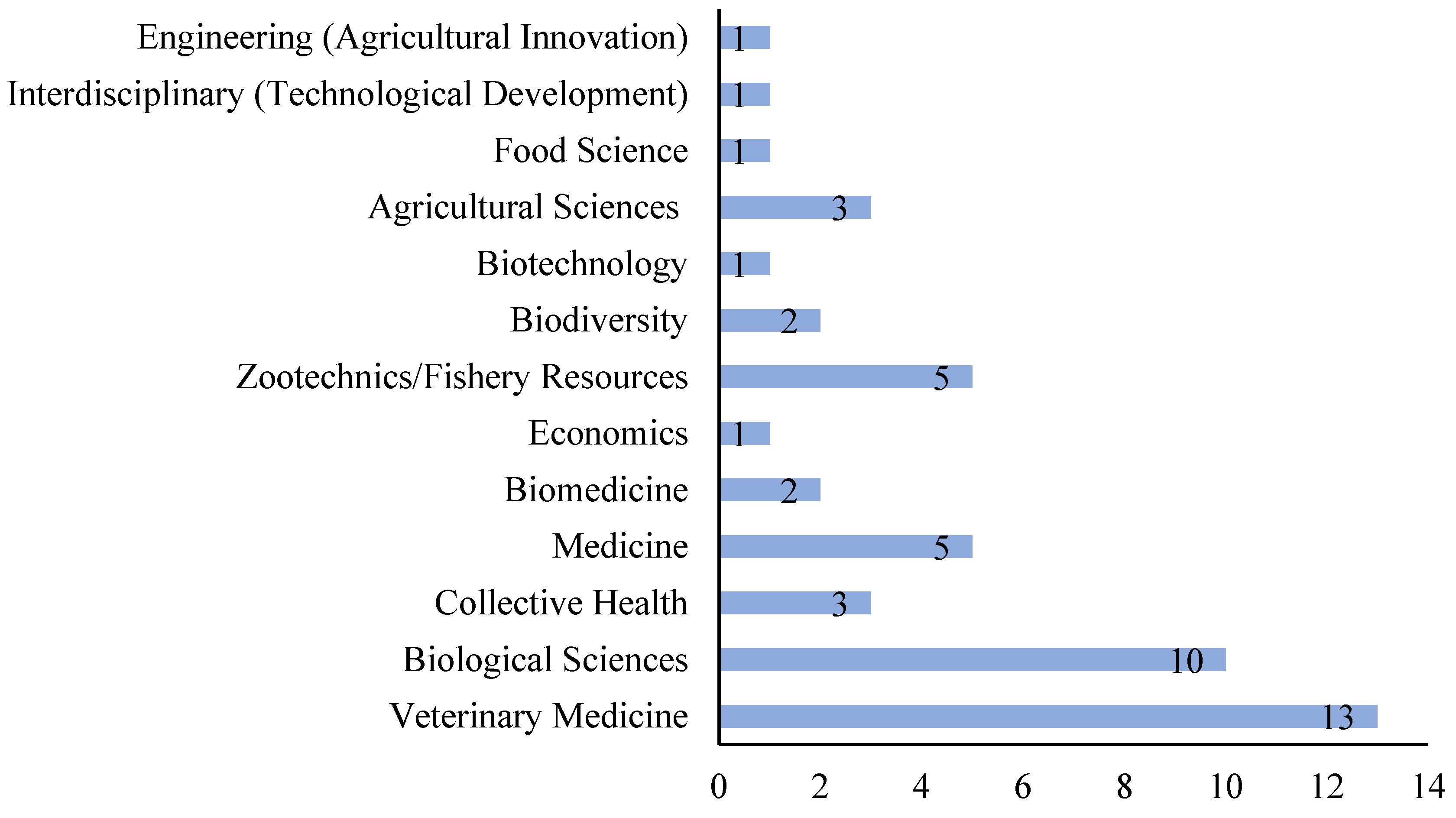
3.3. Institutionality, Affiliation and Interdisciplinarity
4. Discussion
4.1. Publication ID
4.2. Methodological Issues
4.3. Institutionality, Affiliation and Interdisciplinarity
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, A.; Basurto, X.; Virdin, J.; Lin, X.; Betances, S.J.; Smith, M.D.; Allison, E.H.; Best, B.A.; Brownell, K.D.; Campbell, L.M.; et al. Recognize fish as food in policy discourse and development funding. Ambio 2021, 50, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Memesh, R.; Yasir, M.; Ledder, R.G.; Zowawi, H.; McBain, A.J.; Azhar, E.I. An update on the prevalence of colistin and carbapenem-resistant Gram-negative bacteria in aquaculture: An emerging threat to public health. J. Appl. Microbiol. 2024, 135, lxad288. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Ferreira, M.; Machado, M.; Mota, C.S.; Abreu, H.; Silva, J.; Maia, M.R.G.; Kiron, V.; Costas, B.; Valente, L.M.P. Micro-and macroalgae blend modulates the mucosal and systemic immune responses of European seabass (Dicentrarchus labrax) upon infection with Tenacibaculum maritimum. Aquaculture 2023, 566, 739222. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Emran, T.B.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Bateman, I.J.; Hinchliffe, S.J.; Bass, D.; Hartnell, R.; Santos, E.M.; Devlin, M.J.; Feist, S.W.; Taylor, N.G.H.; Verner-Jeffreys, D.W.; et al. Sustainable aquaculture through the One Health lens. Nat. Food 2020, 1, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Fan, L.; Yang, N.; Zeng, J.; Guo, G.; Li, Q.; Wang, P.; Zeng, W.; Zheng, J. Response regulator KdpE contributes to Aeromonas dhakensis virulence. Aquaculture 2023, 568, 739298. [Google Scholar] [CrossRef]
- Fauzi, N.N.F.N.M.; Hamdan, R.H.; Mohamed, M.; Ismail, A.; Zin, A.A.M.; Mohamad, N.F.A. Prevalence, antibiotic susceptibility, and presence of drug resistance genes in Aeromonas spp. isolated from freshwater fish in Kelantan and Terengganu states, Malaysia. Vet. World 2021, 14, 2064–2072. [Google Scholar] [CrossRef]
- Castiglione, D.; Di Maggio, M.; Guardone, L.; Ricci, E.; Tinacci, L.; Guglielmone, G.; Coltraro, M.; Susini, F.; Armani, A. Eustrongylides excisus in fish species caught in the Massaciuccoli Lake (Northwest Tuscany, Italy): Implications for freshwater fish quality and public health. Food Control 2023, 153, 109894. [Google Scholar] [CrossRef]
- Couch, C.E.; Kent, M.L.; Weiss, L.M.; Takvorian, P.M.; Nervino, S.; Cummins, L.; Sanders, J.L. Enterocytozoon schreckii n. sp. infects the enterocytes of adult chinook salmon (Oncorhynchus tshawytscha) and may be a sentinel of immunosenescence. mSphere 2022, 7, e00908-21. [Google Scholar] [CrossRef]
- Barría, A.; Trịnh, T.Q.; Mahmuddin, M.; Peñaloza, C.; Papadopoulou, A.; Gervais, O.; Chadag, V.M.; Benzie, J.A.H.; Houston, R.D. A major quantitative trait locus affecting resistance to Tilapia lake virus in farmed Nile tilapia (Oreochromis niloticus). Heredity 2021, 127, 334–343. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.M.M.