What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions
Abstract
:1. Introduction
2. Organ-Specific Diseases Associated with the COVID-19 Vaccines
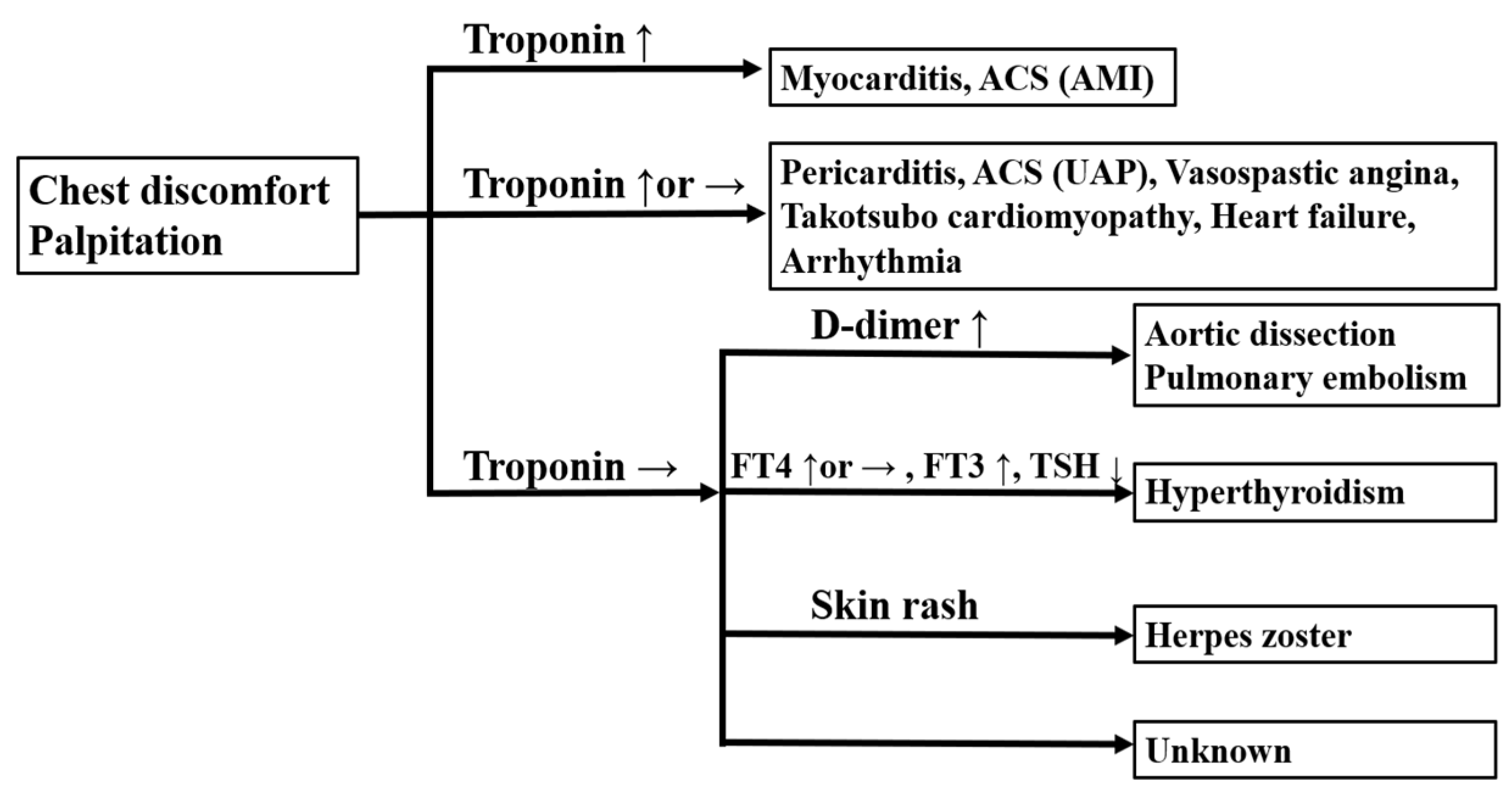
2.1. Cardiovascular Diseases
2.2. Respiratory Diseases
2.3. Gastroenterological Diseases
2.4. Renal Diseases
2.5. Neurological Diseases
2.6. Skin Diseases
2.7. Endocrine Diseases
2.8. Collagen Diseases
2.9. Hematologic Diseases
2.10. Others
3. Plausible Causes of Post-Vaccine Adverse Reactions
3.1. Inflammatory Cytokines
3.2. Autoimmunity
3.3. Eosinophilia
3.4. ACE2 Downregulation
4. Precautionary Measures Including Exercise, Alcohol Intake, Tobacco Smoking, and Baths
4.1. Avoid Strenuous Exercise
4.2. Avoid Consuming Alcohol and Smoking
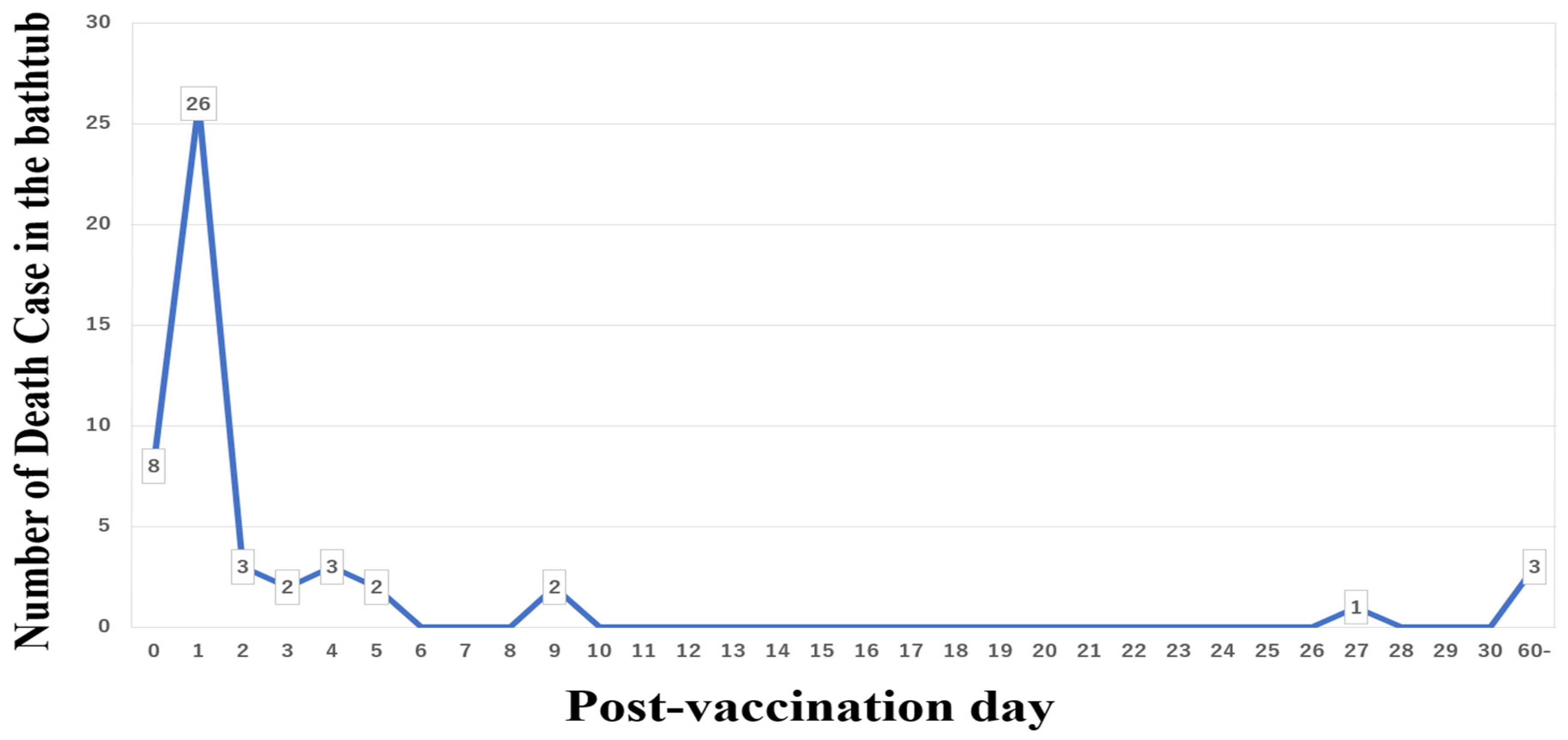
4.3. Take a Shower Instead of Sitting in a Hot Bath
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noor, R.; Shareen, S.; Billah, M. COVID-19 vaccines: Their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants. Bull. Natl. Res. Cent. 2022, 46, 96. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Kim, M.; Kwon, S.; Joo, J.; Kwak, S.H.; Kim, E.O.; Jung, J.; et al. Adverse Reactions of the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers in Korea. J. Korean Med. Sci. 2021, 36, e153. [Google Scholar] [CrossRef]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Yeo, A.; Kuek, B.; Lau, M.; Tan, S.R.; Chan, S. Post COVID-19 vaccine deaths—Singapore’s early experience. Forensic Sci. Int. 2022, 332, 111199. [Google Scholar] [CrossRef]
- Liu, T.; Liang, Y.; Huang, L. Development and Delivery Systems of mRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [Green Version]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyarto, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-gamma, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Wu, H.H.L.; PKalra, A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Vuille-Lessard, E.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021, 123, 102710. [Google Scholar] [CrossRef]
