The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Antigen and Adjuvant
2.2. Location and Study Cohorts
2.3. Clinical and Laboratory Safety Assessment
2.4. Investigation of Immune Responses
2.5. Investigation of Cell-Mediated Immunity
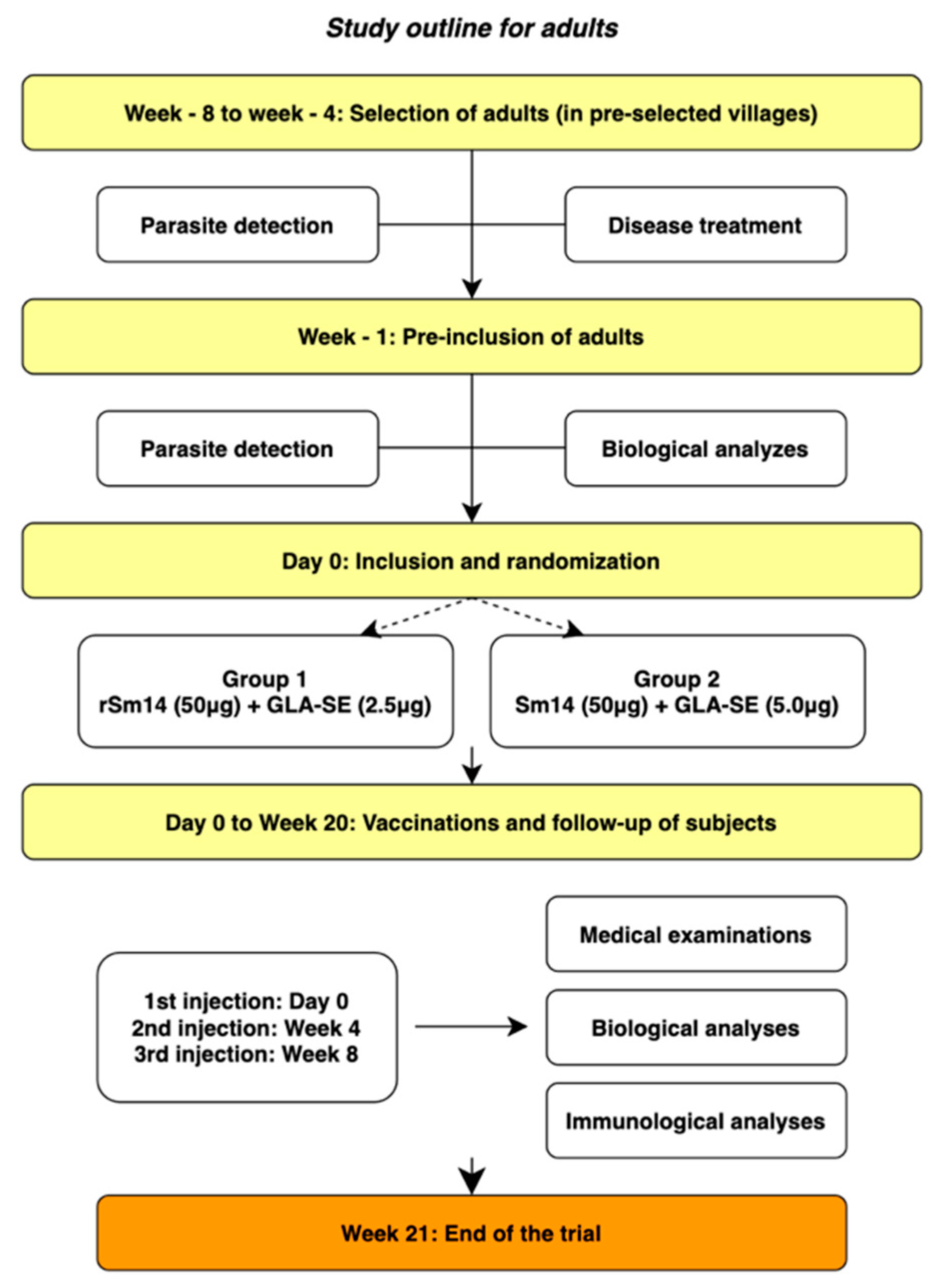
2.6. Vaccination of Adults—Phase IIa
2.6.1. Vaccination of Adults—Extended Phase IIa
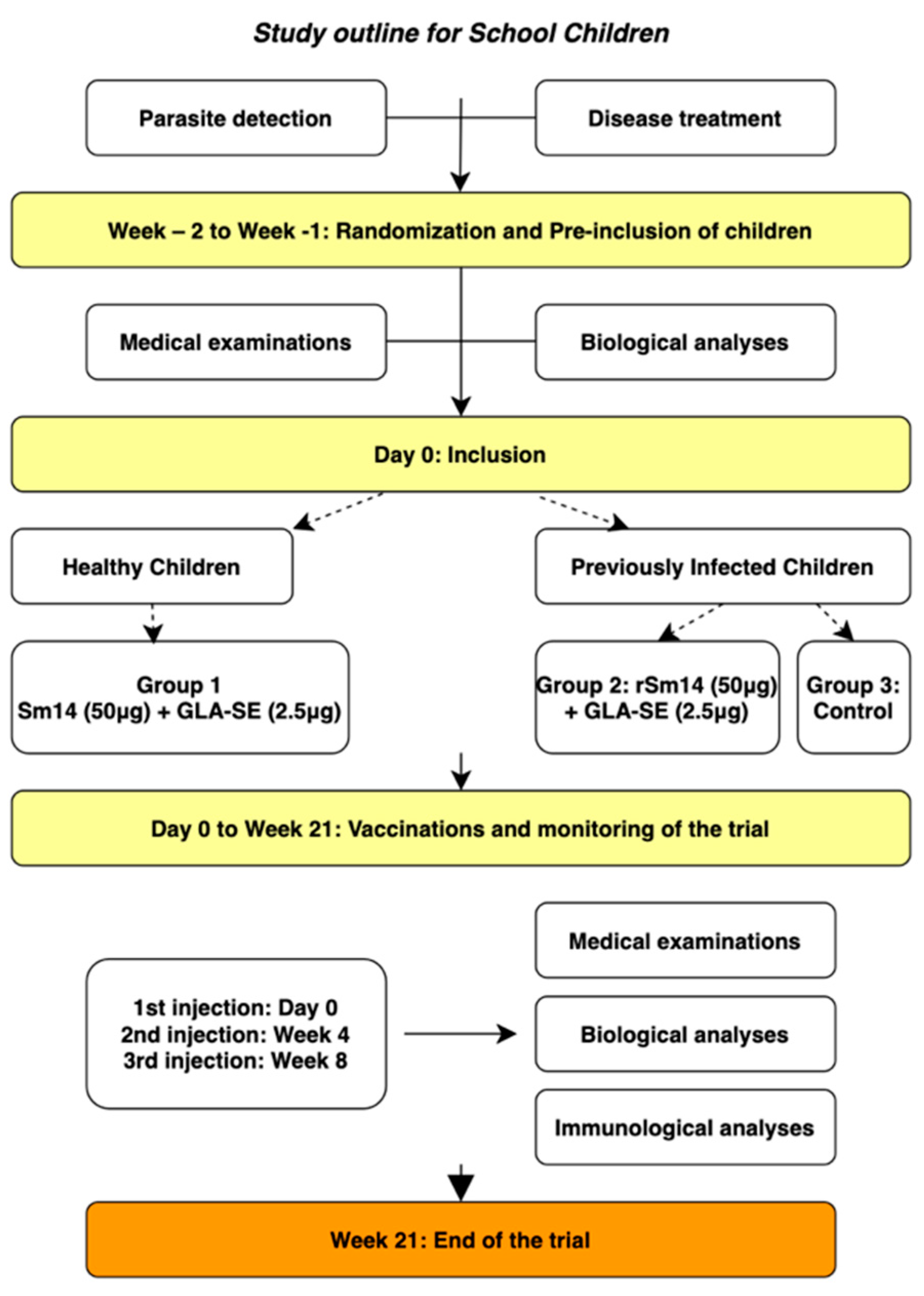
2.6.2. Vaccination of Children—Phase IIb
2.6.3. Safety-Related Measures
3. Results
3.1. Adult Trial (Phase IIa)
3.1.1. Safety Outcome
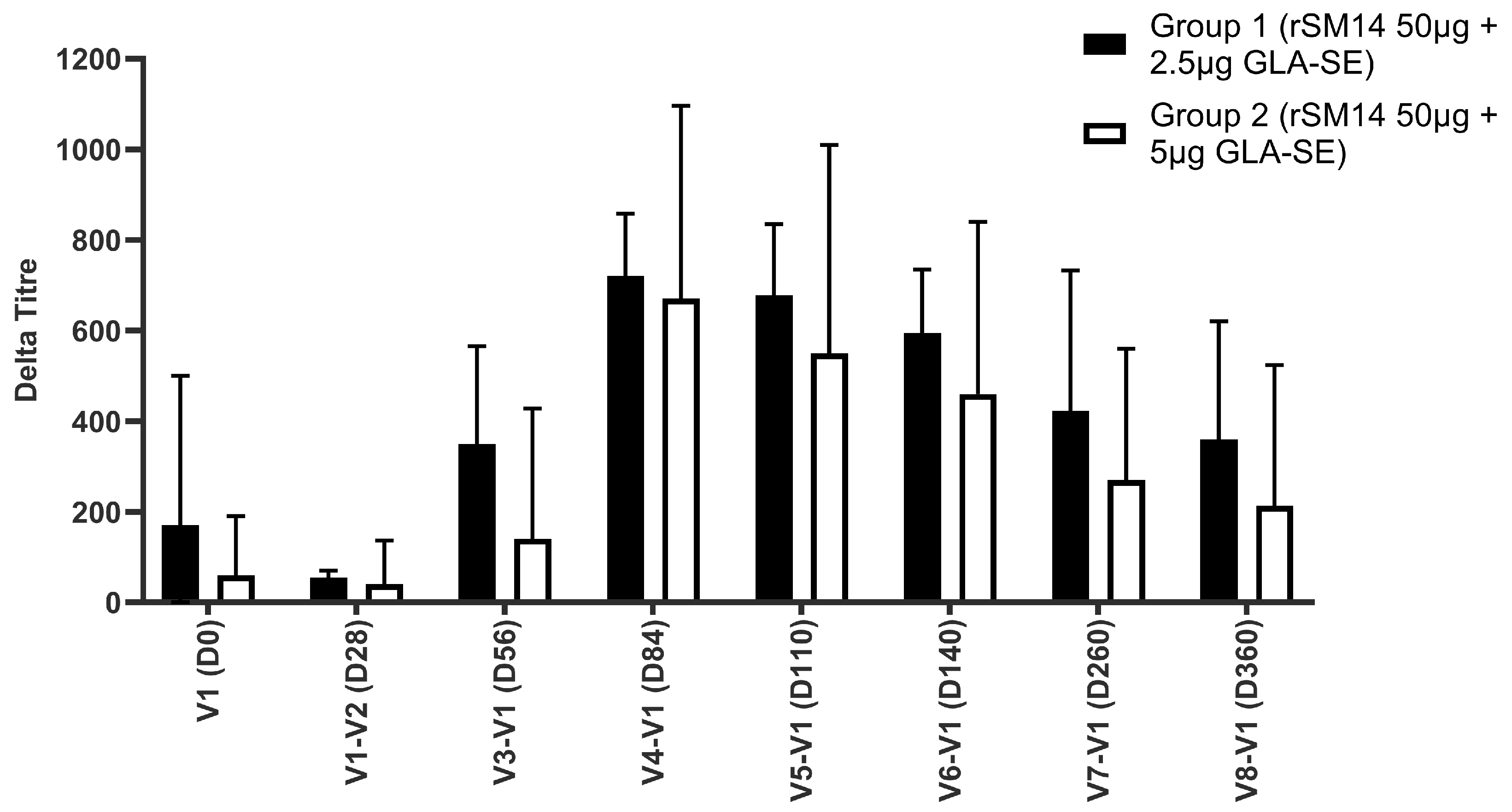
3.1.2. Humoral Immunity
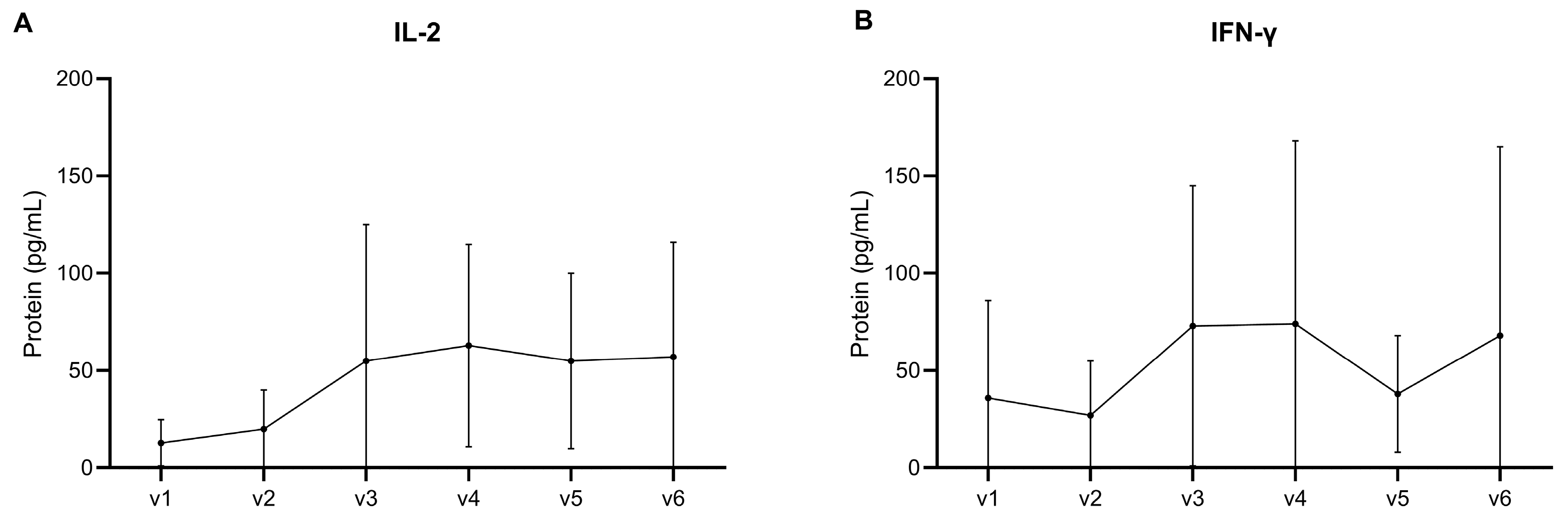
3.1.3. Cell-Mediated Immunity
3.2. Phase IIb
3.2.1. Safety Outcome
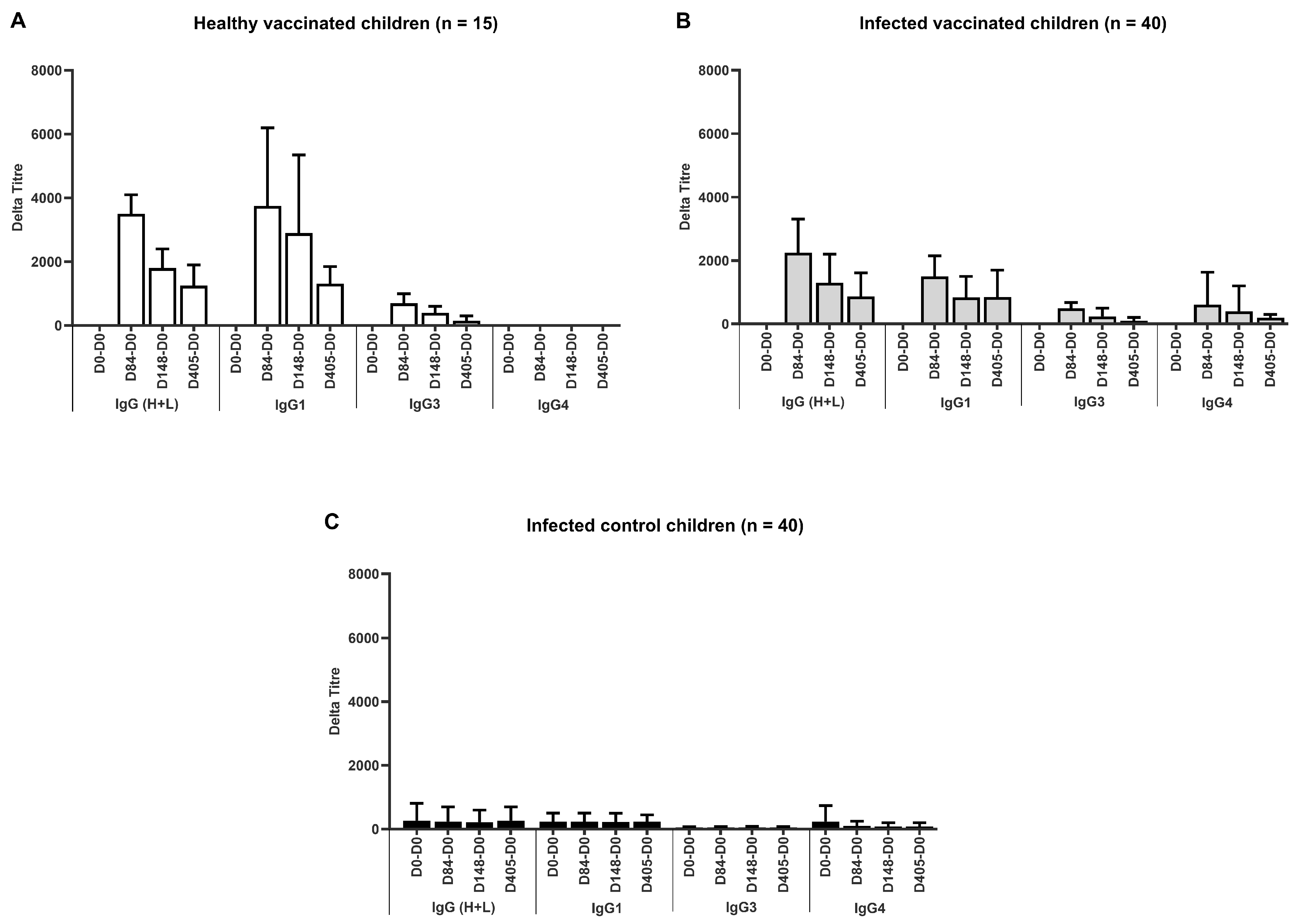
3.2.2. Humoral Immunity
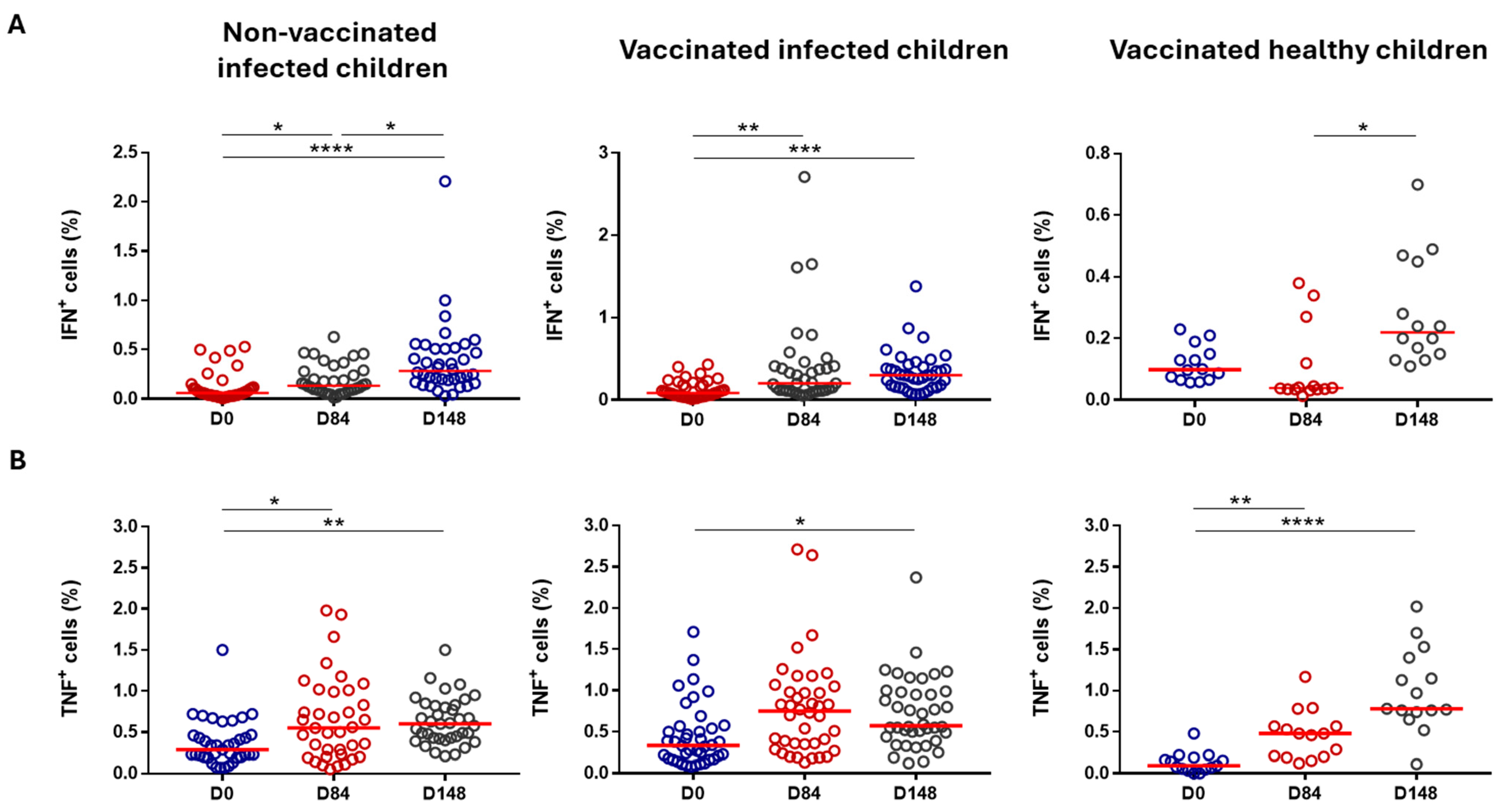
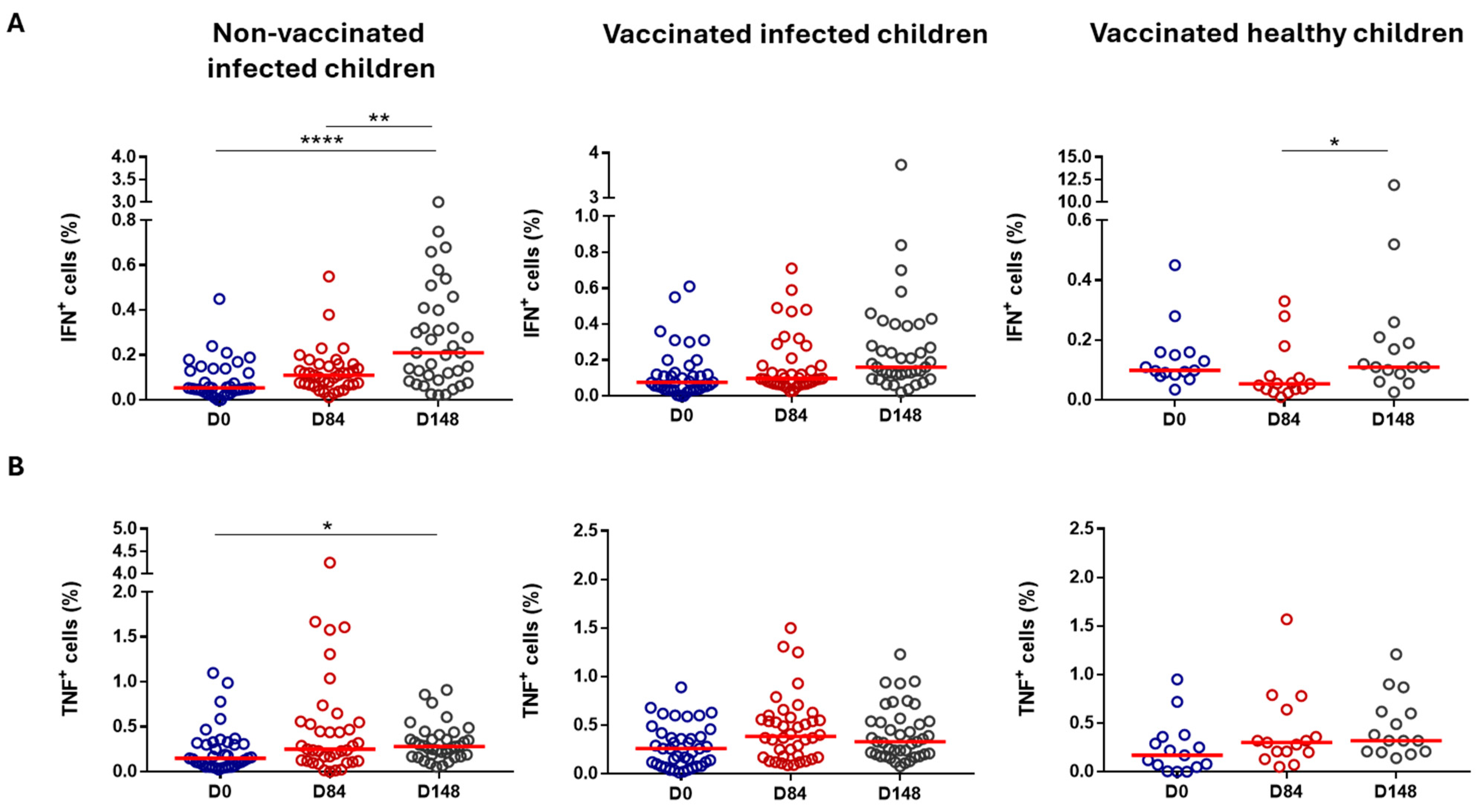
3.2.3. Cell-Mediated Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004160-8. [Google Scholar]
- Wood, C.L.; Sokolow, S.H.; Jones, I.J.; Chamberlin, A.J.; Lafferty, K.D.; Kuris, A.M.; Jocque, M.; Hopkins, S.; Adams, G.; Buck, J.C.; et al. Precision Mapping of Snail Habitat Provides a Powerful Indicator of Human Schistosomiasis Transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 23182–23191. [Google Scholar] [CrossRef]
- Crompton, D.W.T.; World Health Organization. WHO Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; Preventive Chemotherapy: Geneva, Switzerland, 2006. [Google Scholar]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From Immunopathology to Vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Kura, K.; Truscott, J.E.; Toor, J.; Anderson, R.M. Modelling the Impact of a Schistosoma mansoni Vaccine and Mass Drug Administration to Achieve Morbidity Control and Transmission Elimination. PLoS Neglected Trop. Dis. 2019, 13, e0007349. [Google Scholar] [CrossRef] [PubMed]
- Tendler, M.; Almeida, M.S.; Pinto, R.M.; Noronha, D.; Katz, N. Schistosoma mansoni-New Zealand Rabbit Model: Resistance Induced by Infection Followed by Active Immunization with Protective Antigens. J. Parasitol. 1991, 77, 138–141. [Google Scholar] [CrossRef]