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Delghandi, M.R.; El-Matbouli, M.; Menanteau–Ledouble, S. Mycobacteriosis and infections with non-tuberculous mycobacteria in aquatic organisms: A review. Microorganisms 2020, 8, 1368. [Google Scholar] [CrossRef] [PubMed]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; ElSadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet. Q. 2018, 38, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Q.; Tang, M.; Wei, T.; Zou, J. Effects of tlr2/4 signalling pathway in western mosquitofish (Gambusia affinis) after Edwardsiella tarda infection. J. Fish. Dis. 2023, 46, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Novriadi, R. Vibriosis in aquaculture. Omni-Akuatika 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Lim, S.R.; Lee, D.; Park, S.Y.; Lee, S.; Kim, H.Y.; Lee, M.; Lee, J.; Han, J.E.; Kim, H.K.; Kim, J.H. Wild Nutria (Myocastor coypus) Is a Potential Reservoir of Carbapenem-Resistant and Zoonotic Aeromonas spp. in Korea. Microorganisms 2019, 7, 224. [Google Scholar] [CrossRef]
- Grilo, M.L.; Amaro, G.; Chambel, L.; Marques, C.S.; Marques, T.A.; Gil, F.; Sousa-Santos, C.; Robalo, J.I.; Oliveira, M. Aeromonas spp. Prevalence, Virulence, and Antimicrobial Resistance in an Ex Situ Program for Threatened Freshwater Fish—A Pilot Study with Protective Measures. Animals 2022, 12, 436. [Google Scholar] [CrossRef]
- Praveen, K.; Debnath, C.; Shekhar, S.; Dalai, N.; Ganguly, S. Incidence of Aeromonas spp. infection in fish and chicken meat and its related public health hazards: A review. Vet. World 2016, 9, 6–11. [Google Scholar] [CrossRef]
- Borella, L.; Salogni, C.; Vitale, N.; Scali, F.; Moretti, V.M.; Pasquali, P.; Alborali, G.L. Motile aeromonads from farmed and wild freshwater fish in northern Italy: An evaluation of antimicrobial activity and multidrug resistance during 2013 and 2016. Acta Vet. Scand 2020, 62, 6. [Google Scholar] [CrossRef]
- Thomas, S.G.; Abajorga, M.; Glover, M.A.; Wengert, P.C.; Parthasarathy, A.; Savka, M.A.; Wadsworth, C.B.; Shipman, P.A.; Hudson, A.O. Aeromonas hydrophila RIT668 and Citrobacter portucalensis RIT669—Potential Zoonotic Pathogens Isolated from Spotted Turtles. Microorganisms 2020, 8, 1805. [Google Scholar] [CrossRef]
- Park, S.Y.; Lim, S.R.; Son, J.S.; Kim, H.K.; Yoon, S.; Jeong, D.G.; Lee, M.; Lee, J.R.; Lee, D.; Kim, J.H. Complete Genome Sequence of Aeromonas rivipollensis KN-Mc-11N1, Isolated from a Wild Nutria (Myocastor coypus) in South Korea. Microbiol. Resour. Announc. 2018, 7, e00907-18. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, C.; Lam, K.; Chan, K.; Mo, W. Uses of Antibiotics in Ornamental Fish in Hong Kong and the Antibiotic Resistance in the Associated Zoonotic Pathogens. J. Xenobiot. 2022, 12, 365–377. [Google Scholar] [CrossRef] [PubMed]
- El-Hossary, D.; Mahdy, A.; Elariny, E.Y.; Askora, A.; Merwad, A.M.A.; Saber, T.; Dahshan, H.; Hakami, N.Y.; Ibrahim, R.A. Antibiotic Resistance, Virulence Gene Detection, and Biofilm Formation in Aeromonas spp. Isolated from Fish and Humans in Egypt. Biology 2023, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.V.; Cardoso, W.M.; Lopes, E.S.; Teixeira, R.S.C.; Albuquerque, A.H.; Rocha-e-Silva, R.C.; Machado, D.N.; Bezerra, W.G.A. Identification and antimicrobial resistance of members from the Enterobacteriaceae family isolated from canaries (Serinus canaria). Pesq. Vet. Bras. 2015, 35, 552–556. [Google Scholar] [CrossRef]
- Albuquerque, D.D.A.; Figueiredo, F.B.; Brandão, M.L.; Furtado, M.C.; Cordeiro, J.L.P.; Lourenço, M.C.S.; Bruno, S.F. Microbiota oral e retal de calitriquídeos (Callithrix sp.) em área antropizada de Mata Atlântica, Rio de Janeiro, Brasil. Arq. Bras. Med. Vet. Zootec. 2020, 72, 1113–1121. [Google Scholar] [CrossRef]
- Erkinharju, T.; Dalmo, R.A.; Hansen, M.; Seternes, T. Cleaner fish in aquaculture: Review on diseases and vaccination. Rev. Aquacult. 2021, 13, 189–237. [Google Scholar] [CrossRef]
- Magalhães, Â.M.S.; Costa, B.S.; Tavares, G.C.; de Carvalho, S.I.G. Zoonoses parasitárias associadas ao consumo de carne de peixe cru. PubVet 2012, 6, 1411. [Google Scholar]
