Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. qPCR and ELISA
2.3. DNA Sequencing
2.4. Statistical Analysis
3. Results
3.1. Sensitivity of the Three qPCRs
3.2. PVL and the Proportion of BLV-Infected Cells in Whole-Blood Samples by Different qPCRs
3.3. PVL and the Proportion of BLV-Infected Cells in Tumor Tissue Samples by Different qPCRs
3.4. Nucleotide Sequences of BLV Proviruses in Tumor Tissue Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoye, J.P.; Blomberg, J.; Coffin, J.M.; Fan, H.; Hahn, B.; Neil, J.; Quackenbush, S.; Rethwilm, A.; Tristem, M. Family Retroviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M., Lefkowitz, E., Adams, M.J., Carstens, E.B., Eds.; Elsevier: San Diego, CA, USA, 2011; Volume 9, pp. 477–495. [Google Scholar]
- OIE. Enzootic bovine leukosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019; World Health Organization for Animal Health: Paris, France, 2019; pp. 1113–1124. [Google Scholar]
- Tsutsui, T.; Kobayashi, S.; Hayama, Y.; Yamamoto, T. Fraction of bovine leukemia virus-infected dairy cattle developing enzootic bovine leukosis. Prev. Vet. Med. 2016, 124, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Erskine, R.; Bartlett, P.; Byrem, T.; Render, C.; Febvay, C.; Houseman, J. Association between bovine leukemia virus, production, and population age in Michigan dairy herds. J. Dairy Sci. 2012, 95, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.; Johnson, R.; Wells, S.J. Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Prev. Vet. Med. 2003, 61, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Erskine, R.J.; Bartlett, P.C.; Sabo, K.M.; Sordillo, L.M. Bovine leukemia virus infection in dairy cattle: Effect on serological response to immunization against J5 Escherichia coli bacterin. Vet. Med. Int. 2011, 2011, 915747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trainin, Z.; Brenner, J.; Meirom, R.; Ungar-Waron, H. Detrimental effect of bovine leukemia virus (BLV) on the immunological state of cattle. Vet. Immunol. Immunopathol. 1996, 54, 293–302. [Google Scholar] [CrossRef]