- Yamamoto, K.; Mashiba, T.; Takano, K.; Suzuki, T.; Kami, M.; Takita, M.; Kusumi, E.; Mizuno, Y.; Hamaki, T. A Case of Exacerbation of Subclinical Hyperthyroidism after First Administration of BNT162b2 mRNA COVID-19 Vaccine. Vaccines 2021, 9, 1108. [Google Scholar] [CrossRef]
- Patrizio, A.; Ferrari, S.M.; Antonelli, A.; Fallahi, P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J. Autoimmun. 2021, 125, 102738. [Google Scholar] [CrossRef]
- Chen, S.; XFan, R.; He, S.; Zhang, J.W.; Li, S.J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. 2021, 42, 3537–3539. [Google Scholar] [CrossRef]
- McKean, N.; Chircop, C. Guillain-Barre syndrome after COVID-19 vaccination. BMJ Case Rep. 2021, 14, e244125. [Google Scholar] [CrossRef]
- Scollan, M.E.; Breneman, A.; Kinariwalla, N.; Soliman, Y.; Youssef, S.; Bordone, L.A.; Gallitano, S.M. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022, 20, 1–5. [Google Scholar] [CrossRef]
- Saraceno, J.J.F.; GSouza, M.; Finamor, L.P.D.S.; Nascimento, H.M.; Belfort, R., Jr. Vogt-Koyanagi-Harada Syndrome following COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccine. Int. J. Retin. Vitr. 2021, 7, 49. [Google Scholar] [CrossRef]
- Takeda, M.; Ishio, N.; Shoji, T.; Mori, N.; Matsumoto, M.; Shikama, N. Eosinophilic Myocarditis Following Coronavirus Disease 2019 (COVID-19) Vaccination. Circ. J. 2021. [Google Scholar] [CrossRef]
- May, J.; Draper, A.; Aul, R. Eosinophilic pneumonia and COVID-19 vaccination. QJM 2022, 115, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Bertino, L.; Squeri, R.; Genovese, C.; Isola, S.; Spatari, G.; Spina, E.; Cutroneo, P. Early atypical injection-site reactions to COVID-19 vaccine: A case series. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e24–e26. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, K.; Katayama, K.; Kaji, Y.; Tawara, J.; Ohira, Y. Non-episodic angioedema with eosinophilia after BNT162b2 mRNA COVID-19 vaccination. QJM 2021, 114, 745–746. [Google Scholar] [CrossRef]
- Kaikati, J.; Ghanem, A.; el Bahtimi, R.; Helou, J.; Tomb, R. Eosinophilic panniculitis: A new side effect of Sinopharm COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e337–e339. [Google Scholar] [CrossRef]