- Santos, C.A.L.; Howgate, P. Fishborne zoonotic parasites and aquaculture: A review. Aquaculture 2011, 318, 253–261. [Google Scholar] [CrossRef]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]
- Menconi, V.; Lazzaro, E.; Bertola, M.; Guardone, L.; Mazzucato, M.; Prearo, M.; Bilska-Zajac, E.; Cortinovis, L.; Manfrin, A.; Arcangeli, G.; et al. The occurrence of freshwater fish-borne zoonotic helminths in italy and neighbouring countries: A systematic review. Animals 2023, 13, 3793. [Google Scholar] [CrossRef]
- Madsen, H.; Stauffer, J.R. Zoonotic Trematode Infections; Their Biology, Intermediate Hosts and Control. IntechOpen 2022. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Dorny, P.; Nguyen, T.T.G.; Dermauw, V. Helminth infections in fish in vietnam: A systematic review. Int. J. Parasitol. Parasites Wildl. 2021, 14, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.P.; Borges, J.N. Current knowledge of small flukes (Digenea: Heterophyidae) from South America. Korean J. Parasitol. 2020, 58, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Cho, E.H.; Park, K.H.; Jo, S.M.; Park, B.; Huh, S. A case of vocal cord gnathostomiasis diagnosed with sectional morphologies in a histopathological specimen from a Chinese woman living in korea. Parasites Hosts Dis. 2023, 61, 298–303. [Google Scholar] [CrossRef]
- Chaves, C.M.; Chaves, C.; Zoroquiain, P.; Belfort, R., Jr.; Burnier, M.N., Jr. Ocular gnathostomiasis in Brazil: A case report. Ocul. Oncol. Pathol. 2016, 2, 194–196. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquacult. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fungal diseases of marine and estuarine fishes. In Pathobiology of Marine and Estuarine Organisms; CRC Press: Boca Raton, FL, USA, 2021; pp. 85–109. [Google Scholar]
- World Health Organization (WHO). UNEP United Nations Environment Programme, & World Organisation for Animal Health. In One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bordier, M.; Uea-Anuwong, T.; Binot, A.; Hendrikx, P.; Goutard, F.L. Characteristics of One Health surveillance systems: A systematic literature review. Prev. Vet. Med. 2020, 181, 104560. [Google Scholar] [CrossRef]
- Jenkins, M.; Ahmed, S.; Barnes, A.N. A systematic review of waterborne and water-related disease in animal populations of Florida from 1999–2019. PLoS ONE 2021, 16, e0255025. [Google Scholar] [CrossRef]
- Corrales, N.U. The significance of education in the preparedness for zoonotic diseases. Epidemic Preparedness and Control. IntechOpen 2023. [Google Scholar] [CrossRef]
- Pereira, E.Q.; Nascimento, E.P. A interdisciplinaridade nas universidades brasileiras: Trajetória e desafios. REDES Rev. Desenv. Reg. 2016, 21, 209–232. [Google Scholar] [CrossRef][Green Version]
- Oliveira, M.R.; Almeida, J. Programas de pós-graduação interdisciplinares: Contexto, contradições e limites do processo de avaliação Capes. Rev. Bras. Pós-Grad. 2011, 8. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group PRISMA-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Bader, C.; Chelladurai, J.J.; Starling, D.E.; Jones, D.E.; Brewer, M.T. Efficacy of injectable praziquantel for elimination of trematode metacercariae in bluegills (Lepomis macrochirus) and quantification of parasite death by propidium iodide staining. Parasitol. Res. 2018, 117, 365–370. [Google Scholar] [CrossRef]