- Watanabe, A.; Murakami, H.; Kakinuma, S.; Murao, K.; Ohmae, K.; Isobe, N.; Akamatsu, H.; Seto, T.; Hashimura, S.; Konda, K.; et al. Association between bovine leukemia virus proviral load and severity of clinical mastitis. J. Vet. Med. Sci. 2019, 81, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, P.C.; Ruggiero, V.J.; Hutchinson, H.C.; Droscha, C.J.; Norby, B.; Sporer, K.R.B.; Taxis, T.M. Current Developments in the Epidemiology and Control of Enzootic Bovine Leukosis as Caused by Bovine Leukemia Virus. Pathogens 2020, 9, 1058. [Google Scholar] [CrossRef]
- Nakada, S.; Fujimoto, Y.; Kohara, J.; Adachi, Y.; Makita, K. Estimation of economic loss by carcass weight reduction of Japanese dairy cows due to infection with bovine leukemia virus. Prev. Vet. Med. 2022, 198, 105528. [Google Scholar] [CrossRef]
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.-I.; Tsutsui, T. Nationwide Survey of Bovine Leukemia Virus Infection among Dairy and Beef Breeding Cattle in Japan from 2009–2011. J. Vet. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef] [Green Version]
- MAFF. Annual Statistics of Notifiable Diseases. Available online: https://www.maff.go.jp/j/syouan/douei/kansi_densen/attach/pdf/kansi_densen-165.pdf (accessed on 1 January 2022). (in Japanese)
- Kobayashi, S.; Yamamoto, T.; Hayama, Y.; Murak, K.; Tsutsui, T. Descriptive epidemiology of bovine leukemia in Japan. J. Vet. Emidemiol. 2016, 20, 18. [Google Scholar]
- Oliver-Espinosa, O.; Physick-Sheard, P.W.; Wollenberg, G.K.; Taylor, J. Sporadic bovine leukosis associated with ataxia and tibiotarsal joint swelling: A case report. Can. Vet. J. 1994, 35, 777. [Google Scholar]
- Igawa, S. Overview of Guidelines for Biosecurity Measures of Enzootic Bovine Leukosis. J. Vet. Epidemiol. 2015, 19, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Juliarena, M.A.; Gutierrez, S.E.; Ceriani, C. Determination of proviral load in bovine leukemia virus-infected cattle with and without lymphocytosis. Am. J. Vet. Res. 2007, 68, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Mekata, H.; Sekiguchi, S.; Konnai, S.; Kirino, Y.; Honkawa, K.; Nonaka, N.; Horii, Y.; Norimine, J. Evaluation of the natural perinatal transmission of bovine leukaemia virus. Vet. Rec. 2015, 176, 254. [Google Scholar] [CrossRef] [PubMed]
