Isolation and Characterization of a Porcine Getah Virus Strain from Sichuan Province
Simple Summary
Abstract
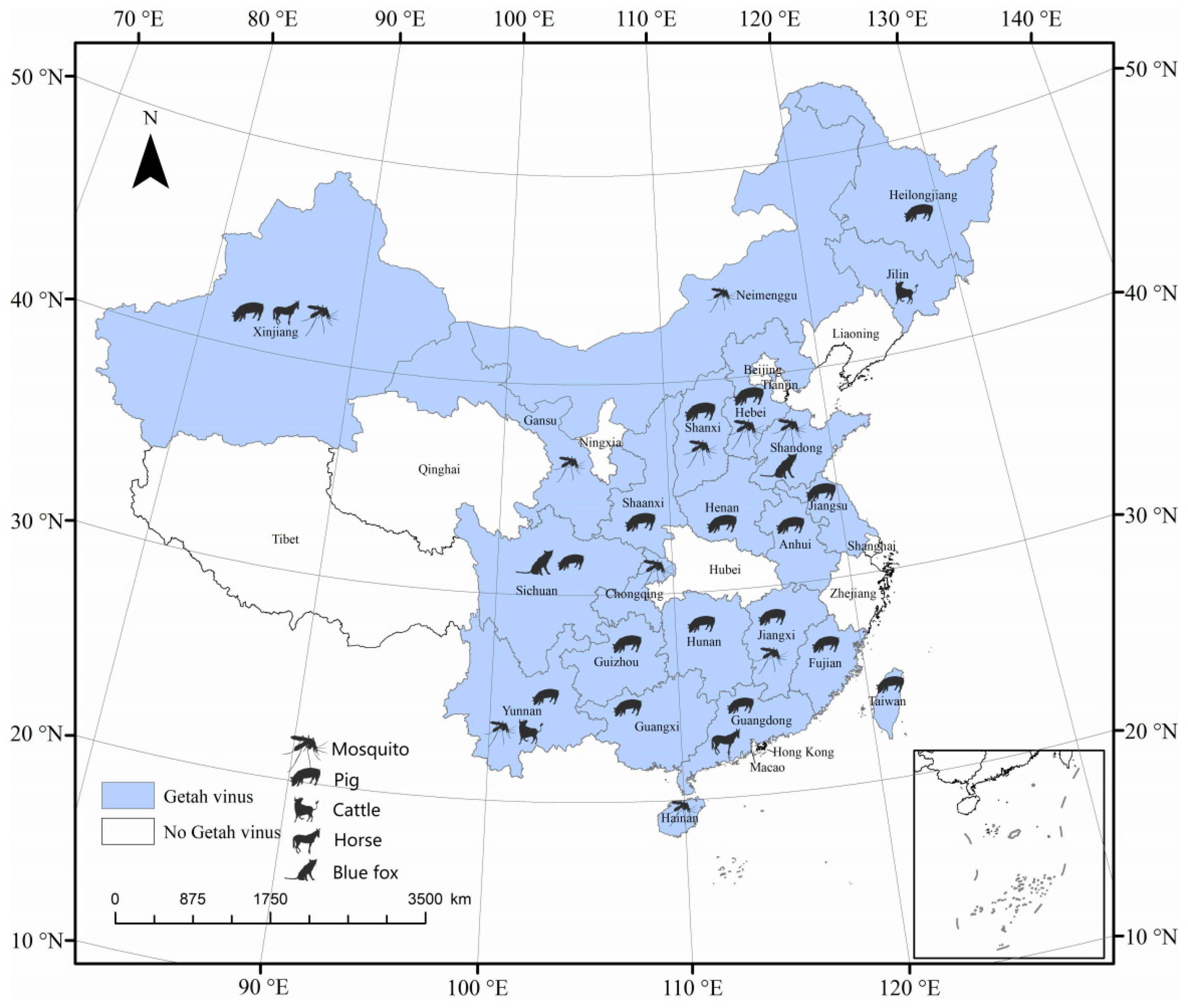
1. Introduction
2. Materials and Methods
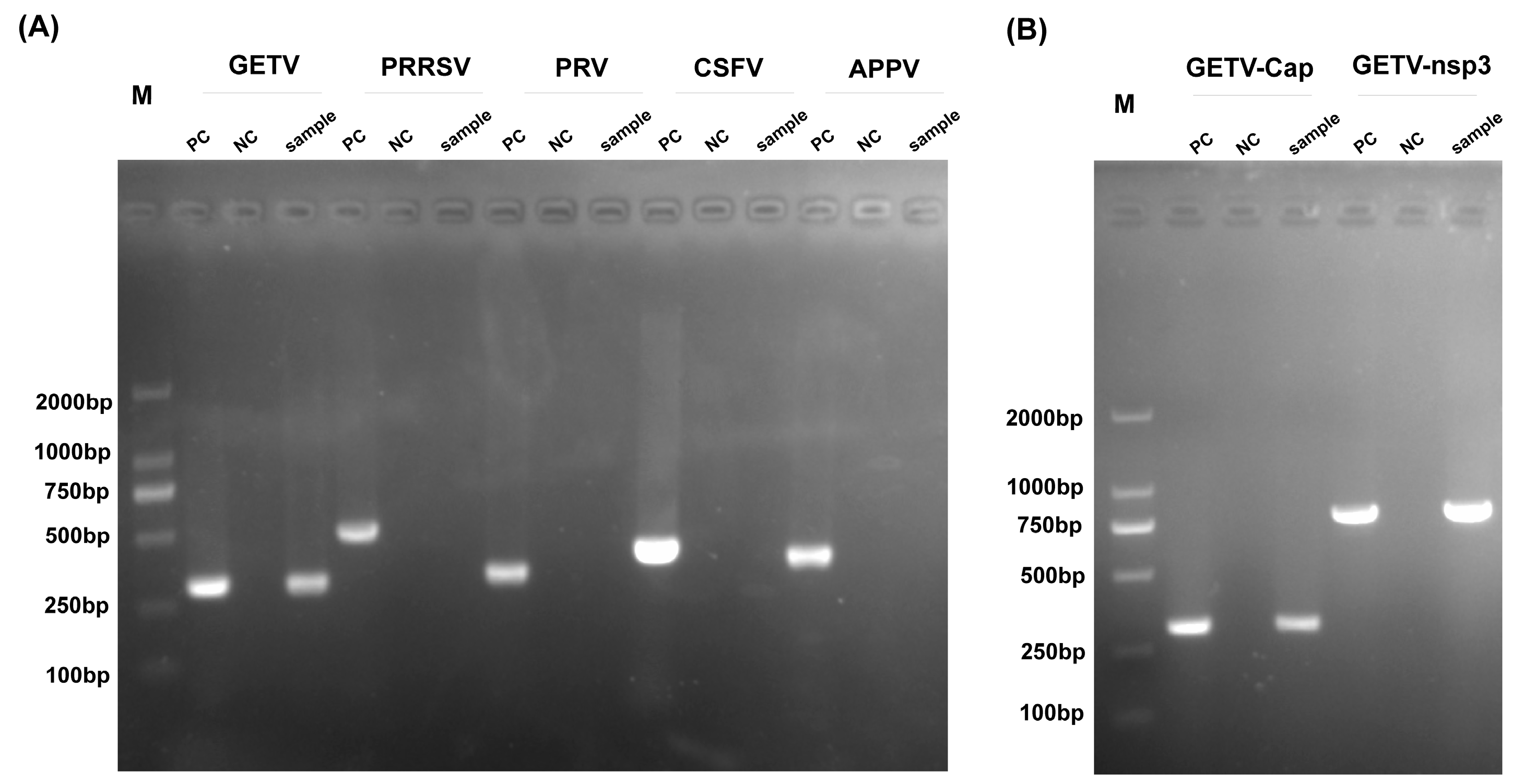
2.1. Sample Collection and Detection
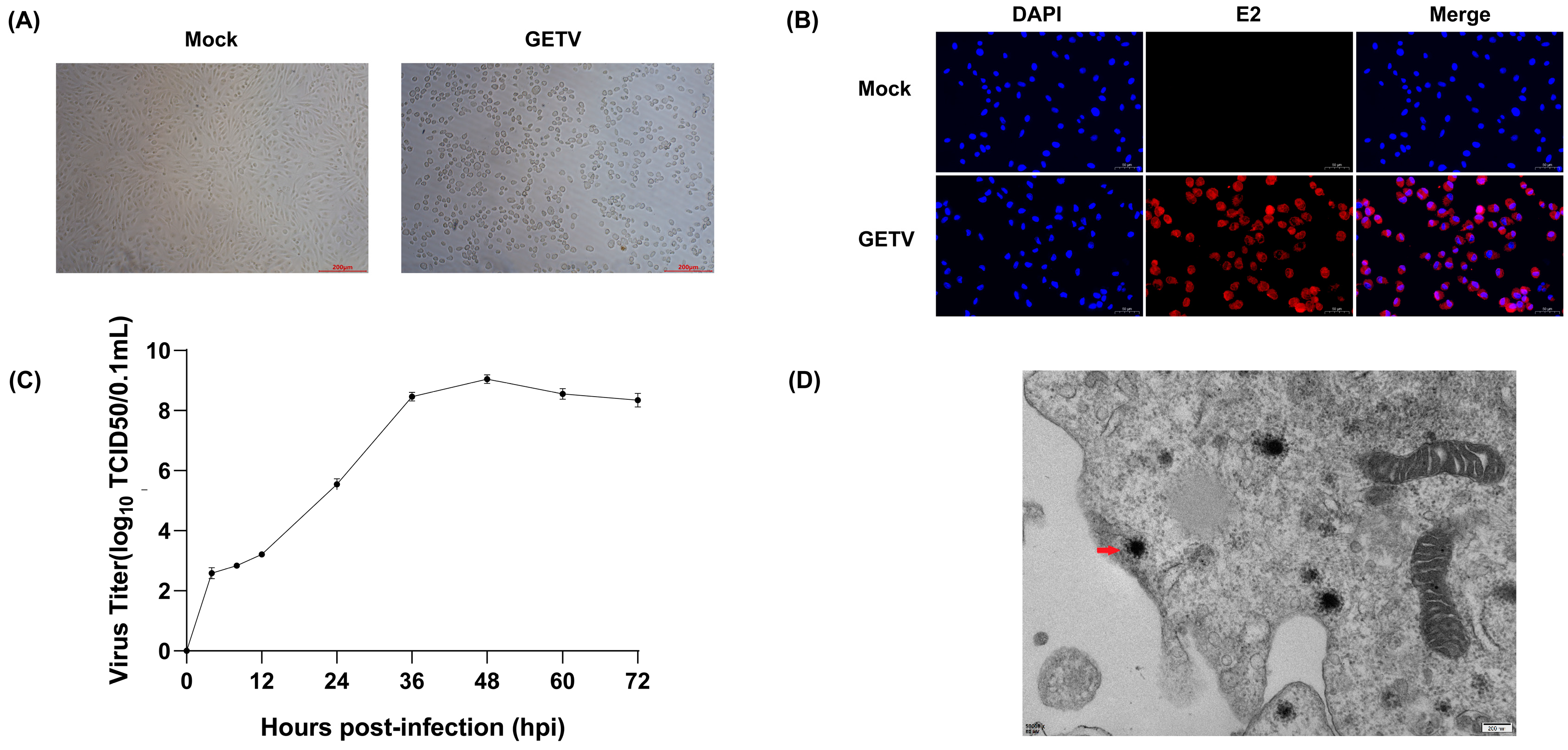
2.2. Viral Growth and Cytopathic Effect Analysis
2.3. Complete GETV SC202009 Sequencing
2.4. Whole-Genome Sequence Splicing and Gene Analysis
3. Results
3.1. Sample Testing and Viral Isolation
3.2. Viral Growth Dynamics and Cytopathic Effects
3.3. The SC202009 Genome
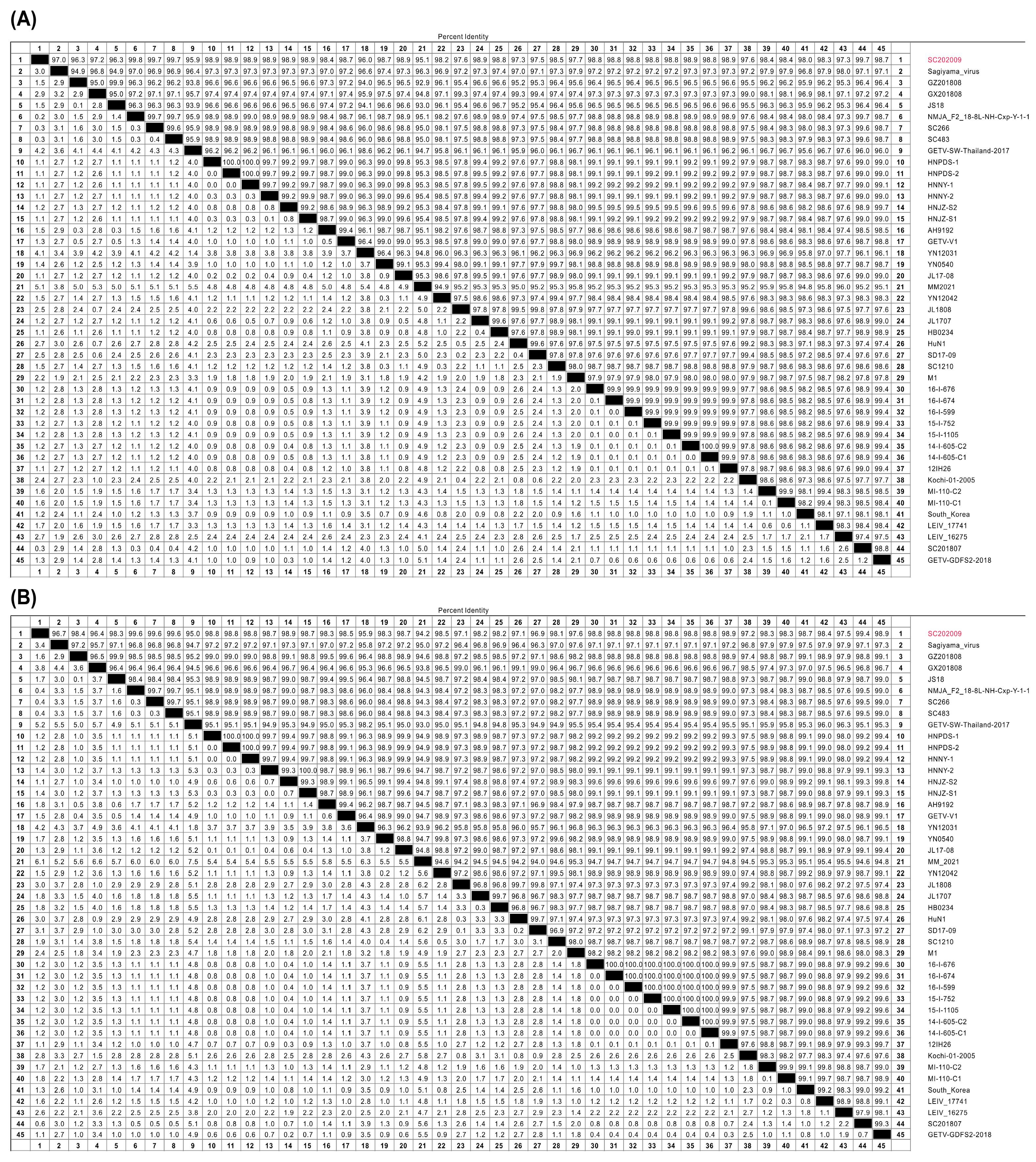
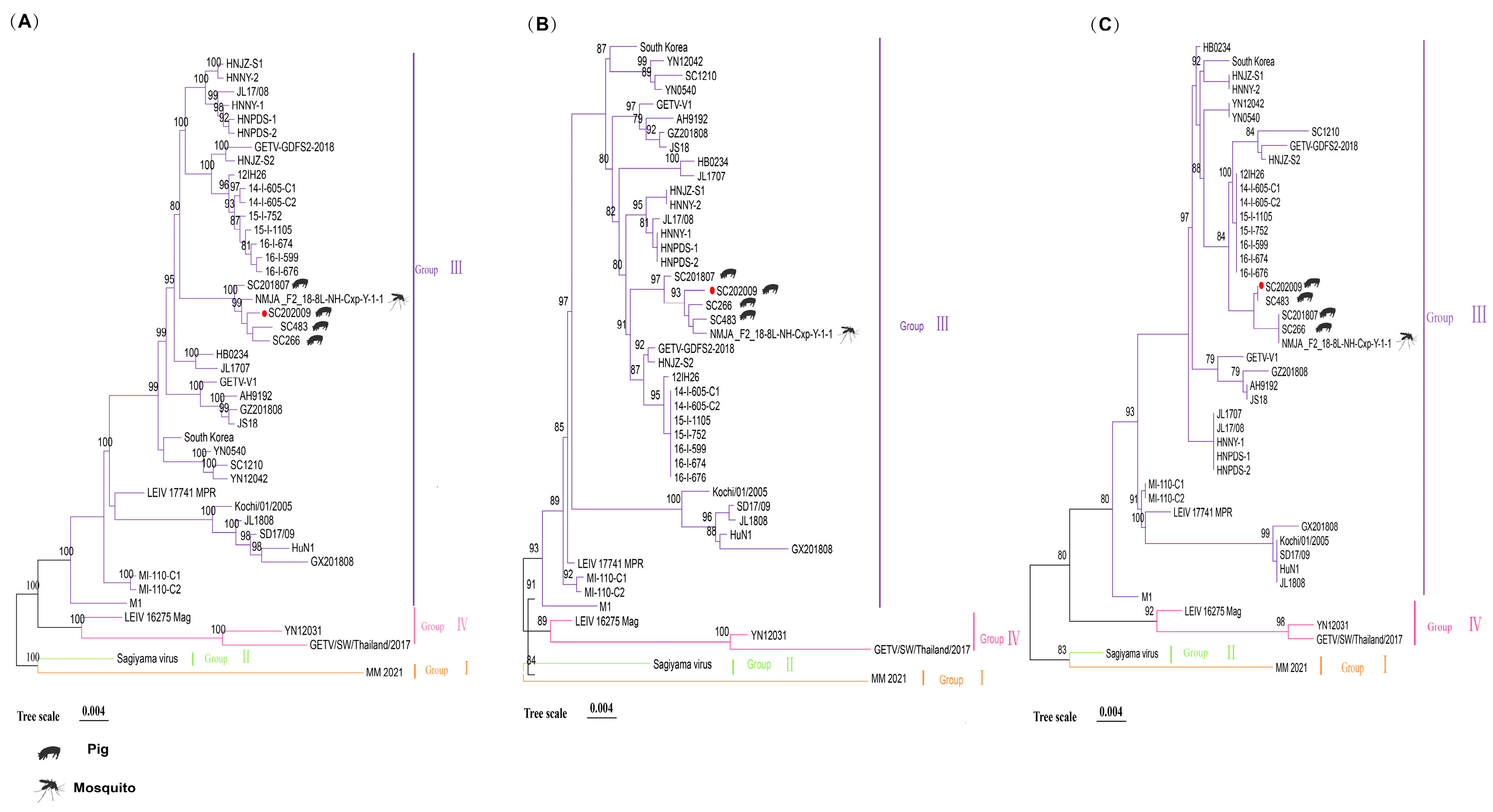
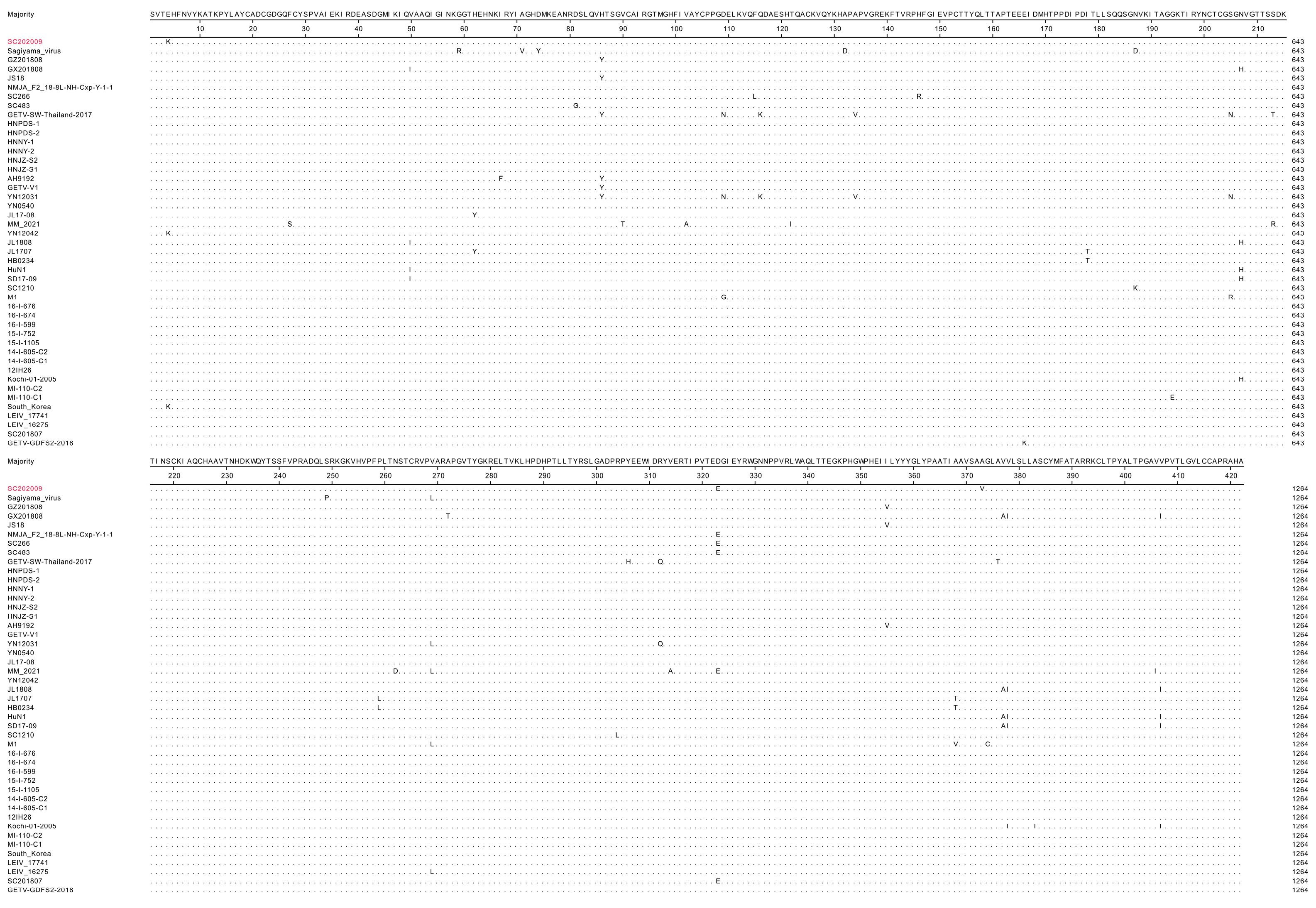
3.4. Gene Sequence Analyses of SC202009
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemoto, M.; Bannai, H.; Tsujimura, K.; Kobayashi, M.; Kikuchi, T.; Yamanaka, T.; Kondo, T. Getah Virus Infection among Racehorses, Japan, 2014. Emerg. Infect. Dis. 2015, 21, 883. [Google Scholar] [CrossRef]
- Sam, S.S.; Mohamed-Romai-Noor, N.A.; Teoh, B.T.; Hamim, Z.R.; Ng, H.Y.; Abd-Jamil, J.; Khor, C.S.; Hassan, S.S.; Ahmad, H.; AbuBakar, S. Group IV getah virus in culex mosquitoes, malaysia. Emerg. Infect. Dis. 2022, 28, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.W.; Mo, Q.R.; Wang, Y.X.; Wang, H.; Nong, Z.R.; Wang, J.L.; Niu, C.X.; Liu, C.; Chen, Y.; Ouyang, K.; et al. Emergence and phylogenetic analysis of a getah virus isolated in Southern China. Front. Vet. Sci. 2020, 7, 552517. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, H.; Fu, S.H.; Li, X.L.; Guo, X.F.; Li, M.H.; Feng, Y.; Chen, W.X.; Wang, L.H.; Lei, W.W. From discovery to spread: The evolution and phylogeny of Getah virus. Infect. Genet. Evol. 2017, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Fu, S.H.; Guo, X.F.; Lei, W.W.; Li, X.L.; Song, J.D.; Cao, L.; Gao, X.Y.; Lyu, Z.; He, Y.; et al. Identification of a newly isolated getah virus in the China-laos border, China. Biomed. Environ. Sci. 2017, 30, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Nan, X.W.; Fan, N.; Fu, S.H.; Si, X.Y.; Zhang, L.; He, Y.; Lei, W.W.; Li, F.; Wang, H.Y.; et al. Emerging of Japanese encephalitis virus and Getah virus from specimen of mosquitoes in Inner Mongolia Autonomous Region. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 571–579. [Google Scholar] [CrossRef]
- Dong, P.; Li, N.; He, Y.W.; Xu, T.G.; Yin, J.Z.; Wang, J.L. Isolation and identification of getah virus from culicoides in Yunnan. Chin. J. Clin. Hepatol. 2017, 31, 405–408. (In Chinese) [Google Scholar]
- Lu, G.; Chen, R.A.; Shao, R.; Dong, N.; Liu, W.Q.; Li, S.J. Getah virus: An increasing threat in China. J. Infect. 2020, 80, 350–371. [Google Scholar] [CrossRef]
- Liu, S.; Hasimu, J.; Xue, X.M.; Ding, M.Y.; Ma, X.Q.; Ye, F.; Ma, J.J.; Yi, X.P.; Gu, W.X.; Zhong, Q. Isolation and identification of Getah virus from Culicoides in Xinjiang. Acta Vet. Et Zootech. Sin. 2017, 48, 1998–2004. (In Chinese) [Google Scholar]
- Kurogi, H.; Inaba, Y.; Goto, Y.; Miura, Y.; Takahashi, H.; Sato, K.; Omori, T.; Matumoto, M. Serologic evidence for etiologic role of Akabane virus in epizootic abortion-arthrogryposis-hydranencephaly in cattle in Japan, 1972–1974. Arch. Virol. 1975, 47, 71–83. [Google Scholar] [CrossRef]
- Yago, K.; Hagiwara, S.; Kawamura, H.; Narita, M. A fatal case in newborn piglets with Getah virus infection: Isolation of the virus. Nihon Juigaku Zasshi 1987, 49, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Izumida, A.; Takuma, H.; Inagaki, S.; Kubota, M.; Hirahara, T.; Kodama, K.; Sasaki, N. Experimental infection of Getah virus in swine. Nihon Juigaku Zasshi 1988, 50, 679–684. [Google Scholar] [CrossRef]
- Brown, C.M.; Timoney, P.J. Getah virus infection of Indian horses. Trop. Anim. Health Prod. 1998, 30, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Guo, X.; Fu, S.; Feng, Y.; Yang, Z.; Wang, H.; He, Y.; Gao, X.; Lyu, Z.; Zhou, H.; et al. Investigation of mosquitoes and arboviruses in the border areas of Yunnan province, 2012. Chin. J. Clin. Hepatol. 2017, 31, 311–314. [Google Scholar] [CrossRef]