- Tendler, M.; Pinto, R.M.; Lima, A.d.O.; Savino, W.; Katz, N. Vaccination in Murine Schistosomiasis with Adult Worm-Derived Antigens: Variables Influencing Protection in Outbred Mice. Int. J. Parasitol. 1991, 21, 299–306. [Google Scholar] [CrossRef]
- Tendler, M.; Brito, C.A.; Vilar, M.M.; Serra-Freire, N.; Diogo, C.M.; Almeida, M.S.; Delbem, A.C.; Da Silva, J.F.; Savino, W.; Garratt, R.C.; et al. A Schistosoma mansoni Fatty Acid-Binding Protein, Sm14, Is the Potential Basis of a Dual-Purpose Anti-Helminth Vaccine. Proc. Natl. Acad. Sci. USA 1996, 93, 269–273. [Google Scholar] [CrossRef]
- Ramos, C.R.; Vilar, M.M.; Nascimento, A.L.; Ho, P.L.; Thaumaturgo, N.; Edelenyi, R.; Almeida, M.; Dias, W.O.; Diogo, C.M.; Tendler, M. R-Sm14-pRSETA Efficacy in Experimental Animals. Mem. Inst. Oswaldo Cruz 2001, 96, 131–135. [Google Scholar] [CrossRef]
- Ramos, C.R.R.; Figueredo, R.C.R.; Pertinhez, T.A.; Vilar, M.M.; do Nascimento, A.L.T.O.; Tendler, M.; Raw, I.; Spisni, A.; Ho, P.L. Gene Structure and M20T Polymorphism of the Schistosoma mansoni Sm14 Fatty Acid-Binding Protein. Molecular, Functioanl, and Immunoprotection Analysis. J. Biol. Chem. 2003, 278, 12745–12751. [Google Scholar] [CrossRef]
- Ramos, C.R.R.; Spisni, A.; Oyama, S.; Sforça, M.L.; Ramos, H.R.; Vilar, M.M.; Alves, A.C.; Figueredo, R.C.R.; Tendler, M.; Zanchin, N.I.T.; et al. Stability Improvement of the Fatty Acid Binding Protein Sm14 from S. Mansoni by Cys Replacement: Structural and Functional Characterization of a Vaccine Candidate. Biochim. Biophys. Acta 2009, 1794, 655–662. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Coler, R.N.; Parra, J.; Veloso, V.; Jayashankar, L.; Pinto, P.M.; Ciol, M.A.; Bergquist, R.; Reed, S.G.; Tendler, M. Schistosomiasis Vaccine Candidate Sm14/GLA-SE: Phase 1 Safety and Immunogenicity Clinical Trial in Healthy, Male Adults. Vaccine 2016, 34, 586–594. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Machado Pinto, P.; dos Santos, T.; Vilar, M.M.; Grinsztejn, B.; Veloso, V.; Paes-de-Almeida, E.C.; Amaral, M.A.Z.; Ramos, C.R.; Marroquin-Quelopana, M.; et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines 2022, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Fundação Oswaldo Cruz. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 in Combination with the Adjuvant Glucopyranosyl Lipid A (GLA-SE) in Adults Living in Endemic Regions for S. mansoni and S. haematobium in Senegal. A Comparative, Randomized, Open-Label Trial. 2016; Recorded by the Senegalese Ethics Committee (CNERS) Under the Reference: SEN 16/26; and Its Extension Under the Reference: SEN 17/22. Available online: https://clinicaltrials.gov/study/NCT03041766 (accessed on 30 October 2024).
- Fundação Oswaldo Cruz. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 Against Schistosomiasis in Senegalese School Children Healthy or Infected with S. mansoni and/or S. haematobium. A Comparative, Randomized, Controlled, Open-Label Trial. 2019; Recorded by the Senegalese Ethics Committee Under the Reference: SEN 18/26. Available online: https://clinicaltrials.gov/study/NCT03799510 (accessed on 30 October 2024).
- International Centers for Tropical Disease Research Network. ICTDR Investigator Manual: Monitoring and Reporting Adverse Events; NIAID: Bethesda, MD, USA, 2003.
- Food and Drug Administration. FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; Center for Biologics Evaluation and Research: Rockville, MD, USA, 2007.
- Borkowf, C.B. Constructing Binomial Confidence Intervals with near Nominal Coverage by Adding a Single Imaginary Failure or Success. Stat. Med. 2006, 25, 3679–3695. [Google Scholar] [CrossRef] [PubMed]
- Queto, T.; Vasconcelos, Z.F.M.; Luz, R.A.; Anselmo, C.; Guiné, A.A.A.; e Silva, P.M.R.; Farache, J.; Cunha, J.M.T.; Bonomo, A.C.; Gaspar-Elsas, M.I.C.; et al. G-CSF Suppresses Allergic Pulmonary Inflammation, Downmodulating Cytokine, Chemokine and Eosinophil Production. Life Sci. 2011, 88, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Silva-Freitas, M.L.; Corrêa-Castro, G.; Cota, G.F.; Giacoia-Gripp, C.; Rabello, A.; Teixeira Dutra, J.; de Vasconcelos, Z.F.M.; Savino, W.; Da-Cruz, A.M.; Santos-Oliveira, J.R. Impaired Thymic Output Can Be Related to the Low Immune Reconstitution and T Cell Repertoire Disturbances in Relapsing Visceral Leishmaniasis Associated HIV/AIDS Patients. Front. Immunol. 2020, 11, 953. [Google Scholar] [CrossRef]