- Costanzo, G.; ALedda, G.; Ghisu, A.; Vacca, M.; Firinu, D.; del Giacco, S. Eosinophilic Granulomatosis with Polyangiitis Relapse after COVID-19 Vaccination: A Case Report. Vaccines 2021, 10, 13. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Penninger, J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013, 77, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.S.; Steele, J.M.; Fonseca, B.; Huang, S.; Shah, S.; Maskatia, S.A.; Buddhe, S.; Misra, N.; Ramachandran, P.; Gaur, L.; et al. COVID-19 Vaccination-Associated Myocarditis in Adolescents. Pediatrics 2021, 148, e2021053427. [Google Scholar] [CrossRef]
- Hieber, M.L.; Sprute, R.; Eichenauer, D.A.; Hallek, M.; Jachimowicz, R.D. Hemophagocytic lymphohistiocytosis after SARS-CoV-2 vaccination. Infection 2022. [Google Scholar] [CrossRef]
- Khakroo Abkenar, I.; Rahmani-Nia, F.; Lombardi, G. The Effects of Acute and Chronic Aerobic Activity on the Signaling Pathway of the Inflammasome NLRP3 Complex in Young Men. Medicina 2019, 55, 105. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Abshire, K.M.; Farokhnia, M.; Akhlaghi, F.; Leggio, L. Effect of oral alcohol administration on plasma cytokine concentrations in heavy drinking individuals. Drug Alcohol Depend. 2021, 225, 108771. [Google Scholar] [CrossRef]
- Jain, D.; Chaudhary, P.; Varshney, N.; Razzak, K.S.B.; Verma, D.; Zahra, T.R.K.; Janmeda, P.; Sharifi-Rad, J.; Dastan, S.D.; Mahmud, S.; et al. Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis. J. Oncol. 2021, 2021, 5905357. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, S.P.; Bishop, N.C.; Faulkner, S.H.; Bailey, S.J.; Leicht, C.A. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J. Appl. Physiol. 2018, 125, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Singapore Ministry of Health. Does the COVID-19 Vaccine Cause Myocarditis or Pericarditis? Available online: https://www.moh.gov.sg/covid-19/vaccination/faqs---children-related-vaccination-matters (accessed on 13 March 2022).