- Yang, T.; Li, R.; Hu, Y.; Yang, L.; Zhao, D.; Du, L.; Li, J.; Ge, M.; Yu, X. An outbreak of Getah virus infection among pigs in China, 2017. Transbound. Emerg. Dis. 2018, 65, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ou, J.J.; Ji, J.Z.; Ren, Z.X.; Hu, X.; Wang, C.Y.; Li, S.J. Emergence of getah virus infection in horse with fever in China, 2018. Front. Microbiol. 2019, 10, 1416. [Google Scholar] [CrossRef]
- Shi, N.; Li, L.X.; Lu, R.G.; Yan, X.J.; Liu, H. Highly pathogenic swine getah virus in blue foxes, Eastern China, 2017. Emerg. Infect. Dis. 2019, 25, 1252–1254. [Google Scholar] [CrossRef]
- Li, Y.Y.; Fu, S.H.; Guo, X.F.; Li, X.L.; Li, M.H.; Wang, L.H.; Gao, X.Y.; Lei, W.W.; Cao, L.; Lü, Z.; et al. Serological survey of getah virus in domestic animals in Yunnan province, China. Vector Borne Zoonotic Dis. 2019, 19, 59–61. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.P.; Kuang, Z.P.; Lin, L.M.; Zhang, H.; Yin, L.J.; Hong, J.B.; Ren, B.H.; Li, Q.H.; Wang, L.X. Isolation and pathogenicity of a highly virulent group III porcine Getah virus in China. Front. Cell. Infect. Microbiol. 2024, 14, 1494654. [Google Scholar] [CrossRef]
