Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Experimental Animals
2.2. Experimental Design (Individual Methods Are Outlined in Section 2.3, Section 2.4, Section 2.5, Section 2.6, Section 2.7, Section 2.8 and Section 2.9)
2.2.1. Trial 1—Local and Systemic Inflammatory Responses to Intradermal (i.d.) GF-Pulp Injections of Lipopolysaccharide (LPS) in 10-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
2.2.2. Trial 2—Local and Systemic Inflammatory Responses to i.d. GF-Pulp Injections of Peptidoglycan (PGN) in 11-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
2.2.3. Trial 3—Local and Systemic Innate and Adaptive Immune Responses to a First i.d. GF-Pulp Immunization with a Formalin-Killed Salmonella Enteritidis Vaccine (SEV) in 14-Week-Old Broiler Breeder Pullets from the MRB, LWCR, and HWCR Lines
2.3. Growing Feather Bioassay: Intradermal Injections and Sample Collection
Blood Sampling
2.4. Preparation of GF-Pulp and Blood Cell Suspensions and Immunofluorescent (IF) Staining Procedures
2.5. RNA Isolation, Quantification, cDNA Synthesis, and Relative Expression of Inflammatory Cytokines
2.6. Reactive Oxygen Species Generation Assay
2.7. Plasma Alpha-1 Acid Glycoprotein-1 Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA) to Detect Plasma Levels of Salmonella Enteritidis-Specific Antibodies
2.9. Statistical Analysis
3. Results
3.1. Trial 1—Local and Systemic Inflammatory Responses to i.d. GF-Pulp Injections of Lipopolysaccharide (LPS) in 10-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
3.1.1. Leukocyte Population Response Profiles in GF-Pulps and Peripheral Blood Following i.d. GF-Pulp Injection of LPS