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Siegel, S. Estatística não-paramétrica: Para as ciências do comportamento. In Estatística Não-Paramétrica: Para as Ciências do Comportamento; Mc Graw-Hill: São Paulo, Brazil, 1975; p. 350. [Google Scholar]
- Katz, N.; Chaves, A.; Pellegrino, J. A Simple Device for Quantitative Stool Thick-Smear Technique in Schistosomiasis Mansoni. Rev. Inst. Med. Trop. São Paulo 1972, 14, 397–400. [Google Scholar]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From Mouse to Man: Safety, Immunogenicity and Efficacy of a Candidate Leishmaniasis Vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef]
- Windish, H.P.; Duthie, M.S.; Misquith, A.; Ireton, G.; Lucas, E.; Laurance, J.D.; Bailor, R.H.; Coler, R.N.; Reed, S.G. Protection of Mice from Mycobacterium Tuberculosis by ID87/GLA-SE, a Novel Tuberculosis Subunit Vaccine Candidate. Vaccine 2011, 29, 7842–7848. [Google Scholar] [CrossRef]
- Duthie, M.S.; Coler, R.N.; Laurance, J.D.; Sampaio, L.H.; Oliveira, R.M.; Sousa, A.L.M.; Stefani, M.M.A.; Maeda, Y.; Matsuoka, M.; Makino, M.; et al. Protection against Mycobacterium Leprae Infection by the ID83/GLA-SE and ID93/GLA-SE Vaccines Developed for Tuberculosis. Infect. Immun. 2014, 82, 3979–3985. [Google Scholar] [CrossRef]
- Reed, S.G.; Carter, D.; Casper, C.; Duthie, M.S.; Fox, C.B. Correlates of GLA Family Adjuvants’ Activities. Semin. Immunol. 2018, 39, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.-A. 2—Vaccine Immunology. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 16–34.e7. ISBN 978-0-323-35761-6. [Google Scholar]
- Yamey, G.; McDade, K.K.; Anderson, R.M.; Bartsch, S.M.; Bottazzi, M.E.; Diemert, D.; Hotez, P.J.; Lee, B.Y.; McManus, D.; Molehin, A.J.; et al. Vaccine Value Profile for Schistosomiasis. Vaccine 2024, 126020. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, N.R.; Leonardo, L.R.; Mitchell, G.F. Vaccine-Linked Chemotherapy: Can Schistosomiasis Control Benefit from an Integrated Approach? Trends Parasitol. 2005, 21, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D.C.; Maayan, N.; Donegan, S.; Chaplin, M.; Garner, P. Public Health Deworming Programmes for Soil-transmitted Helminths in Children Living in Endemic Areas. Cochrane Database Syst. Rev. 2019, 2019, CD000371. [Google Scholar] [CrossRef]