- Hayes, L.; Robinson, G.; Chalmers, R.M.; Ormerod, S.J.; Paziewska-Harris, A.; Chadwick, E.A.; Durance, I.; Cable, J. The occurrence and zoonotic potential of Cryptosporidium species in freshwater biota. Parasit. Vectors 2023, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, Z.; Conn, D.B.; Giese, E.G.; Pinheiro, R.H.S.; Miquel, J. Functional ultrastructure and cytochemistry of vitellogenesis stages of Rohdella amazonica (Aspidogastrea, Aspidogastridae, Rohdellinae), a parasite of the Amazoninan banded puffer fish Colomesus psittacus. Zool. Anz. 2021, 294, 106–113. [Google Scholar] [CrossRef]
- Shamsi, S.; Day, S.; Zhu, X.; McLellan, M.; Barton, D.P.; Dang, M.; Nowak, B.F. Wild fish as reservoirs of parasites on Australian Murray cod farms. Aquaculture 2021, 539, 736584. [Google Scholar] [CrossRef]
- Palomba, M.; Santoro, M.; Albuquerque, R.A.; Cipriani, P.; Mattiucci, S. First molecular detection of the parasites Molicola uncinatus and Hepatoxylon trichiuri (Cestoda: Trypanorhyncha) infecting the silver scabbardfish Lepidopus caudatus from the Central Mediterranean Sea: Implications for the seafood quality and safety. Food Control 2021, 122, 107807. [Google Scholar] [CrossRef]
- Casal, G.; Silva, T.J.; Soares, E.C.; Oliveira, E.; Santos, M.; Rocha, S. Morphological, histopathological, ultrastructural and phylogenetic analysis of Henneguya archosargus n. sp. (Cnidaria, Myxosporea) infecting the sparid fish Archosargus probatocephalus in Brazilian Waters. Microb. Pathog. 2023, 184, 106366. [Google Scholar] [CrossRef] [PubMed]
- Di Azevedo, M.I.N.; Iñiguez, A.M. Nematode parasites of commercially important fish from the southeast coast of Brazil: Morphological and genetic insight. Int. J. Food. Microbiol. 2018, 267, 29–41. [Google Scholar] [CrossRef]
- Kumagai, T.; Nishino, Y. Occurrence and prevalence of third-stage larvae of the Anisakidae family in Macrouridae species captured from bathyal depths of Sagami Bay, Japan. Deep-Sea Res. Part I 2023, 200, 104151. [Google Scholar] [CrossRef]
- Riera-Ferrer, E.; Del Pozo, R.; Piazzon, C.; Sitja-Bobadilla, A.; Estensoro, I.; Palenzuela, O. Sparicotyle chrysophrii experimental infection of gilthead seabream (Sparus aurata): Establishment of an in vivo model reproducing the pathological outcomes of sparicotylosis. Aquaculture 2023, 573, 739588. [Google Scholar] [CrossRef]
- Kang, G.; Choi, K.; Cho, D.; Joo, M.; Heo, M.; Woo, W.; Park, C. The First Detection of Kudoa hexapunctata in Farmed Pacific Bluefin Tuna in South Korea, Thunnus orientalis (Temminck and Schlegel, 1844). Animals 2020, 10, 1705. [Google Scholar] [CrossRef]
- Suthar, J.; Shamsi, S. The occurrence and abundance of infective stages of zoonotic nematodes in selected edible fish sold in Australian fish markets. Microb. Pathog. 2021, 154, 104833. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public. Health 2019, 66, 826–834. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Yardimci, B.; Altun, S.; Duman, M. A comprehensive perspective on a Vagococcus salmoninarum outbreak in rainbow trout broodstock. Aquaculture 2021, 545, 737224. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yokota, M.; Jinnai, M.; Minh, D.T.N.; Hoang, O.N.; Thi, H.L.; Thanh, P.N.; Hoai, P.H.; Do, P.N.; Van, C.D.; et al. Detection of chromosome-mediated blaNDM-1-carrying Aeromonas spp. in the intestinal contents of fresh water river fish in Ho Chi Minh City, Vietnam. Mar. Pollut. Bull. 2024, 198, 115812. [Google Scholar] [CrossRef]
- Moya-Salazar, J.; Díaz, C.R.; Cañari, B.; Badillo, R.X.; Verano-Zelada, M.; Chicoma-Flores, K.; Contreras-Pulache, H. Detection of pathogenic Aeromonas hydrophila from two rainbow trout (Oncorhyncus mykiss) farms in Peru. Braz. J. Vet. Med. 2022, 44, e000922. [Google Scholar] [CrossRef]