3.1.2. Cytokine mRNA Expression in GF-Pulps Following i.d. GF-Pulp Injection of LPS
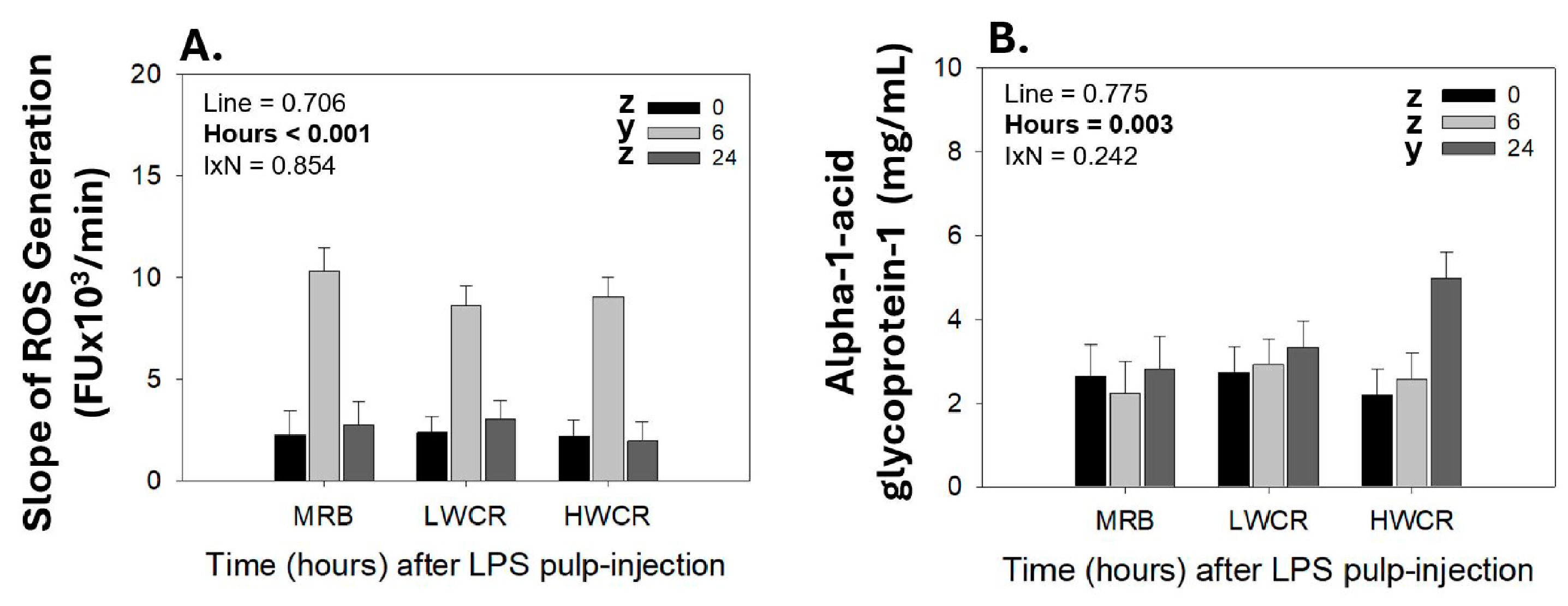
3.1.3. Reactive Oxygen Species (ROS) Generation in GF-Pulps and Alpha-1-Acid Glycoprotein-1 (AGP-1) Plasma Concentrations Following i.d. GF-Pulp Injection of LPS
3.2. Trial 2—Local and Systemic Inflammatory Responses to i.d. GF-Pulp Injections of Peptidoglycan (PGN) in 11-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
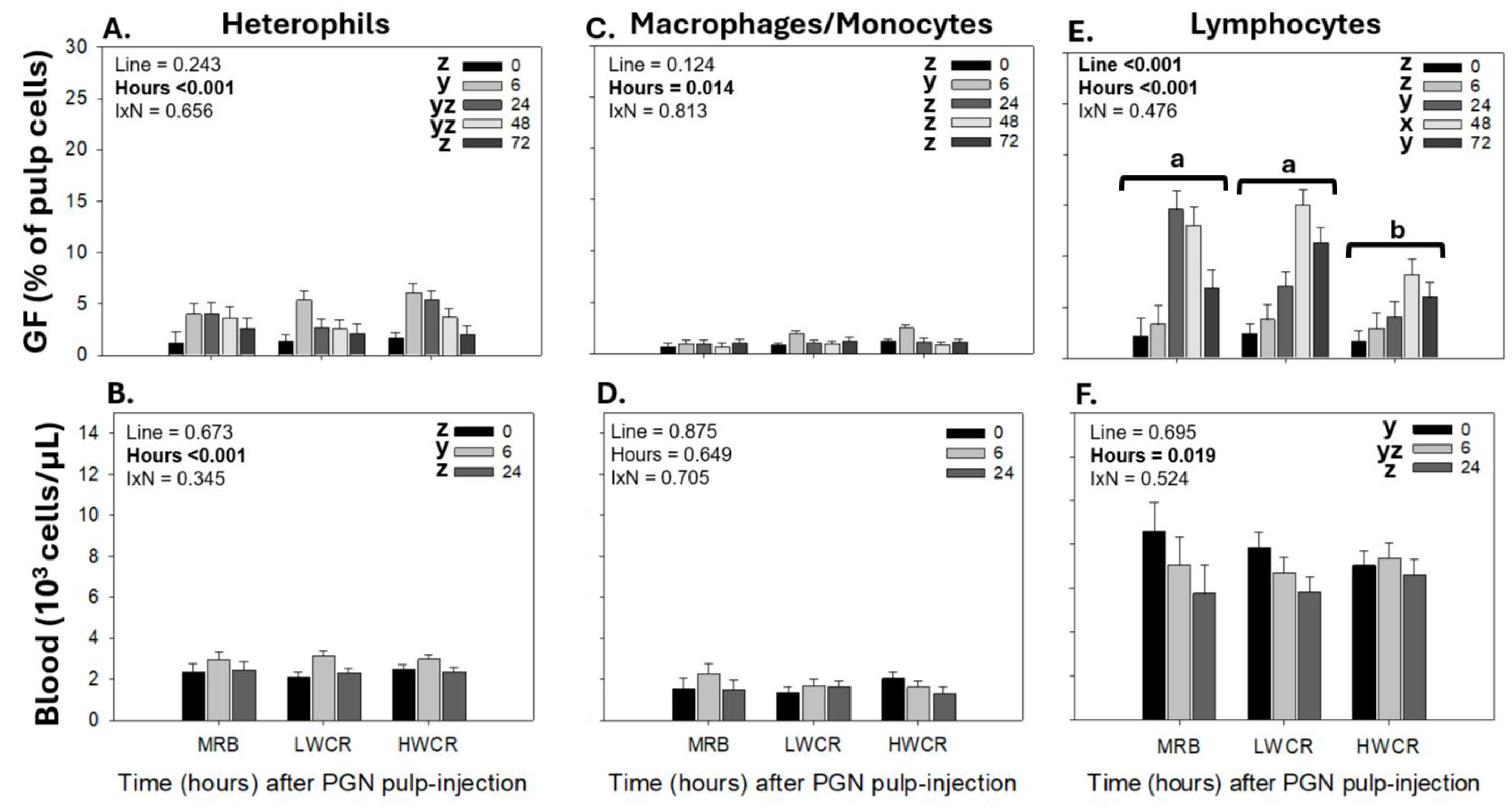
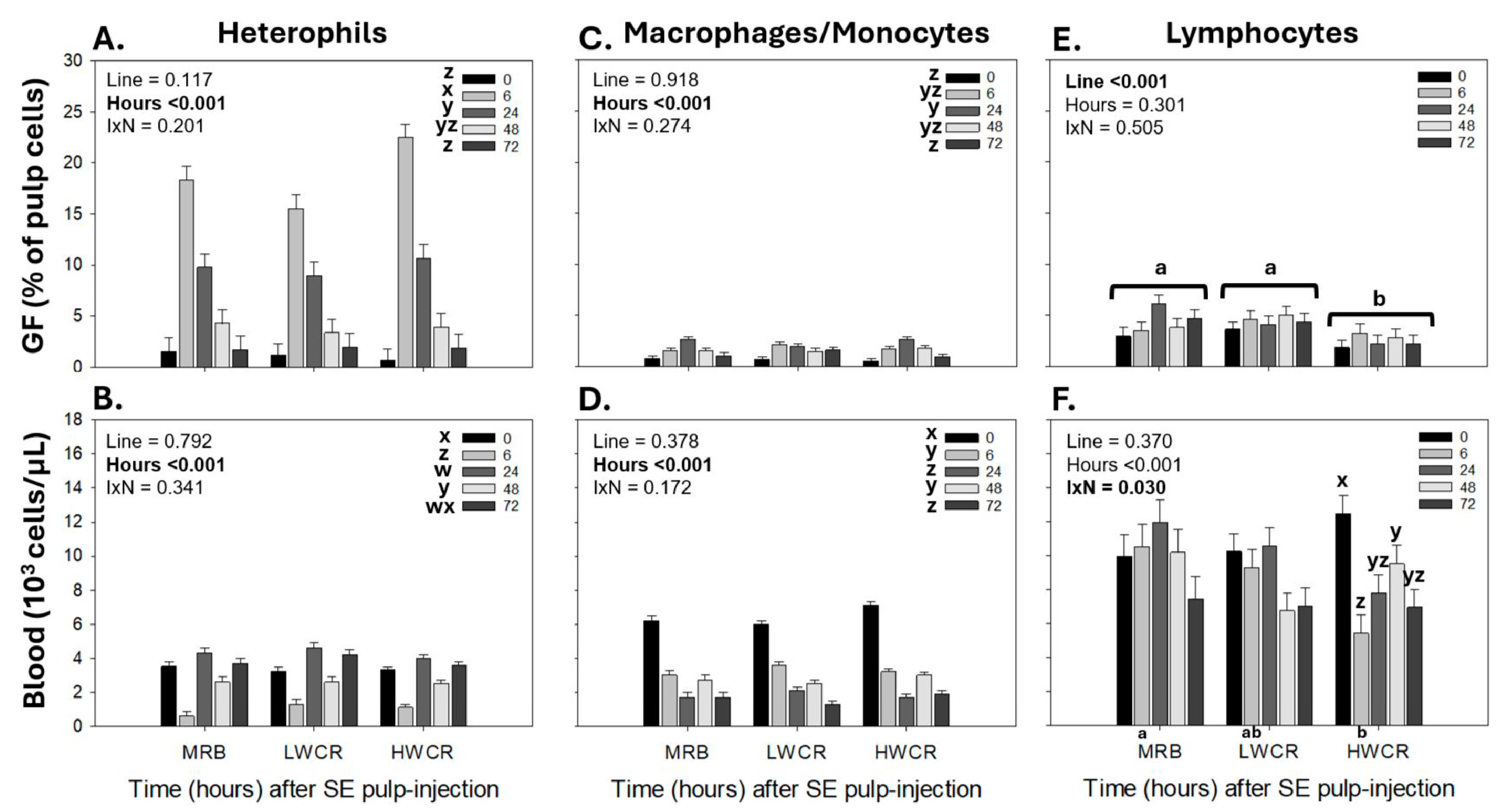
3.2.1. Leukocyte Population Responses Profiles in GF-Pulps and Peripheral Blood Following i.d. GF-Pulp Injection of PGN
3.2.2. Cytokine mRNA-Expression in GF-Pulps Following i.d. GF-Pulp Injection of PGN
3.2.3. Plasma Alpha-1-Acid Glycoprotein-1 (AGP-1) Concentrations Before and After i.d. GF-Pulp Injection of PGN
3.3. Trial 3—Local and Systemic Innate and Adaptive Immune Responses to a First i.d. GF-Pulp Immunization with a Formalin-Killed Salmonella Enteritidis Vaccine (SEV) in 14-Week-Old Broiler Breeder Pullets from the MRB, LWCR, and HWCR Lines
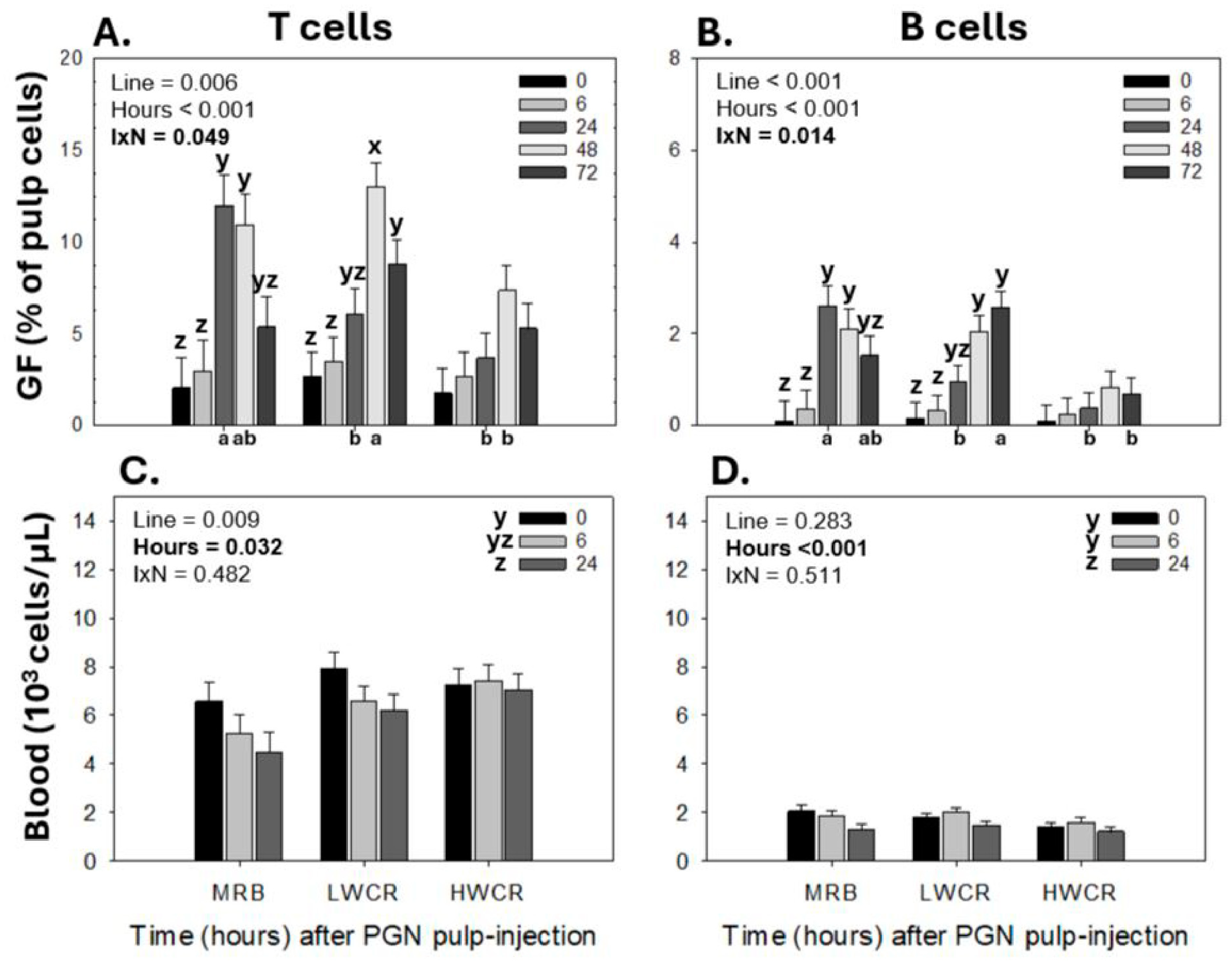
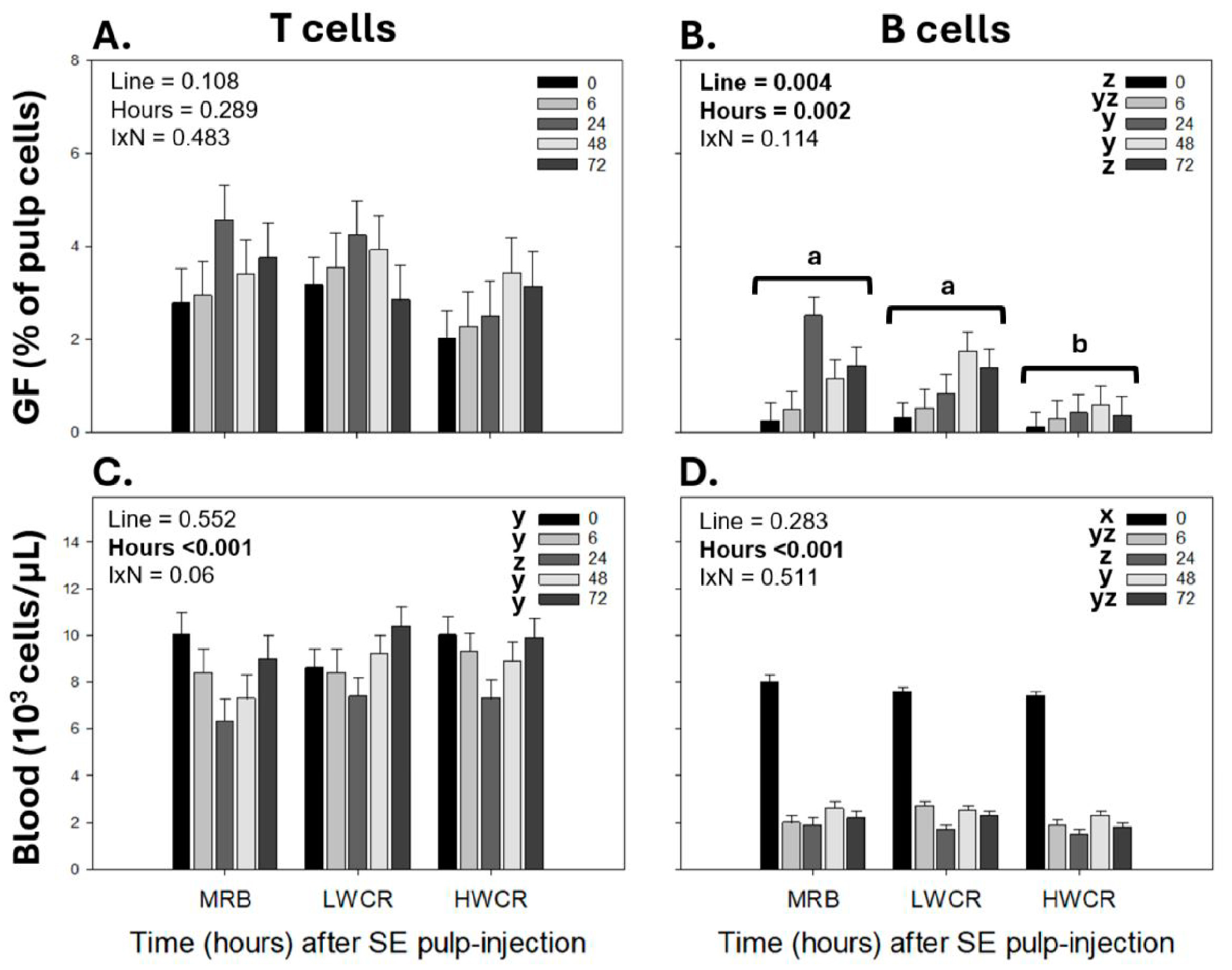
3.3.1. Leukocyte Population Profiles in GF-Pulps Before and After i.d. GF-Pulp Injection of SEV
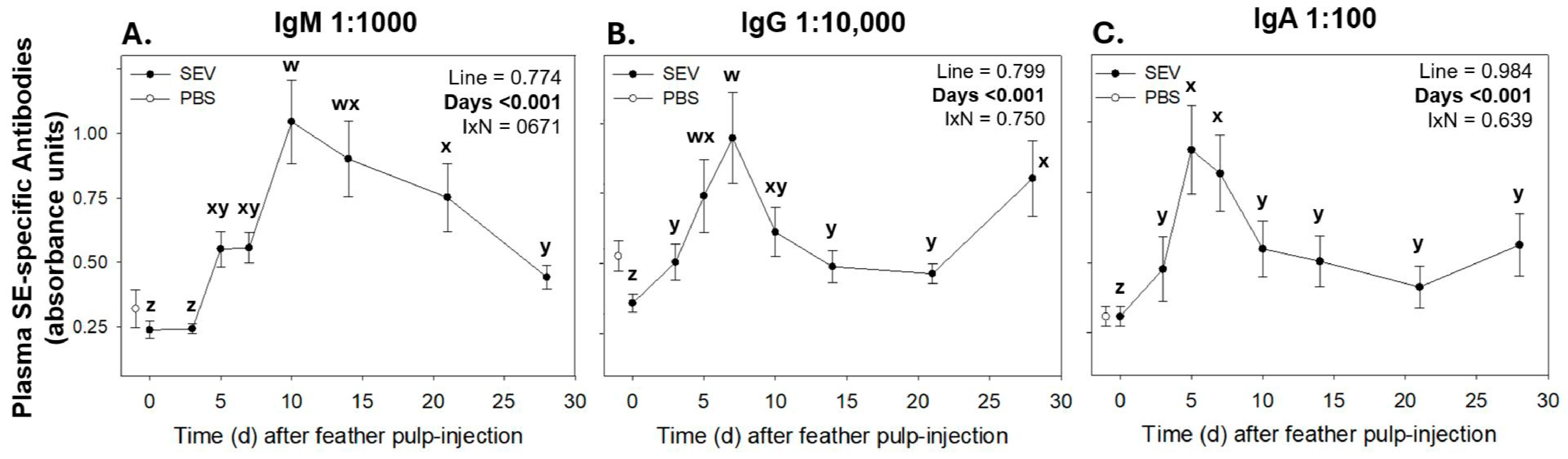
3.3.2. Salmonella Enteritidis-Specific IgM, IgG, and IgA Antibody Levels in Plasma Before and After Primary GF-Pulp Immunization with Formalin-Killed Salmonella Enteritidis Vaccine (SEV)