- World Health Organization. Global Vaccine Action Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-150498-0. [Google Scholar]
| Trial Characteristic | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | |
|---|---|---|---|---|
| Sample size | 15 | 40 | 40 | |
| Age | ||||
| Years | Mean (SD) | 9.3 (1.2) | 9.4 (1.1) | 9.2 (1.1) |
| Median | (min, max) | 9 (8, 11) | 9.5 (8, 11) | 9.0 (8, 11) |
| Sex % | males (n) | 40.0 (6) | 50.0 (20) | 42.5 (17) |
| Infection type | ||||
| S. haematobium only | % (n) | 0 (0) | 50.0 (20) | 62.5 (25) |
| S. mansoni only | % (n) | 0 (0) | 35.0 (14) | 27.5 (11) |
| Mixed infection | % (n) | 0 (0) | 15.0 (6) | 10.0 (4) |
| Healthy group: first vaccine injection (D0) | |||||||
|---|---|---|---|---|---|---|---|
| Week 1 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | 1 | 2 | 3 | ||||
| Group 2 (Previously infected children + rSm14) | |||||||
| Group 3 (Unvaccinated, previously infected children) | |||||||
| Inclusion | Week 1 = 6 subjects | ||||||
| Vaccination | Week 1 = 6 subjects | ||||||
| Week 2 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | 3 | 3 | 3 | ||||
| Group 2 (Previously infected children + rSm14) | |||||||
| Group 3 (Unvaccinated, previously infected children) | |||||||
| Inclusion | Week 2 = 9 subjects | ||||||
| Vaccination | Week 2 = 9 subjects | ||||||
| Infected group: first vaccine injection (D0) | |||||||
| Week 3 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 1 | 2 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 1 | 2 | 3 | ||||
| Inclusions | Week 3 = 12 subjects | ||||||
| Vaccination | Week 3 = 6 subjects | ||||||
| Week 4 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 4 = 18 subjects | ||||||
| Vaccination | Week 4 = 9 subjects | ||||||
| Week 5 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 5 = 18 subjects | ||||||