- Cheng, C.; Park, S.C.; Giri, S.S. Effect of Pandanus tectorius extract as food additive on oxidative stress, immune status, and disease resistance in Cyprinus carpio. Fish. Shellfish. Immunol. 2022, 120, 287–294. [Google Scholar] [CrossRef]
- Vaneci-Silva, D.; Assane, I.M.; Alves, L.O.; Gomes, F.C.; Moro, E.B.; Kotzent, S.; Pitondo-Silva, A.; Pilarski, F. Klebsiella pneumoniae causing mass mortality in juvenile Nile tilapia in Brazil: Isolation, characterization, pathogenicity and phylogenetic relationship with other environmental and pathogenic strains from livestock and human sources. Aquaculture 2022, 546, 737376. [Google Scholar] [CrossRef]
- Sibinga, N.A.; Lee, M.; Johnson, E.L.; Selvaraj, V.; Marquis, H. Longitudinal Sampling of the Rainbow Trout (Oncorhynchus mykiss) Microbiome Reveals Effects of Dietary Cecropin A and Yersinia ruckeri Infection. Front. Mar. Sci. 2022, 9, 901389. [Google Scholar] [CrossRef]
- Atlas, R.M. One Health: Its origins and future. In One Health: The Human-Animal-Environment Interfaces in Emerging Infectious Diseases: The Concept and Examples of a One Health Approach; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–13. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health approach—Why is it so important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Senthamarai, M.D.; Rajan, M.R.; Bharathi, P.V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef]
- Shamsi, S. Seafood-borne parasitic diseases: A “one-health” approach is needed. Fishes 2019, 4, 9. [Google Scholar] [CrossRef]
- Okumura, M.P.M.; de Pérez, A.C.A.; Espíndola Filho, A. Principais zoonoses parasitárias transmitidas por pescado-revisão. Rev. Educ. Contin. Med. Vet. Zootec. CRMV-SP 1999, 2, 66–80. [Google Scholar] [CrossRef]
- Raja, R.A.; Patil, P.K.; Avunje, S.; Kumaran, M.; Solanki, H.G.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Efficacy of emamectin benzoate in controlling natural infestations of ectoparasites in economically important fish species of India. Aquaculture 2022, 551, 737940. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, S.W.; Kim, J.; Seo, K.S.; You, K.W.; Chung, J.H.; Moon, H.C.; Hong, G.Y. A case of endoscopically treated laryngopharyngitis resulting fromclinostomum complanatuminfection. Korean J. Gastroenterol. 2017, 69, 177–180. [Google Scholar] [CrossRef]
- Tachibana, T.; Watari, T. Novel case of food poisoning caused by the consumption of pacific bluefin tuna infected with Kudoa hexapunctata. Clin. Case Rep. 2021, 9, e04222. [Google Scholar] [CrossRef]
- Yokoyama, H.; Suzuki, J.; Shirakashi, S. Kudoa hexapunctata n. sp. (Myxozoa: Multivalvulida) from the somatic muscle of pacific bluefin tuna Thunnus orientalis and re-description of K. neothunni in yellowfin tuna T. albacares. Parasitol. Int. 2014, 63, 571–579. [Google Scholar] [CrossRef]
- Bashirullah, A.; Díaz, M. Temporal distribution and population structure of two congeneric species of Cucullanus (Nematoda: Cucullanidae) in Orthopristis ruberin venezuela. J. Helminthol. 2008, 82, 69–76. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, S.; Xu, S.; Wang, J.; Wang, S.; Hu, X.; Zhang, L.; Ren, L.; Zhang, J.; Liu, X.; et al. Porcine epidemic diarrhea virus replication in human intestinal cells reveals potential susceptibility to cross-species infection. Viruses 2023, 15, 956. [Google Scholar] [CrossRef]