- Japanese Government Statistics by Ministy of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/content/10601000/000928692.pdf (accessed on 26 April 2022). (In Japanese)
- Japanese Government Statistics by Ministy of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/content/10601000/000928694.pdf (accessed on 26 April 2022). (In Japanese)
- Aye, Y.N.; Mai, A.S.; Zhang, A.; Lim, O.Z.H.; Lin, N.; Ng, C.H.; Chan, M.Y.; Yip, J.; Loh, P.H.; Chew, N.W.S. Acute Myocardial Infarction and Myocarditis following COVID-19 Vaccination. QJM 2021, hcab252. [Google Scholar] [CrossRef]
- Koizumi, T.; Awaya, T.; Yoshioka, K.; Kitano, S.; Hayama, H.; Amemiya, K.; Enomoto, Y.; Yazaki, Y.; Moroi, M.; Nakamura, M. Myocarditis after COVID-19 mRNA vaccines. QJM 2021, 114, 741–743. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Uesako, H.; Fujikawa, H.; Hashimoto, S.; Wakabayashi, T. Prominent J waves and ventricular fibrillation due to myocarditis and pericarditis after BNT162b2 mRNA COVID-19 vaccination. Can. J. Cardiol. 2022. [Google Scholar] [CrossRef]
- Krug, A.; Stevenson, J.; Hoeg, T.B. BNT162b2 Vaccine-Associated Myo/Pericarditis in Adolescents: A Stratified Risk-Benefit Analysis. Eur. J. Clin. Invest. 2022, 52, e13759. [Google Scholar] [CrossRef]
- Bews, H.; Bryson, A.; Bortoluzzi, T.; Tam, J.W.; Jassal, D.S. COVID-19 vaccination induced myopericarditis: An imager’s perspective. CJC Open 2022, 4, 497–500. [Google Scholar] [CrossRef]
- Toida, R.; Uezono, S.; Komatsu, H.; Toida, T.; Imamura, A.; Fujimoto, S.; Kaikita, K. Takotsubo cardiomyopathy after vaccination for coronavirus disease 2019 in a patient on maintenance hemodialysis. CEN Case Rep. 2021, 11, 220–224. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, P.Y.; Su, Y.J. Paroxysmal supra-ventricular ventricular tachycardia after AstraZeneca COVID-19 vaccine injection. New Microbes New Infect. 2022, 45, 100965. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Sulemankhil, I.; Abdelrahman, M.; Negi, S.I. Temporal association between the COVID-19 Ad26.COV2.S vaccine and acute myocarditis: A case report and literature review. Cardiovasc. Revasc. Med. 2021, 38, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.B.; Gregersen, I.; Sandanger, O.; Yang, K.; Sokolova, M.; Halvorsen, B.E.; Gullestad, L.; Broch, K.; Aukrust, P.; Louwe, M.C. Targeting the Inflammasome in Cardiovascular Disease. JACC Basic Transl. Sci. 2022, 7, 84–98. [Google Scholar] [CrossRef]
- Lu, J.; He, Y.; Terkeltaub, R.; Sun, M.; Ran, Z.; Xu, X.; Wang, C.; Li, X.; Hu, S.; Xue, X.; et al. Colchicine prophylaxis is associated with fewer gout flares after COVID-19 vaccination. Ann. Rheum. Dis. 2022. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sueyoshi, K.; Nakamura, Y.; Okamoto, K.; Tanaka, H. Can the coronavirus disease-2019 vaccine induce an asthma attack? Acute Med. Surg. 2021, 8, e714. [Google Scholar] [CrossRef]
- Sharma, A.; Upadhyay, B.; Banjade, R.; Poudel, B.; Luitel, P.; Kharel, B. A Case of Diffuse Alveolar Hemorrhage with COVID-19 Vaccination. Cureus 2022, 14, e21665. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Ishioka, K.; Masuzawa, Y.; Suda, S.; Murata, S.; Uwamino, Y.; Fujino, M.; Miyahara, H.; Hasegawa, N.; Ryuzaki, M.; et al. COVID-19 vaccine induced interstitial lung disease. J. Infect. Chemother. 2022, 28, 95–98. [Google Scholar] [CrossRef]
- Matsuo, T.; Honda, H.; Tanaka, T.; Uraguchi, K.; Kawahara, M.; Hagiya, H. COVID-19 mRNA Vaccine-Associated Uveitis Leading to Diagnosis of Sarcoidosis: Case Report and Review of Literature. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221086450. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Kantar, A.; Seminara, M.; Odoni, M.; Verde, I.D. Acute Mild Pancreatitis Following COVID-19 mRNA Vaccine in an Adolescent. Children 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Fronhoffs, F.; Dold, L.; Strassburg, C.P.; Weismuller, T.J. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis—Should we be more vigilant? J. Hepatol. 2022, 76, 218–220. [Google Scholar] [CrossRef] [PubMed]
- López Romero-Salazar, F.; Lista, M.V.; Gómez-Domínguez, E.; Ibarrola-Andrés, C.; Gómez, R.M.; Vázquez, I.F. SARS-CoV-2 vaccine, a new autoimmune hepatitis trigger? Rev. Esp. Enferm. Dig. 2022. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.R.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Janes, F.; Gigli, G.L.; Curcio, F.; Negro, I.D.; D’Agostini, S.; Fabris, M.; Valente, M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021, 208, 106839. [Google Scholar] [CrossRef]