| Vaccination | Week 5 = 9 subjects | ||||||
| Week 6 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 6 = 18 subjects | ||||||
| Vaccination | Week 6 = 9 subjects | ||||||
| Week 7 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 1 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 1 | ||||
| Inclusion | Week 7 = 14 subjects | ||||||
| Vaccination | Week 7 = 7 subjects | ||||||
| Adverse Events | GLA-SE Content | AE After First Injection * (95% CI) | AE After Second Injection ** (95% CI) | AE After Third Injection *** (95% CI) |
|---|---|---|---|---|
| Serious | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | |
| Pain at site of injection | 2.5 μg | 0.13 (0.02, 0.40) | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.20 (0.04, 0.48) | 0.20 (0.04, 0.48) | 0.13 (0.02, 0.40) | |
| Heavy arm post injection | 2.5 μg | 0.00 (0.00, 0.22) | 0.20 (0.04, 0.48) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.07 (0.002, 0.32) | 0.27 (0.08, 0.55) | 0.20 (0.04, 0.48) | |
| Pruritus | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.13 (0.02, 0.40) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | |
| Dizziness and/or headache | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) | 0.00 (0.00, 0.22) | |
| Acute gastroenteritis | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| Adverse Event (AE) | Group | No. a | AE After First Injection b Event (95% CI) | No. a | AE After Second Injection c Event (95% CI) | No. a | AE After Third Injection d Event (95% CI) |
|---|---|---|---|---|---|---|---|
| Serious (Grades 3–4) | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | |
| Pain at site of infection | Healthy | 0/15 | 0.00 (0.00, 0.22) | 2/15 | 0.13 (0.02, 0.40) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 2/40 | 0.05 (0.01, 0.17) | 3/40 | 0.08 (0.02, 0.20) | 3/40 | 0.08 (0.02, 0.20) | |
| Heavy arm post inject. | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Pruritus | Healthy | 1/15 | 0.00 (0.00, 0.22) | 1/15 | 0.00 (0.00, 0.22) | 1/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Swelling | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Fever | Healthy | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | 1/40 | 0.02 (0.001, 0.13) | |