- Chu, P.P.; Guo, H.Y.; Zhou, X.; Chen, S.N.; Sun, X.R.; Tian, S.C.; Zou, Y.G.; Li, C.L.; Zhang, R.; Zhai, S.L. Emergence of a novel GIII Getah virus variant in pigs in Guangdong, China, 2023. Microbiol. Spectr. 2024, 12, e0048324. [Google Scholar] [CrossRef]
- Lan, J.H.; Fang, M.T.; Duan, L.L.; Liu, Z.; Wang, G.G.; Wu, Q.; Ke, F.; Huang, D.Y.; Ye, Y.; Wan, G.; et al. Novel porcine getah virus from diarrheal piglets in Jiangxi province, China: Prevalence, genome sequence, and pathogenicity. Animals 2024, 14, 2980. [Google Scholar] [CrossRef]
- Mohamed-Romai-Noor, N.; Sam, S.S.; Teoh, B.; Hamim, Z.; AbuBakar, S. Genomic and in vitro phenotypic comparisons of epidemic and non-epidemic getah virus strains. Viruses 2022, 14, 942. [Google Scholar] [CrossRef]
- Shi, N.; Qiu, X.S.; Cao, X.Y.; Mai, Z.H.; Zhu, X.Y.; Li, N.; He, Z.; Zhang, J.Y.; Li, Z.X.; Shaya, N.; et al. Molecular and serological surveillance of Getah virus in the Xinjiang Uygur Autonomous Region, China, 2017–2020. Virol. Sin. 2022, 37, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dellicour, S.; Yan, Z.; Veit, M.; Gill, M.S.; He, W.; Zhai, X.; Ji, X.; Suchard, M.A.; Lemey, P.; et al. Early genomic surveillance and phylogeographic analysis of getah virus, a reemerging arbovirus, in livestock in China. J. Virol. 2023, 97, e109122. [Google Scholar] [CrossRef]