- Ancau, M.; Liesche-Starnecker, F.; Niederschweiberer, J.; Krieg, S.M.; Zimmer, C.; Lingg, C.; Kumpfmuller, D.; Ikenberg, B.; Ploner, M.; Hemmer, B.; et al. Case Series: Acute Hemorrhagic Encephalomyelitis After SARS-CoV-2 Vaccination. Front. Neurol. 2021, 12, 820049. [Google Scholar] [CrossRef]
- Zlotnik, Y.; Gadoth, A.; Abu-Salameh, I.; Horev, A.; Novoa, R.; Ifergane, G. Case Report: Anti-LGI1 Encephalitis Following COVID-19 Vaccination. Front. Immunol. 2021, 12, 813487. [Google Scholar] [CrossRef]
- Takeyama, R.; Fukuda, K.; Kouzaki, Y.; Koga, T.; Hayashi, S.; Ohtani, H.; Inoue, T. Intracerebral hemorrhage due to vasculitis following COVID-19 vaccination: A case report. Acta Neurochir. 2022, 164, 543–547. [Google Scholar] [CrossRef]
- de Gregorio, C.; Colarusso, L.; Calcaterra, G.; Bassareo, P.P.; Ieni, A.; Mazzeo, A.T.; Ferrazzo, G.; Noto, A.; Koniari, I.; Mehta, J.L.; et al. Cerebral Venous Sinus Thrombosis following COVID-19 Vaccination: Analysis of 552 Worldwide Cases. Vaccines 2022, 10, 232. [Google Scholar] [CrossRef]
- Suri, V.; Pandey, S.; Singh, J.; Jena, A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021, 14, e245816. [Google Scholar] [CrossRef]
- Shapiro Ben David, S.; Potasman, I.; Rahamim-Cohen, D. Rate of Recurrent Guillain-Barré Syndrome after mRNA COVID-19 Vaccine BNT162b2. JAMA Neurol. 2021, 78, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Havla, J.; Schultz, Y.; Zimmermann, H.; Hohlfeld, R.; Danek, A.; Kumpfel, T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2022, 269, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Lupica, A.; di Stefano, V.; Iacono, S.; Pignolo, A.; Quartana, M.; Gagliardo, A.; Fierro, B.; Brighina, F. Impact of COVID-19 in AChR Myasthenia Gravis and the Safety of Vaccines: Data from an Italian Cohort. Neurol. Int. 2022, 14, 406–416. [Google Scholar] [CrossRef]
- Mahajan, S.; Zhang, F.; Mahajan, A.; Zimnowodzki, S. Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve 2021, 64, E3–E4. [Google Scholar] [CrossRef]
- Syed, K.; Chaudhary, H.; Donato, A. Central Venous Sinus Thrombosis with Subarachnoid Hemorrhage Following an mRNA COVID-19 Vaccination: Are These Reports Merely Co-Incidental? Am. J. Case Rep. 2021, 22, e933397. [Google Scholar] [CrossRef]
- Ikechi, D.; Hashimoto, H.; Nakano, H.; Nakamura, K. A Case of Suspected COVID-19 Vaccine-related Thrombophlebitis. Intern. Med. 2022, 61, 1631. [Google Scholar] [CrossRef]
- Pagenkopf, C.; Sudmeyer, M. A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J. Neuroimmunol. 2021, 358, 577606. [Google Scholar] [CrossRef]
- Takeshita, Y.; Obermeier, B.; Cotleur, A.C.; Spampinato, S.F.; Shimizu, F.; Yamamoto, E.; Sano, Y.; Kryzer, T.J.; Lennon, V.A.; Kanda, T.; et al. Effects of neuromyelitis optica-IgG at the blood-brain barrier in vitro. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e311. [Google Scholar] [CrossRef] [Green Version]
- Uzawa, A.; Mori, M.; Arai, K.; Sato, Y.; Hayakawa, S.; Masuda, S.; Taniguchi, J.; Kuwabara, S. Cytokine and chemokine profiles in neuromyelitis optica: Significance of interleukin-6. Mult. Scler. J. 2010, 16, 1443–1452. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, H.; Hwang, W.Z. Moyamoya disease with Sjogren disease and autoimmune thyroiditis presenting with left intracranial hemorrhage after messenger RNA-1273 vaccination: A case report. Medicine 2022, 101, e28756. [Google Scholar] [CrossRef]
- Bardazzi, F.; Guglielmo, A.; Abbenante, D.; Sacchelli, L.; Sechi, A.; Starace, M.V.R. New insights into alopecia areata during COVID-19 pandemic: When infection or vaccination could play a role. J. Cosmet. Dermatol. 2022, 21, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Dell’Antonia, M.; Anedda, S.; Usai, F.; Atzori, L.; Ferreli, C. Bullous pemphigoid triggered by COVID-19 vaccine: Rapid resolution with corticosteroid therapy. Dermatol. Ther. 2022, 35, e15208. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- de Las Vecillas, L.; Lopez, J.; Morchon, E.; Rodriguez, F.; Drake, M.; Martino, M. Viral-like Reaction or Hypersensitivity? Erythema Multiforme Minor Reaction and Moderate Eosinophilia after the Pfizer-BioNTech BNT162b2 (mRNA-Based) SARS-CoV-2 Vaccine. J. Investig. Allergol. Clin. Immunol. 2021, 32, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Cotter, D.; Basa, J.; Greenberg, H.L. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J. Cosmet. Dermatol. 2021, 20, 1960–1964. [Google Scholar] [CrossRef]