| Headache | Healthy | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 2/40 | 0.05 (0.01, 0.17) | 0/40 | 0.00 (0.00, 0.09) | |
| Abdominal pain | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | |
| Vomiting | Healthy | 0/15 | 0.00 (0.00, 0.22) | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) |
| Blood Collection (Day) | Group | ||||
|---|---|---|---|---|---|
| IgG Response | Healthy, Vaccinated a Group 1 | Previously Infected, Vaccinated b Group 2 | Previously Infected, Non-Vaccinated c Group 3 | p-Value d | |
| 0 | Mean (SD) | 56 (176) | 321 (627) | 324 (751) | |
| Median (min, max) | 0 (0, 688) | 2 (0, 2306) | 0 (0, 3370) | ||
| Responder % (n) | 6.7% (1) | 25.0% (10) | 20.0% (8) | 0.40 | |
| 84 | Mean (SD) | 3533 (1778) | 2555 (1672) | 262 (591) | |
| Median (min, max) | 3118 (1763, 9180) | 2211 (22, 7326) | 0 (0, 2332) | ||
| Responder % (n) | 100 (15) | 92.5 (37) | 20.0 (8) | <0.001 | |
| 148 | Mean (SD) | 2078 (1210) | 1626 (1177) | 231 (536) | |
| Median (min, max) | 1620 (920, 5624) | 1605 (1, 4998) | 0 (0, 2072) | ||
| Responder % (n) | 100 (15) | 90.0 (36) | 20.0 (8) | <0.001 | |
| 400+ e | Mean (SD) | 1322 (973) | 1205 (1014) | 317 (604) | |
| Median (min, max) | 1203 (61, 3476) | 1110 (0, 3336) | 1 (0, 2454) | ||
| Responder % (n) | 92.9 (13) | 73.7 (28) | 33.3 (13) | <0.001 | |
| Cell Population | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | |||
|---|---|---|---|---|---|---|
| T cells (CD3+) | Not significant | ↓ Day 84 (%) | p = 0.0036 | ↑ Day 148 (%) | p = 0.0349 | |
| CD4+ T cells | Not significant | Not significant | Not significant | |||
| CD8+ T cells | ↓ Day 84 (AN) | p = 0.0105 | Not significant | Not significant | ||
| B cells | ↑ Day 84 (%) | p = 0.0105 | ↓ Day 84 (%) | p = 0.0003 | ↓ Day 148 (%) | p = 0.0017 |
| Monocytes | Not significant | ↑ Day 84/Day 148 (%/AN) | p = 0.0029/0.0063 p = 0.0110/0.0417 | ↑ Day 148 (%) | p = 0.0250 | |
| CD4 T-Cell Subpopulation | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | ||
|---|---|---|---|---|---|
| Naïve T cells | ↑ Day 84 | p = 0.0016 | Not significant | ↑ Day 148 | p = 0.0250 |