- Feng, Y.; Li, S.G.; Zhang, H.L.; Fu, S.H.; Kang, X.H.; Fu, D.Q.; Guo, C.; Zhang, Y.Z.; Yang, W.H.; Wang, H.Y.; et al. Molecular characterization of Japanese encephalitis virus and Getah virus strains newly isolated in Tengchong County, Yunnan Province, China. Chin. J. Zoonoses 2014, 30, 353–357+363. (In Chinese) [Google Scholar]
- Liang, Y.; Zhang, L.S.; Liu, Y.W.; Dong, Y.Q.; Wang, Z.Q.; Wang, J.W.; Liang, G.D. Investigation on getah virus infecting pigs in She County of Hebei Province. J. Med. Pest Control. 2010, 26, 793–794. (In Chinese) [Google Scholar]
- Wen, J.S.; Zhao, W.Z.; Liu, J.W.; Zhou, H.; Tao, J.P.; Yan, H.Y.; Yu, L.; Zhou, J.J.; Jiang, L.F. Genomic analysis of a Chinese isolate of Getah-like virus and its phylogenetic relationship with other Alphaviruses. Virus Genes 2007, 35, 597–603. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.Y.; Liang, G.D. Getah Virus (Alphavirus): An emerging, spreading Zoonotic virus. Pathogens 2022, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.C.; Deng, H.D.; Zhou, Y.C.; Sun, X.G.; Huang, Y.; Zhu, L.; Xu, Z.W. Development of a reverse transcription recombinase-aided amplification assay for detection of Getah virus. Sci. Rep. 2021, 11, 20060. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, Y.C.; Liu, Z.Y.; Zhang, J.Z.; Wu, F.; You, D.; Ge, L.P.; Liu, Z.H.; Sun, J.; Zeng, X. Prevalence and genetic diversity of porcine epidemic diarrhea virus in Southwest China during 2020–2022. Sci. Rep. 2024, 14, 29124. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of determining fifty percent end points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Jian, Z.J.; Jiang, C.Y.; Zhu, L.; Li, F.Q.; Deng, L.S.; Ai, Y.R.; Lai, S.Y.; Xu, Z.W. Infectivity and pathogenesis characterization of getah virus (GETV) strain via different inoculation routes in mice. Heliyon 2024, 10, e33432. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhang, H.Q.; Chen, X.Q.; Zeng, M.Y.; Han, H.; Guo, Y.J.; Li, J.M.; Luo, S.C.; Yan, G.Z.; Chen, S.N.; et al. Evolutionary characterization and pathogenicity of Getah virus from pigs in Guangdong Province of China. Arch. Virol. 2023, 168, 258. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Zhu, X.Y.; Qiu, X.S.; Cao, X.Y.; Jiang, Z.Y.; Lu, H.J.; Jin, N.Y. Origin, genetic diversity, adaptive evolution and transmission dynamics of Getah virus. Transbound. Emerg. Dis. 2022, 69, e1037–e1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Zhai, X.F.; Li, X.L.; Wang, Y.; He, W.T.; Jiang, Z.W.; Veit, M.; Su, S. Attenuation of getah virus by a single amino acid substitution at residue 253 of the E2 protein that might be part of a new heparan sulfate binding site on alphaviruses. J. Virol. 