3.4. Leukocyte Response Profiles in GF-Pulps and Peripheral Blood Following Intradermal Pulp Injections of Endotoxin-Free Phosphate-Buffered Saline (PBS-Vehicle) in Broiler Breeders from the MRB, LWCR, and HWCR Lines from Three Trials
4. Discussion
4.1. Assessment of Local and Systemic Inflammatory Responses to i.d. GF-Pulp Injections of Lipopolysaccharide (LPS) in 10-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
4.2. Local and Systemic Inflammatory Responses to i.d. GF-Pulp Injections of Peptidoglycan (PGN) in 11-Week-Old Male Broiler Breeders from the MRB, LWCR, and HWCR Lines
4.3. Local and Systemic Innate and Adaptive Immune Responses to a First i.d. GF-Pulp Immunization with a Formalin-Killed Salmonella Enteritidis Vaccine (SEV) in 14-Week-Old Broiler Breeder Pullets from the MRB, LWCR, and HWCR Lines
4.4. Responses to Sterile, Endotoxin-Free PBS-Vehicle Injections
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving water use in crop production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; Belmonte-Ureña, L.J.; Román-Sánchez, I.M. Sustainable water use in agriculture: A review of worldwide research. Sustainability 2018, 10, 1084. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Tang, Q.; Liu, B.; Wada, Y.; Yang, H. Global Agricultural Water Scarcity Assessment Incorporating Blue and Green Water Availability Under Future Climate Change. Earth's Future 2022, 10, e2021EF002567. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water. 2019, 2, 15. [Google Scholar] [CrossRef]
- Walker, S.; Baum, J.I. Eggs as an affordable source of nutrients for adults and children living in food-insecure environments. Nutr. Rev. 2022, 80, 178–186. [Google Scholar] [CrossRef]