- Fukuoka, H.; Fukuoka, N.; Kibe, T.; Tubbs, R.S.; Iwanaga, J. Oral Herpes Zoster Infection Following COVID-19 Vaccination: A Report of Five Cases. Cureus 2021, 13, e19433. [Google Scholar] [CrossRef]
- Cohen, S.R.; Prussick, L.; Kahn, J.S.; Gao, D.X.; Radfar, A.; Rosmarin, D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int. J. Dermatol. 2021, 60, 1032–1033. [Google Scholar] [CrossRef]
- Durmaz, I.; Turkmen, D.; Altunisik, N.; Toplu, S.A. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID-19 vaccine: A report of three cases. Dermatol. Ther. 2022, 35, e15331. [Google Scholar] [CrossRef]
- Dash, S.; Sirka, C.S.; Mishra, S.; Viswan, P. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin. Exp. Dermatol. 2021, 46, 1615–1617. [Google Scholar] [CrossRef]
- Kreuter, A.; Licciardi-Fernandez, M.J.; Burmann, S.N.; Burkert, B.; Oellig, F.; Michalowitz, A.L. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA-based or adenoviral vector-based SARS-CoV-2 vaccination. Clin. Exp. Dermatol. 2022, 47, 161–163. [Google Scholar] [CrossRef]
- Zengarini, C.; Pileri, A.; Salamone, F.P.; Piraccini, B.M.; Vitale, G.; la Placa, M. Subacute cutaneous lupus erythematosus induction after SARS-CoV-2 vaccine in a patient with primary biliary cholangitis. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e179–e180. [Google Scholar] [CrossRef]
- Tomaszewska, K.; Kozlowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN-gamma, IL-1beta, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid Med. Cell Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef]
- Moseley, I.; Yang, E.J.; Mathieu, R.J.; Elco, C.; Massoud, C.M. Wells syndrome as a presenting sign of COVID-19 in the setting of allergic rhinitis and iron deficiency anemia. JAAD Case Rep. 2022, 23, 27–30. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Sriphrapradang, C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine 2021, 74, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Murvelashvili, N.; Tessnow, A. A Case of Hypophysitis Following Immunization with the mRNA-1273 SARS-CoV-2 Vaccine. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211043386. [Google Scholar] [CrossRef] [PubMed]
- Oyibo, S.O. Subacute Thyroiditis After Receiving the Adenovirus-Vectored Vaccine for Coronavirus Disease (COVID-19). Cureus 2021, 13, e16045. [Google Scholar] [CrossRef]
- Lindner, G.; Ryser, B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: Case report. BMC Infect. Dis. 2021, 21, 1000. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins with Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2020, 11, 617089. [Google Scholar] [CrossRef]
- Obata, S.; Hidaka, S.; Yamano, M.; Yanai, M.; Ishioka, K.; Kobayashi, S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin. Kidney J. 2022, 15, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Jinno, S.; Naka, I.; Nakazawa, T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: In search of the underlying mechanism. Rheumatol. Adv. Pract. 2021, 5, rkab096. [Google Scholar] [CrossRef] [PubMed]
- Gouda, W.; Albasri, A.; Alsaqabi, F.; al Sabah, H.Y.; Alkandari, M.; Abdelnaby, H. Dermatomyositis Following BNT162b2 mRNA COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e32. [Google Scholar] [CrossRef] [PubMed]
- Sauret, A.; Stievenart, J.; Smets, P.; Olagne, L.; Guelon, B.; Aumaitre, O.; Andre, M.; Trefond, L. Case of Giant Cell Arteritis After SARS-CoV-2 Vaccination: A Particular Phenotype? J. Rheumatol. 2022, 49, 120. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Natale, M.; Castagna, A. Polymyalgia rheumatica as uncommon adverse event following immunization with COVID-19 vaccine: A case report and review of literature. Aging Med. 2021, 4, 234–238. [Google Scholar] [CrossRef]
- Patil, S.; Patil, A. Systemic lupus erythematosus after COVID-19 vaccination: A case report. J. Cosmet. Dermatol. 2021, 20, 3103–3104. [Google Scholar] [CrossRef]
- Cole, A.; Thomas, R.; Goldman, N.; Howell, K.; Chakravarty, K.; Denton, C.P.; Ong, V.H. Diffuse cutaneous systemic sclerosis following SARS-Co V-2 vaccination. J. Autoimmun. 2022, 128, 102812. [Google Scholar] [CrossRef]