2022, 96, e0175121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Nie, M.; Liu, B.; Li, F.; Xu, T.; Xu, L.; Deng, L.; Li, H.; Zhao, L.; Li, Y.; et al. Isolation and Characterization of a Porcine Getah Virus Strain from Sichuan Province. Vet. Sci. 2025, 12, 276. https://doi.org/10.3390/vetsci12030276
Shao L, Nie M, Liu B, Li F, Xu T, Xu L, Deng L, Li H, Zhao L, Li Y, et al. Isolation and Characterization of a Porcine Getah Virus Strain from Sichuan Province. Veterinary Sciences. 2025; 12(3):276. https://doi.org/10.3390/vetsci12030276
Chicago/Turabian StyleShao, Lina, Mincai Nie, Baoling Liu, Fengqin Li, Tong Xu, Lei Xu, Lishuang Deng, Hanyu Li, Lei Zhao, Youyou Li, and et al. 2025. "Isolation and Characterization of a Porcine Getah Virus Strain from Sichuan Province" Veterinary Sciences 12, no. 3: 276. https://doi.org/10.3390/vetsci12030276
APA StyleShao, L., Nie, M., Liu, B., Li, F., Xu, T., Xu, L., Deng, L., Li, H., Zhao, L., Li, Y., Zhang, L., Yan, Y., Xu, Z., & Zhu, L. (2025). Isolation and Characterization of a Porcine Getah Virus Strain from Sichuan Province. Veterinary Sciences, 12(3), 276. https://doi.org/10.3390/vetsci12030276