- Sajiki, Y.; Konnai, S.; Nishimori, A.; Okagawa, T.; Maekawa, N.; Goto, S.; Nagano, M.; Kohara, J.; Kitano, N.; Takahashi, T. Intrauterine infection with bovine leukemia virus in pregnant dam with high viral load. J. Vet. Med. Sci. 2017, 17–0391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, A.; Laydon, D.J.; Bain, B.J.; Fields, P.A.; Taylor, G.P. Pre-morbid human T-lymphotropic virus type I proviral load, rather than percentage of abnormal lymphocytes, is associated with an increased risk of aggressive adult T-cell leukemia/lymphoma. Haematologica 2013, 98, 385–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.-R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: A nationwide prospective study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Heenemann, K.; Lapp, S.; Teifke, J.P.; Fichtner, D.; Mettenleiter, T.C.; Vahlenkamp, T.W. Development of a Bovine leukemia virus polymerase gene-based real-time polymerase chain reaction and comparison with an envelope gene-based assay. J. Vet. Diagn. Investig. 2012, 24, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Jimba, M.; Takeshima, S.N.; Matoba, K.; Endoh, D.; Aida, Y. BLV-CoCoMo-qPCR: Quantitation of bovine leukemia virus proviral load using the CoCoMo algorithm. Retrovirology 2010, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Kuckleburg, C.J.; Chase, C.C.; Nelson, E.A.; Marras, S.A.E.; Dammen, M.A.; Christopher-Hennings, J. Detection of Bovine Leukemia Virus in Blood and Milk by Nested and Real-Time Polymerase Chain Reactions. J. Vet. Diagn. Investig. 2003, 15, 72–76. [Google Scholar] [CrossRef]
- Rola-Łuszczak, M.; Finnegan, C.; Olech, M.; Choudhury, B.; Kuźmak, J. Development of an improved real time PCR for the detection of bovine leukaemia provirus nucleic acid and its use in the clarification of inconclusive serological test results. J. Virol. Methods 2013, 189, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.P.; Pluta, A.; Rola-Łuszczak, M.; McGowan, S.L.; Finnegan, C.; Heenemann, K.; Carignano, H.A.; Alvarez, I.; Murakami, K.; Willems, L.; et al. Interlaboratory Comparison of Six Real-Time PCR Assays for Detection of Bovine Leukemia Virus Proviral DNA. J. Clin. Microbiol. 2018, 56, e00304-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duck, P.; Alvarado-Urbina, G.; Burdick, B.; Collier, B. Probe amplifier system based on chimeric cycling oligonucleotides. Biotechniques 1990, 9, 142–148. [Google Scholar] [PubMed]