- Kaur, K.; Jansen, P.A.; Aspehaug, V.T.; Horsberg, T.E. Phe362Tyr in AChE: A major factor responsible for azamethiphos resistance in Lepeophtheirus salmonis in Norway. PLoS ONE 2016, 11, e0149264. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Control of parasitic diseases in aquaculture. Parasitology 2022, 149, 1985–1997. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Pelletier, C.; Boussaha, M.; Douet, D.; Lautraite, A.; Tailliez, P. Diversity of lactic acid bacteria associated with fish and the fish farm environment, established by amplified rrna gene restriction analysis. Appl. Environ. Microbiol. 2007, 73, 2947–2955. [Google Scholar] [CrossRef]
- Gatesoupe, F. Updating the importance of lactic acid bacteria in fish farming: Natural occurrence and probiotic treatments. Microbial. Physiol. 2007, 14, 107–114. [Google Scholar] [CrossRef]
- Jaffrès, E.; Prévost, H.; Rossero, A.; Joffraud, J.; Dousset, X. Vagococcus penaei sp. nov., isolated from spoilage microbiota of cooked shrimp (Penaeus vannamei). Int. J. Syst. Evol. Microbiol. 2010, 60, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Dai, F.; Duan, B.; Dong, G.; Zu, F.; Yang, Z.; Xiao, X. Isolation and characterization of Vagococcus carniphilus from diseased crucian carp. Biotechnol. Biotechnol. Equip. 2017, 32, 936–941. [Google Scholar] [CrossRef]
- Sibinga, N.; Marquis, H. Tissue-specific differences in detection of yersinia ruckeri carrier status in rainbow trout (Oncorhynchus mykiss). J. Fish. Dis. 2021, 44, 2013–2020. [Google Scholar] [CrossRef]
- Fan, S.; Lin, J.; Wu, S.; Mu, X.; Guo, J. Random forest model can predict the prognosis of hospital-acquired klebsiella pneumoniae infection as well as traditional logistic regression model. PLoS ONE 2022, 17, e0278123. [Google Scholar] [CrossRef]
- Jung, G.; Moon, S.; Oh, J.; Choi, H.; You, J. Acute osteomyelitis of the mandible by extended-spectrum β-lactamase producing klebsiella pneumoniae: A case report. J. Oral. Med. Pain. 2021, 46, 88–92. [Google Scholar] [CrossRef]
- Kumar, G.; Menanteau–Ledouble, S.; Saleh, M.; El-Matbouli, M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet. Res. 2015, 46, 103. [Google Scholar] [CrossRef]
- Resende, D.; Costas, B.; Sá, T.; Golfetto, U.; Machado, M.; Pereira, M.; Marques, B.; Rocha, C.M.R.; Pintado, M.; Valente, L.M.P. Innovative swine blood hydrolysates as promising ingredients for European seabass diets: Impact on growth performance and resistance to Tenacibaculum maritimum infection. Aquaculture 2022, 561, 738657. [Google Scholar] [CrossRef]
- Midtlyng, P.J. Current use and need for new fish vaccines. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Springer: Berlin/Heidelberg, Germany, 2022; pp. 599–608. [Google Scholar]
- Kaplan, M.; Karaoğlu, M. National and international legislations of combating viral fish diseases. Su Urunleri Dergisi 2018, 35, 353–360. [Google Scholar] [CrossRef]
- Amroabadi, M.A.; Rahimi, E.; Shakerian, A.; Momtaz, H. Incidence of hepatitis A and hepatitis E viruses and norovirus and rotavirus in fish and shrimp samples caught from the Persian Gulf. Arq. Bras. Med. Vet. Zootec. 2021, 73, 169–178. [Google Scholar] [CrossRef]
- Picot, S.; Beugnet, F.; Leboucher, G.; Bienvenu, A.L. Drug resistant parasites and fungi from a one-health perspective: A global concern that needs transdisciplinary stewardship programs. One Health 2022, 14, 100368. [Google Scholar] [CrossRef]
- Villanueva-Cabezas, J.P.; Winkel, K.D.; Campbell, P.T.; Wiethoelter, A.; Pfeiffer, C. One Health education should be early, inclusive, and holistic. Lancet Planet. Health 2022, 6, e188–e189. [Google Scholar] [CrossRef]
- Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Becerra, N.C.; Charron, D.F.; Chaudhary, A.; Zanella, J.R.C.; et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar] [CrossRef]
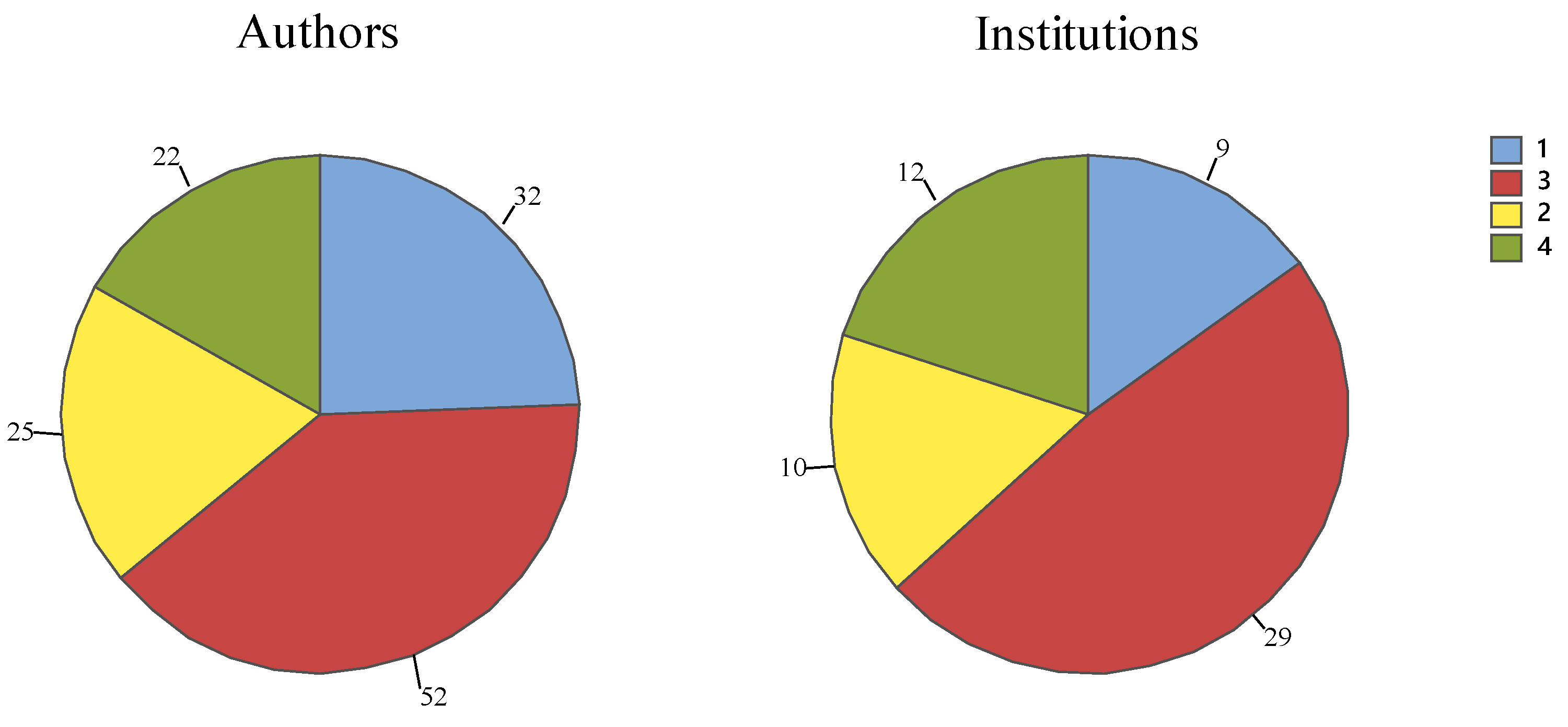
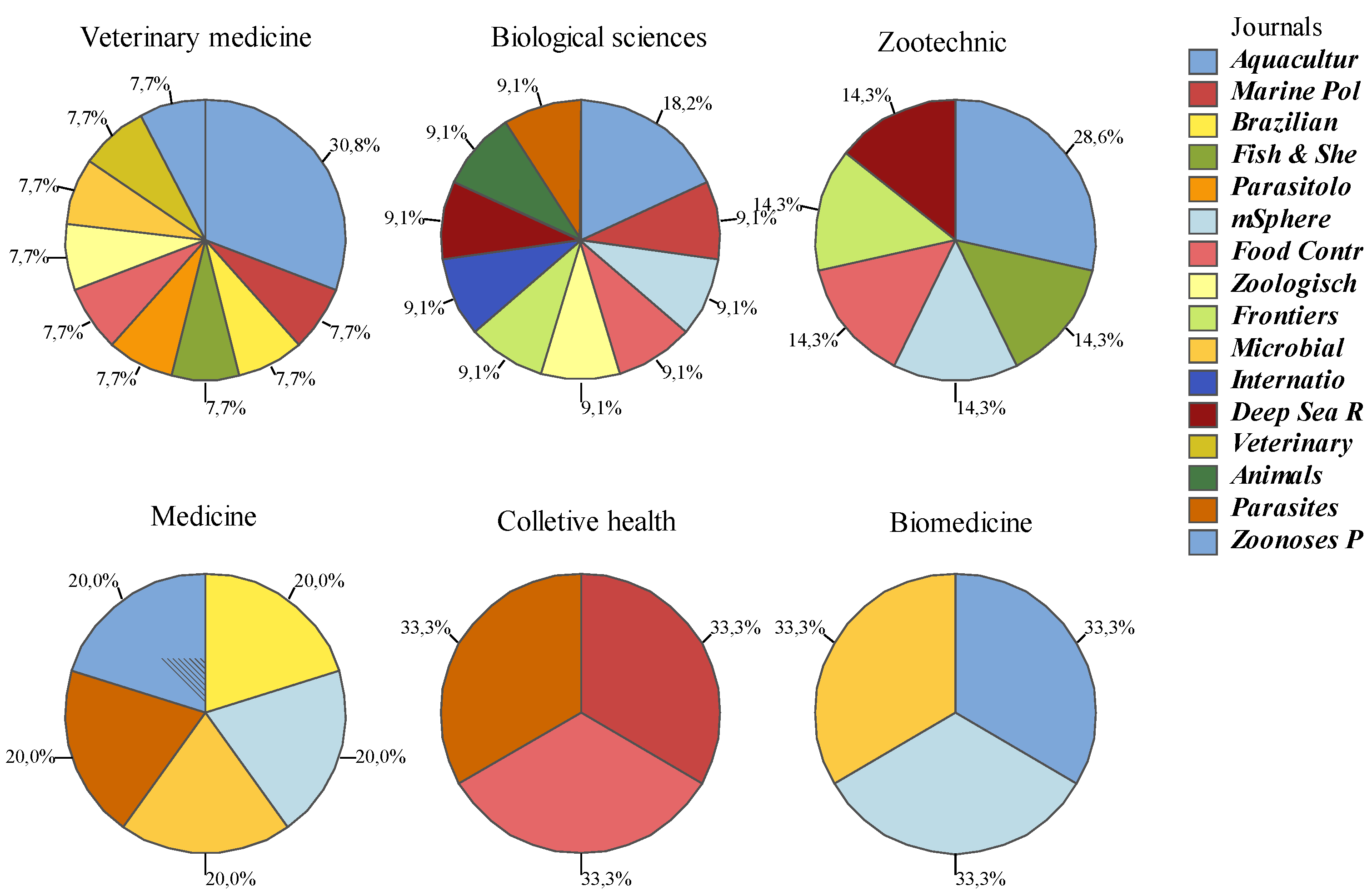
| Authors | Location | Zoonotic Agents | Water | Fish Species |
|---|---|---|---|---|
| Bader et al. [46] | USA * | Posthodiplostomum minimum | Fresh | Lepomis macrochirus |
| Couch et al. [10] | USA * | Enterocytozoon schreckii | Fresh | Oncorhynchus tshawytscha |
| Castiglione et al. [9] | Italy | Eustrongylides excisus | Fresh | Ameiurus melas, Carassius auratus, Silurus glanis, Lepomis gibbosus, Cyprinus carpio, Anguilla anguilla, Tinca tinca, Micropterus salmoides, Atherina boyeri, Chelon ramada and Pseudorasbora parva |
| Hayes et al. [47] | United Kingdom | Cryptosporidium spp. | Fresh | Salmo salar, Salmo truttan and Cottus gobio |
| Swiderski et al. [48] | Brazil | Rohdella amazonica | Fresh | Colomesus psittacus |
| Shamsi et al. [49] | Australia | Apatemon hypseleotris, Clinostomum spp., Parvitaenia and Camallanus | Fresh | Retropinna semoni and Hypseleotris spp. |
| Palomba et al. [50] | Mediterranean Sea ** | Trypanorhyncha spp. | Marine | Lepidopus caudatus |
| Casal et al. [51] | Brazil | Henneguya archosargus | Marine | Archosargus probatocephalus |
| Di Azevedo et al. [52] | Brazil | Cucculanus genypteri, Cucculanus pulcherrimus, Dichelyne sciaenidicola and Procamallanus halitrophus | Marine | Genypterus brasiliensis, Micropogonias furnieri and Mullus argentinae |
| Kumagai and Nishino [53] | Japan | Anisakis simplex | Marine | Coryphaenoides acrolepis, Coelorhynchus japonicus and Coelorhynchus macrochir |
| Riera-Ferrer et al. [54] | Mediterranean Sea | Sparicotyle chrysophrii | Marine | Sparus aurata |
| Kang et al. [55] | South Korea | Kudoa hexapunctata | Marine | Thunnus orientalis |
| Suthar et al. [56] | Australia | Anisakis spp., Contracaecum spp. and Hysterothylacium spp. | Marine | Platycephalus richardsoni, Scomber australasicus, Pagrus auratus and Sillago flindersi |
| Marino et al. [57] | Italy | Toxoplasma gondii | Marine | Esfiraena argentina, Arnoglossus lateral, Boops boops, Congro congro, Diplodus sargus, Engraulis encrasicolus, Merluccius merluccius, Mullus barbatus, Pagellus acarne, Pagellus erytrinus, Raja clavata, Sardina pilchardus, Sarpa salpa, Scorpaena scrofa, Serrano cabrilla, Spicara maena and Trachurus trachurus |
| Authors | Location | Zoonotic Agents | Water | Fish Species |
|---|---|---|---|---|
| Saticioglu et al. [58] | Turkey | Vagococcus salmoninarum | Fresh | Oncorhynchus mykiss |
| Yamaguchi et al. [59] | Vietnam | Aeromonas spp. | Fresh | Anabas testudineus, Channa striata, Clarias fuscus, Cynoglossidae spp., Cyprinus carpio, Mastacembelus spp., Mugil spp., Oreochromis spp. and Pangasius bocourti |
| Fauzi et al. [8] | Malaysia | Aeromonas spp. | Fresh | Oreochromis spp., Clarias gariepinus and Pangasianodon hypophthalmus |
| Moya-Salazar et al. [60] | Peru | Aeromonas hydrophila | Fresh | Oncorhynchus mykiss |
| Cheng et al. [61] | China | Aeromonas hydrophila | Fresh | Cyprinus carpio |
| Vaneci-Silva et al. [62] | Brazil | Klebsiella pneumoniae | Fresh | Oreochromis niloticus |
| Sibinga et al. [63] | USA * | Yersinia ruckeri | Fresh | Oncorhynchus mykiss |
| Authors | Journal | NA | NI |
|---|---|---|---|
| Saticioglu et al. [58]; Vaneci-Silva et al. [62]; Riera-Ferrer et al. [54]; Shamsi et al. [49] | Aquaculture | 25 | 12 |
| Yamaguchi et al. [59] | Marine Pollution Bulletin | 16 | 7 |
| Moya-Salazar et al. [60] | Brazilian Journal of Veterinary Medicine | 7 | 6 |