- Morris, S.S.; Beesabathuni, K.; Headey, D. An egg for everyone: Pathways to universal access to one of nature’s most nutritious foods. Matern. Child Nutr. 2018, 14, e12679. [Google Scholar] [CrossRef]
- Kleyn, F.J.; Ciacciariello, M. Future demands of the poultry industry: Will we meet our commitments sustainably in developed and developing economies? World’s Poult. Sci. J. 2021, 77, 267–278. [Google Scholar] [CrossRef]
- Orlowski, S.K.; Cauble, R.; Tabler, T.; Hiltz, J.Z.; Greene, E.S.; Anthony, N.B.; Dridi, S. Processing evaluation of random bred broiler populations and a common ancestor at 55 days under chronic heat stress conditions. Poult. Sci. 2020, 99, 3491–3500. [Google Scholar] [CrossRef]
- Hiltz, J.Z.; Orlowski, S.K.; Harrington, L.N.; Maynard, C.W.; Tabler, T.W.; Anthony, N.B. Applied research note: Development of a novel low flow water monitoring system in poultry/agriculture systems. J. Appl. Poult. Res. 2021, 30, 100151. [Google Scholar] [CrossRef]
- Rodrigues, D.R.; Wilson, K.M.; Bielke, L.R. Proper immune response depends on early exposure to gut microbiota in broiler chicks. Front. Physiol. 2021, 12, 758183. [Google Scholar] [CrossRef]
- Verwoolde, M.B.; van Baal, J.; Jansen, C.A.; Graat, E.A.; Lamot, D.M.; Lammers, A.; van Eck, L. Transgenerational effects of innate immune activation in broiler breeders on growth performance and immune responsiveness. Poult. Sci. 2021, 100, 101413. [Google Scholar] [CrossRef] [PubMed]