| ↑ Day 148 | p < 0.0001 | ||||
| Effector T cells | ↓ Day 84 | p = 0.0030 | Not significant | Not significant | |
| ↓ Day 148 | p = 0.0030 | ||||
| EMT cells | ↓ Day 84 | p < 0.0001 | Not significant | Not significant | |
| ↓ Day 148 | p = 0.0105 | ||||
| CMT cells | Not significant | Not significant | Not significant | ||
| CD8 T-Cell Subpopulation | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | ||
|---|---|---|---|---|---|
| Naïve T cells | ↑ Day 84 | p = 0.0001 | Not significant | Not significant | |
| ↑ Day 148 | p = 0.0411 | ||||
| Effector T cells | ↓ Day 148 | p = 0.0185 | Not significant | Not significant | |
| EMT cells | ↓ Day 84 | p = 0.0008 | ↓ Day 84 | p = 0.0052 | Not significant |
| CMT cells | Not significant | ↓ Day 84 | p = 0.0024 | Not significant | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, A.T.; Diop, D.; Diop, M.; Schacht, A.-M.; Mbengue, A.; Diagne, R.; Guisse, M.; Dompnier, J.-P.; Messias, C.; Coler, R.N.; et al. The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines 2025, 13, 316. https://doi.org/10.3390/vaccines13030316
Ly AT, Diop D, Diop M, Schacht A-M, Mbengue A, Diagne R, Guisse M, Dompnier J-P, Messias C, Coler RN, et al. The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines. 2025; 13(3):316. https://doi.org/10.3390/vaccines13030316
Chicago/Turabian StyleLy, Amadou Tidjani, Doudou Diop, Modou Diop, Anne-Marie Schacht, Abdoulaye Mbengue, Rokhaya Diagne, Marieme Guisse, Jean-Pierre Dompnier, Carolina Messias, Rhea N. Coler, and et al. 2025. "The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa" Vaccines 13, no. 3: 316. https://doi.org/10.3390/vaccines13030316
APA StyleLy, A. T., Diop, D., Diop, M., Schacht, A.-M., Mbengue, A., Diagne, R., Guisse, M., Dompnier, J.-P., Messias, C., Coler, R. N., Ramos, C. R., Tendeng, J.-N., Ndiaye, S., Marroquin-Quelopana, M., de Carvalho Parra, J., dos Santos, T., Sirianni dos Santos Almeida, M., Mendes-da-Cruz, D. A., Reed, S., ... Tendler, M. (2025). The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines, 13(3), 316. https://doi.org/10.3390/vaccines13030316