- Tabata, S.; Hosoi, H.; Murata, S.; Takeda, S.; Mushino, T.; Sonoki, T. Severe aplastic anemia after COVID-19 mRNA vaccination: Causality or coincidence? J. Autoimmun. 2022, 126, 102782. [Google Scholar] [CrossRef]
- Radwi, M.; Farsi, S. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1515–1518. [Google Scholar] [CrossRef]
- Fatima, Z.; Reece, B.R.A.; Moore, J.S.; Means, R.T., Jr. Autoimmune Hemolytic Anemia after mRNA COVID Vaccine. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096211073258. [Google Scholar] [CrossRef]
- Julian, J.A.; Mathern, D.R.; Fernando, D. Idiopathic Thrombocytopenic Purpura and the Moderna COVID-19 Vaccine. Ann. Emerg. Med. 2021, 77, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources. J. Thromb. Haemost. 2022, 20, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects among Saudi Vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Saginoya, T.; Ishiwata, K.; Nakasato, T.; Munechika, H. [18F]FDG uptake in axillary lymph nodes and deltoid muscle after COVID-19 mRNA vaccination: A cohort study to determine incidence and contributing factors using a multivariate analysis. Ann. Nucl. Med. 2022, 36, 340–350. [Google Scholar] [CrossRef]
- Minamimoto, R.; Kiyomatsu, T. Effects of COVID-19 vaccination on FDG-PET/CT imaging: A literature review. Glob. Health Med. 2021, 3, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Chung, H.; Dhayaparan, Y.; Nyein, A.; Acevedo, B.J.; Chicos, C.; Zheng, D.; Barras, M.; Mohamed, M.; Alfishawy, M.; et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab. Syndr. 2021, 15, 102170. [Google Scholar] [CrossRef]
- Sahu, D.; Shetty, G. Frozen shoulder after COVID-19 vaccination. JSES Int. 2022, 4, 234–238. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, H. Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Suhr, O.B.; Coelho, T.; Buades, J.; Pouget, J.; Conceicao, I.; Berk, J.; Schmidt, H.; Waddington-Cruz, M.; Campistol, J.M.; Bettencourt, B.R.; et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: A phase II multi-dose study. Orphanet J. Rare Dis. 2015, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Tseng, C.T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.G.; Rattis, B.; Ottaviani, G.; Celes, M.R.N.; Dias, E.P. ACE2 Down-Regulation May Act as a Transient Molecular Disease Causing RAAS Dysregulation and Tissue Damage in the Microcirculatory Environment among COVID-19 Patients. Am. J. Pathol. 2021, 191, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Spiller, L.; Wolke, C.; Lendeckel, U.; Weinert, S.; Hoffmann, J.; Bornfleth, P.; Kutschka, I.; Gardemann, A.; Isermann, B.; et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017, 242, 1412–1423. [Google Scholar] [CrossRef]
- Agha-Alinejad, H.; Hekmatikar, A.H.A.; Ruhee, R.T.; Shamsi, M.M.; Rahmati, M.; Khoramipour, K.; Suzuki, K. A Guide to Different Intensities of Exercise, Vaccination, and Sports Nutrition in the Course of Preparing Elite Athletes for the Management of Upper Respiratory Infections during the COVID-19 Pandemic: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 1888. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Mizuno, Y.; Hokimoto, S.; Harada, E.; Kinoshita, K.; Yoshimura, M.; Yasue, H. Variant Aldehyde Dehydrogenase 2 (ALDH2*2) in East Asians Interactively Exacerbates Tobacco Smoking Risk for Coronary Spasm-Possible Role of Reactive Aldehydes. Circ. J. 2016, 81, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Voskoboinik, A.; Kalman, J.M.; de Silva, A.; Nicholls, T.; Costello, B.; Nanayakkara, S.; Prabhu, S.; Stub, D.; Azzopardi, S.; Vizi, D.; et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N. Engl. J. Med. 2020, 382, 20–28. [Google Scholar] [CrossRef]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861.e1–1861.e5. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]
- Tochihara, Y. A review of Japanese-style bathing: Its demerits and merits. J. Physiol. Anthropol. 2022, 41, 5. [Google Scholar] [CrossRef] [PubMed]
- Japanese Government Statistics by Ministy of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/content/10601000/000675088.pdf (accessed on 26 April 2022). (In Japanese)
- US Centers for Disease Control and Prevention. Science Brief: Evidence Used to Update the List of Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html (accessed on 20 May 2022).