- Wlaźlak, S.; Pietrzak, E.; Biesek, J.; Dunislawska, A. Modulation of the immune system of chickens: A key factor in maintaining poultry production—A review. Poult. Sci. 2023, 102, 102785. [Google Scholar] [CrossRef] [PubMed]
- Erf, G.F.; Kong, H.R.; Falcon, D.M.; Byrne, K.A. Two-window approach to monitor and assess cellular and humoral immune responses in poultry. Poultry 2023, 2, 82–97. [Google Scholar] [CrossRef]
- French, C.E.; Sales, M.A.; Rochell, S.J.; Rodriguez, A.; Erf, G.F. Local and systemic inflammatory responses to lipopolysaccharide in broilers: New insights using a two-window approach. Poult. Sci. 2020, 99, 6593–6605. [Google Scholar] [CrossRef]
- Rocchi, A.J.; Santamaria, J.M.; Beck, C.N.; Sales, M.A.; Hargis, B.M.; Tellez-Isaias, G.; Erf, G.F. The immuno-suppressive effects of cyclic, environmental heat stress in broiler chickens: Local and systemic inflammatory responses to an intradermal injection of lipopolysaccharide. Vet. Sci. 2023, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, J.M.; Beck, C.N.; Erf, G.F. Local inflammatory and systemic antibody responses initiated by a first intradermal administration of autogenous Salmonella-killed vaccines and their components in pullets. Vaccines 2024, 12, 1159. [Google Scholar] [CrossRef]
- Byrne, K.A.; Erf, G.F. The bacterial cell wall components lipopolysaccharide and peptidoglycan initiate divergent local tissue and systemic inflammatory response profiles in the chicken model. Animals 2024, 14, 3661. [Google Scholar] [CrossRef]
- Seliger, C.; Schaerer, B.; Kohn, M.; Pendl, H.; Weigend, S.; Kaspers, B.; Härtle, S. A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens. Vet. Immunol. Immunopathol. 2012, 145, 86–99. [Google Scholar] [CrossRef]