| Cheng et al. [61] | Fish & Shellfish Immunology | 3 | 2 |
| Bader et al. [46] | Parasitology Research | 5 | 1 |
| Couch et al. [10] | MSphere | 7 | 3 |
| Castiglione et al. [9]; Palomba et al. [50] | Food Control | 14 | 7 |
| Swiderski et al. [48] | Zoologischer Anzeiger | 5 | 7 |
| Sibinga et al. [63] | Frontiers in Marine Science | 5 | 1 |
| Casal et al. [51]; Suthar et al. [56] | Microbial Pathogenesis | 8 | 3 |
| Di Azevedo et al. [52] | International Journal of Food Microbiology | 2 | 1 |
| Kumagai and Nishino [53] | Deep Sea Research Part I: Oceanographic Research Papers | 2 | 2 |
| Fauzi et al. [8] | Veterinary World | 6 | 1 |
| Kang et al. [55] | Animals | 7 | 1 |
| Hayes et al. [47] | Parasites & Vectors | 8 | 4 |
| Marino et al. [57] | Zoonoses and Public Health | 11 | 2 |
| Total | 131 | 60 |
| Journal | Interdisciplinary Scope | Area(s) With Publication in the Quadrennium | Parent Area |
|---|---|---|---|
| Aquaculture | Y | Biodiversity, Geosciences and Veterinary Medicine | Zootechnics/Fishery Resources |
| Marine Pollution Bulletin | N | Interdisciplinary | Biodiversity |
| Brazilian Journal of Veterinary Medicine | N | Interdisciplinary | Veterinary Medicine |
| Fish & Shellfish Immunology | N | Interdisciplinary | Zootechnics/Fishery Resources |
| Parasitology Research | N | Interdisciplinary | Veterinary Medicine |
| MSphere | N | Interdisciplinary | Biological Sciences |
| Food Control | N | Interdisciplinary | Food Science |
| Zoologischer Anzeiger | N | Interdisciplinary | Biodiversity |
| Frontiers in Marine Science | Y | Interdisciplinary | Biodiversity |
| Microbial Pathogenesis | N | Interdisciplinary | Biological Sciences |
| International Journal of Food Microbiology | N | Interdisciplinary | Food Science |
| Deep Sea Research Part I: Oceanographic Research Papers | N | Environmental Sciences, Biological Sciences, Engineering, Geoscience, Mathematics/Probability and Statistics | Biodiversity |
| Veterinary World | N | Interdisciplinary | Veterinary Medicine |
| Animals | Y | Interdisciplinary | Zootechnics/Fishery Resources |
| Parasites & Vectors | N | Interdisciplinary | Biological Sciences |
| Zoonoses and Public Health | Y | Interdisciplinary | Veterinary Medicine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauad, J.R.C.; da Silva, M.C.; Araújo, C.M.C.; Silva, R.M.M.F.; Caleman, S.M.d.Q.; Russo, M.R. Zoonotic Agents in Farmed Fish: A Systematic Review from the Interdisciplinary Perspective of the One Health Concept. Vet. Sci. 2025, 12, 437. https://doi.org/10.3390/vetsci12050437
Mauad JRC, da Silva MC, Araújo CMC, Silva RMMF, Caleman SMdQ, Russo MR. Zoonotic Agents in Farmed Fish: A Systematic Review from the Interdisciplinary Perspective of the One Health Concept. Veterinary Sciences. 2025; 12(5):437. https://doi.org/10.3390/vetsci12050437
Chicago/Turabian StyleMauad, Juliana Rosa Carrijo, Marcelo Corrêa da Silva, Carolina Marques Costa Araújo, Rosilda Mara Mussury Franco Silva, Silvia Morales de Queiroz Caleman, and Márcia Regina Russo. 2025. "Zoonotic Agents in Farmed Fish: A Systematic Review from the Interdisciplinary Perspective of the One Health Concept" Veterinary Sciences 12, no. 5: 437. https://doi.org/10.3390/vetsci12050437
APA StyleMauad, J. R. C., da Silva, M. C., Araújo, C. M. C., Silva, R. M. M. F., Caleman, S. M. d. Q., & Russo, M. R. (2025). Zoonotic Agents in Farmed Fish: A Systematic Review from the Interdisciplinary Perspective of the One Health Concept. Veterinary Sciences, 12(5), 437. https://doi.org/10.3390/vetsci12050437