- Machado, P.M.; Lawson-Tovey, S.; Strangfeld, A.; Mateus, E.F.; Hyrich, K.L.; Gossec, L.; Carmona, L.; Rodrigues, A.; Raffeiner, B.; Duarte, C.; et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: Results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann. Rheum. Dis. 2022, 81, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Japanese Government Statistics by Ministy of Health, Labour and Welfare. Available online: https://www.cov19-vaccine.mhlw.go.jp/qa/0028.html (accessed on 20 May 2022). (In Japanese)
| 1. Cardiovascular diseases | 6. Skin diseases |
| Acute coronary syndrome (ACS) | Alopecia areata (AA) |
| Aortic dissection (AD) | Bullous pemphigoid |
| Arrhythmia | COVID arm |
| Heart failure (HF) | Eosinophilic cellulitis (EC) |
| Myocarditis/Pericarditis | Eosinophilic panniculitis (EP) |
| Pulmonary embolism (PE) | Erythema multiforme (EM) |
| Takotsubo cardiomyopathy (TCM) | Herpes zoster (skin, oral and facial palsy) |
| Vasospastic angina (VSA) | Leukocytoclastic vasculitis |
| 2. Respiratory diseases | Non-episodic angioedema with eosinophilia |
| Asthma attack | Psoriasis |
| Diffuse alveolar hemorrhage (DAH) | Pyoderma gangrenosum (PG) |
| Eosinophilic pneumonia (EP) | Steven-Johnson syndrome (SJS) |
| Interstitial lung disease (ILD) | Subacute cutaneous lupus erythematosus (SCLE) |
| Sarcoidosis | Urticaria |
| 3. Gastroenterological diseases | 7. Endocrine diseases |
| Appendicitis | Graves’ Disease |
| Autoimmune hepatitis (AIH) | Hypophysitis |
| Bleeding duodenal ulcer | Hypothyroidism |
| Intestinal obstruction/perforation | Syndrome of inappropriate antidiuresis (SIADH) |
| Mesenteric ischemia | Type 1 diabetes mellitus |
| Pancreatitis | Thyroiditis (painful, silent, subacute) |
| 4. Renal diseases | 8. Collagen diseases |
| Acute rejection of kidney transplant | Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis |
| IgA nephropathy | Antiphospholipid syndrome (APS) |
| IgG4 nephritis | Dermatomyositis (DM) |
| Membranous nephropathy (MN) | Eosinophilic granulomatosis (EGPA) |
| Minimal change disease (MCD) | Giant cell arteritis (GCA) |
| Renal thrombotic microangiopathy | Polymyalgia rheumatica (PMR) |
| Scleroderma renal crisis | Rheumatoid arthritis (RA) |
| Vasculitis | Systemic lupus erythematosus (SLE) |
| 5. Neurological diseaes | Systemic sclerosis (SSc) |
| Acute disseminated encephalomyelitis (ADEM) | 9. Hematologic diseases |
| Acute hemorrhagic leukoencephalitis (AHEM) | Aplastic anemia (AA) |
| Acute meningoencephalitis | Acquires hemophilia A (AHA) |
| Bells’ palsy | Autoimmune hemolytic anemia (AIHA) |
| Cerebral hemorrhage (CH) | Hemophagocytic lymphohistiocytosis (HLH) |
| Cerebral infarction (CI) | Immune thrombocytopenia (ITP) |
| Cerebral venous sinus thrombosis (CVST) | Vaccine-induced immune thrombotic thrombocytopenia (VITT) |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) | 10. Others |
| Guillain–Barré syndrome (GBS) | Abnormal menstrual cycle |
| Multiple sclerosis (MS) | Anaphylaxis |
| Myasthenia gravis (MG) | Gout flares |
| Neuromyelitis optica spectrum disorder (NMOSD) | Lymphadenopathy |
| Parsonage-Turner syndrome (Neuralgic amyotrophy) | Rhabdomyolysis |
| Subarachnoid hemorrhage (SAH) | Shoulder injury related to vaccine administration (SIRVA) |
| Thrombophlebitis | Vogt-Koyanagi-Harada syndrome |
| Transverse myelitis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awaya, T.; Moroi, M.; Enomoto, Y.; Kunimasa, T.; Nakamura, M. What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions. Vaccines 2022, 10, 866. https://doi.org/10.3390/vaccines10060866
Awaya T, Moroi M, Enomoto Y, Kunimasa T, Nakamura M. What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions. Vaccines. 2022; 10(6):866. https://doi.org/10.3390/vaccines10060866
Chicago/Turabian StyleAwaya, Toru, Masao Moroi, Yoshinari Enomoto, Taeko Kunimasa, and Masato Nakamura. 2022. "What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions" Vaccines 10, no. 6: 866. https://doi.org/10.3390/vaccines10060866
APA StyleAwaya, T., Moroi, M., Enomoto, Y., Kunimasa, T., & Nakamura, M. (2022). What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions. Vaccines, 10(6), 866. https://doi.org/10.3390/vaccines10060866