- Sreekantapuram, S.; Berens, C.; Barth, S.A.; Methner, U.; Berndt, A. Interaction of Salmonella Gallinarum and Salmonella Enteritidis with peripheral leucocytes of hens with different laying performance. Vet. Res. 2021, 52, 23. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, N.B.; Elleder, D.; Schusser, B.; Krchlíková, V.; Göbel, T.W.; Härtle, S.; Kaspers, B. The discovery of chicken Foxp3 demands redefinition of avian regulatory T cells. J. Immunol. 2022, 208, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.C.; Huff, G.R.; Balog, J.M.; Huff, W.E. Fluorescein isothiocyanate staining and characterization of avian heterophils. Vet. Immunol. Immunopathol. 1998, 64, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, A.; Hsieh, J.C.; Schmidt, C.J.; Lamont, S.J. Heat stress and lipopolysaccharide stimulation of chicken macrophage-like cell line activates expression of distinct sets of genes. PLoS ONE 2016, 11, e0164575. [Google Scholar] [CrossRef]
- Mora, Z.V.D.L.; Macías-Rodríguez, M.E.; Arratia-Quijada, J.; Gonzalez-Torres, Y.S.; Nuño, K.; Villarruel-López, A. Clostridium perfringens as foodborne pathogen in broiler production: Pathophysiology and potential strategies for controlling necrotic enteritis. Animals 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, G.M.; Szeleszczuk, P.; Dolka, B. Review on skeletal disorders caused by Staphylococcus spp. in poultry. Vet. Q. 2022, 42, 21–40. [Google Scholar] [CrossRef]
- Jung, A.; Chen, L.R.; Suyemoto, M.M.; Barnes, H.J.; Borst, L.B. A review of Enterococcus cecorum infection in poultry. Avian Dis. 2018, 62, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.; Sukumaran, A.T. Avian Pathogenic Escherichia coli and Clostridium perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef]
- Mak, P.H.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production Systems and Important Antimicrobial Resistant-Pathogenic Bacteria in Poultry: A Review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Ferro, P.J.; Pevzner, I.Y.; Kogut, M.H. Heterophils are associated with resistance to systemic Salmonella enteritidis infections in genetically distinct chicken lines. FEMS Immunol. Med. Microbiol. 2005, 43, 149–154. [Google Scholar] [CrossRef]
- He, H.; Farnell, M.B.; Kogut, M.H. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 135, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology; Elsevier Health Sciences: Philadelphia, PA, USA, 2023. [Google Scholar]
- Leseigneur, C.; Lê-Bury, P.; Pizarro-Cerdá, J.; Dussurget, O. Emerging Evasion Mechanisms of Macrophage Defenses by Pathogenic Bacteria. Front. Cell. Infect. Microbiol. 2020, 10, 577559. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.K.; Powers, C.; Wigley, P.; Barrow, P.A.; Smith, A.L. Temporal dynamics of the cellular, humoral, and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 2004, 33, 25–33. [Google Scholar] [CrossRef] [PubMed]



| GF-Pulp 1 (% Pulp Cells) | Heterophils | Monocytes/ Macrophages | Lymphocytes |
| Time 4 (h) | |||
| 0 h | 1.34 ± 0.09 z | 0.76 ± 0.08 z | 2.24 ± 0.28 |
| 6 h | 7.45 ± 1.17 y | 2.92 ± 0.45 y | 2.54 ± 0.32 |
| 24 h | 3.38 ± 0.38 z | 1.41 ± 0.15 z | 2.41 ± 0.20 |
| 48 h | 2.90 ± 0.41 z | 1.06 ± 0.13 z | 2.56 ± 0.27 |
| 72 h | 2.22 ± 0.34 z | 1.14 ± 0.14 z | 2.19 ± 0.23 |
| Effects (p-value) 5 | |||
| Time | <0.001 | <0.001 | 0.814 |
| Blood 2 (103 cells/µL) | |||
| Time (h) | |||
| 0 h | 2.49 ± 0.21 z | 3.72 ± 0.39 z | 7.68 ± 0.57 |
| 6 h | 3.61 ± 0.45 y | 5.35 ± 0.48 y | 9.39 ± 0.72 |
| 24 h | 2.68 ± 0.24 yz | 2.50 ± 0.33 z | 7.87 ± 0.55 |
| Effects (p-value) | |||
| Time | <0.001 | <0.001 | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaria, J.M.; Beck, C.N.; Orlowski, S.K.; Maqueda, M.; Bottje, W.G.; Erf, G.F. Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine. Vet. Sci. 2025, 12, 279. https://doi.org/10.3390/vetsci12030279
Santamaria JM, Beck CN, Orlowski SK, Maqueda M, Bottje WG, Erf GF. Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine. Veterinary Sciences. 2025; 12(3):279. https://doi.org/10.3390/vetsci12030279
Chicago/Turabian StyleSantamaria, Jossie M., Chrysta N. Beck, Sara K. Orlowski, Maricela Maqueda, Walter G. Bottje, and Gisela F. Erf. 2025. "Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine" Veterinary Sciences 12, no. 3: 279. https://doi.org/10.3390/vetsci12030279
APA StyleSantamaria, J. M., Beck, C. N., Orlowski, S. K., Maqueda, M., Bottje, W. G., & Erf, G. F. (2025). Selection for Improved Water Efficiency in Broiler Breeder Lines Does Not Negatively Impact Immune Response Capabilities to Gram− and Gram+ Bacterial Components and a Killed-Salmonella Enteritidis Vaccine. Veterinary Sciences, 12(3), 279. https://doi.org/10.3390/vetsci12030279