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. Detection of specific polymerase chain reaction product by utilizing the 5′–3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Jackman, S.D.; Vandervalk, B.P.; Mohamadi, H.; Chu, J.; Yeo, S.; Hammond, S.A.; Jahesh, G.; Khan, H.; Coombe, L.; Warren, R.L. ABySS 2.0: Resource-efficient assembly of large genomes using a Bloom filter. Genome Res. 2017, 27, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Akagami, M.; Oya, S.; Kashima, Y.; Seki, S.; Ouchi, Y.; Hayama, Y. A hematologic key for bovine leukemia virus screening in Japanese black cattle. J. Vet. Diagn. Investig. 2019, 31, 568–571. [Google Scholar] [CrossRef]
- Mekata, H.; Yamamoto, M.; Kirino, Y.; Sekiguchi, S.; Konnai, S.; Horii, Y.; Norimine, J. New hematological key for bovine leukemia virus-infected Japanese Black cattle. J. Vet. Med. Sci. 2018, 80, 316–319. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, H.; Ono, M.; Saruyama, Y.; Mukai, M.; Kohiruimaki, M.; Kawamura, S. Comparison of the peripheral blood leukocyte population between Japanese Black and Holstein calves. Anim. Sci. J. 2011, 82, 93–98. [Google Scholar] [CrossRef]
- Somura, Y.; Sugiyama, E.; Fujikawa, H.; Murakami, K. Comparison of the copy numbers of bovine leukemia virus in the lymph nodes of cattle with enzootic bovine leukosis and cattle with latent infection. Arch. Virol. 2014, 159, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Akbarin, M.M.; Rahimi, H.; Hassannia, T.; Shoja Razavi, G.; Sabet, F.; Shirdel, A. Comparison of HTLV-I Proviral Load in Adult T Cell Leukemia/Lymphoma (ATL), HTLV-I-Associated Myelopathy (HAM-TSP) and Healthy Carriers. Iran J. Basic Med. Sci. 2013, 16, 208–212. [Google Scholar] [PubMed]
- Tamiya, S.; Matsuoka, M.; Etoh, K.; Watanabe, T.; Kamihira, S.; Yamaguchi, K.; Takatsuki, K. Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood 1996, 88, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.I.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and epigenetic inactivation oftax gene in adult T-cell leukemia cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef]
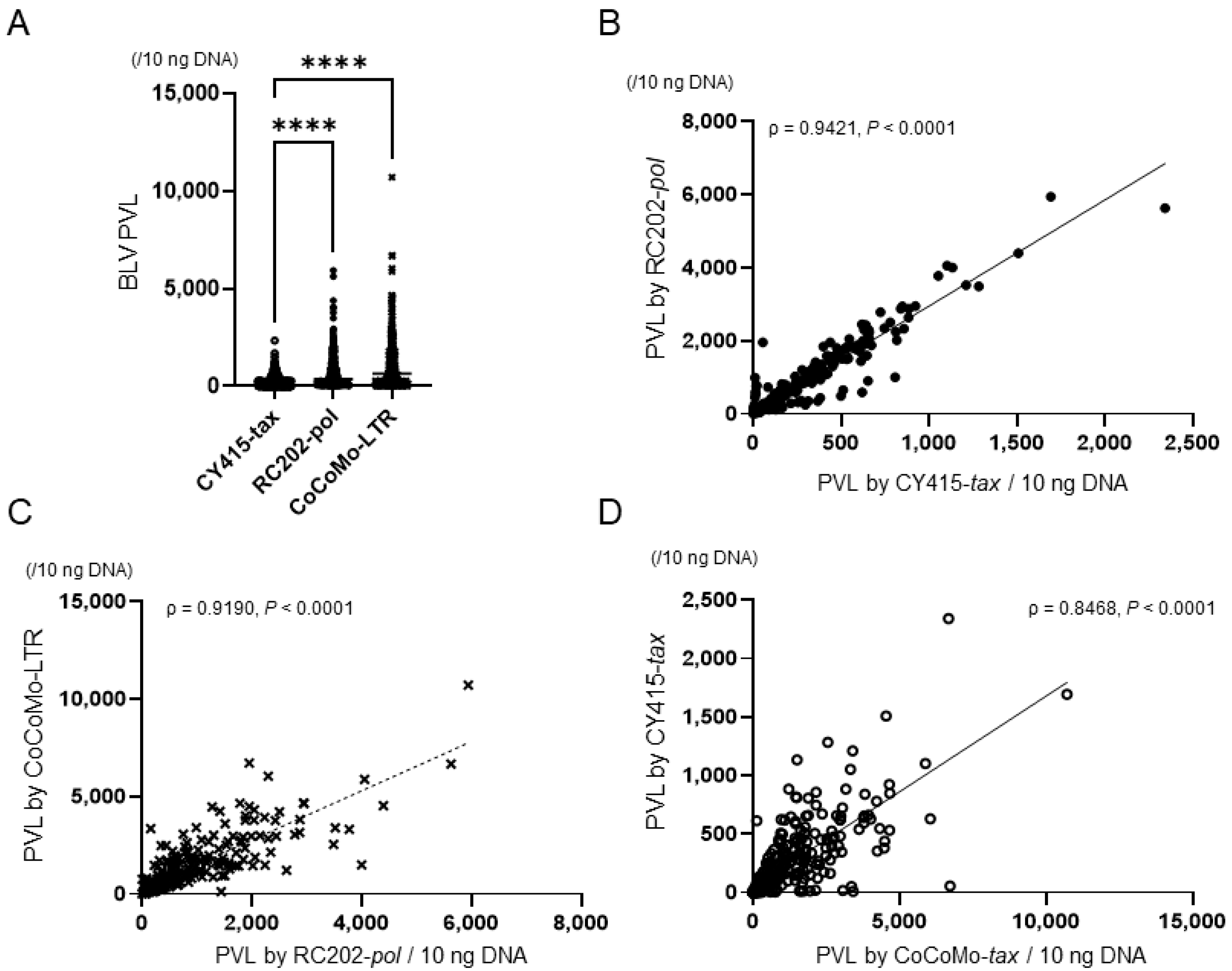


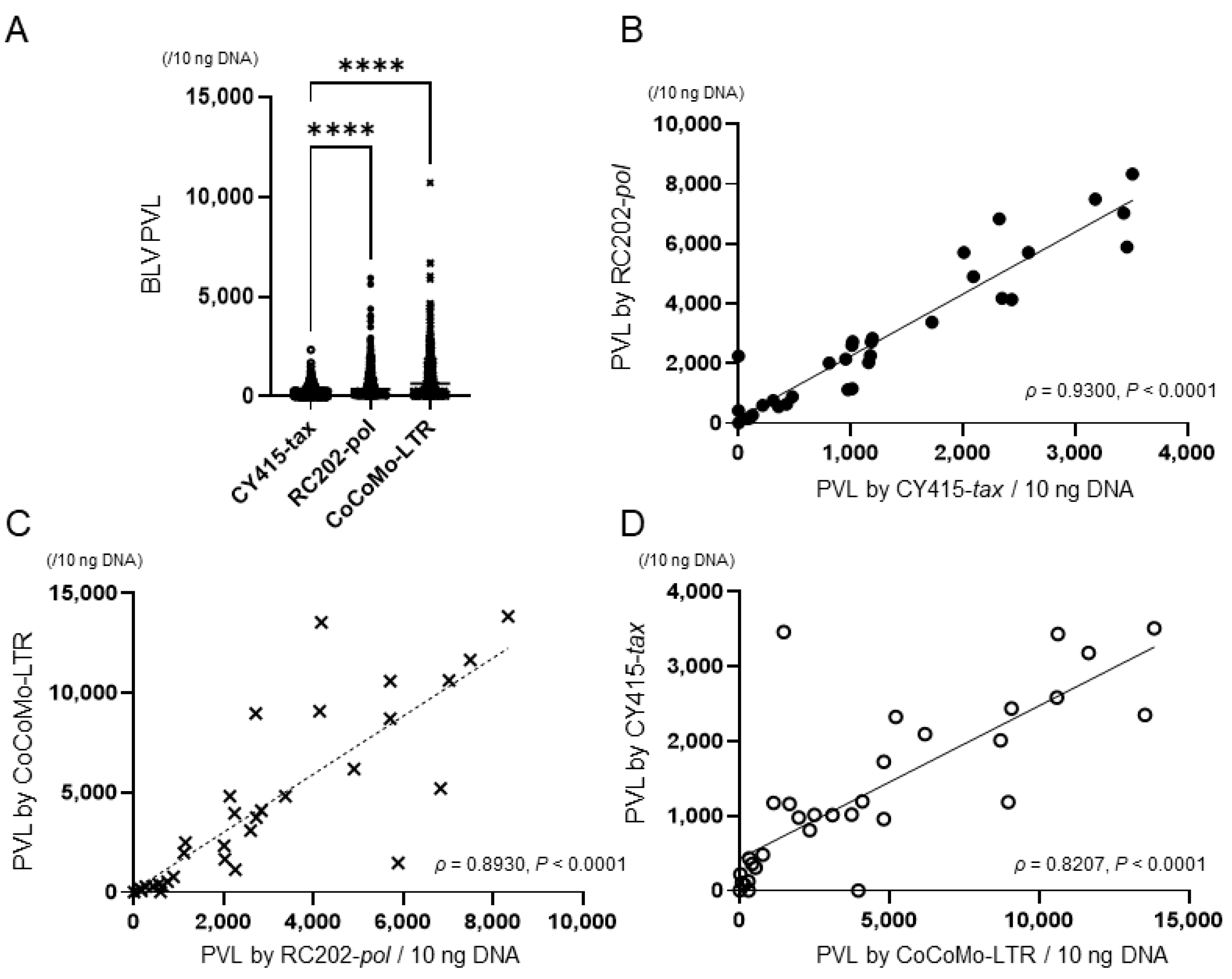

| Type of Reagent | 0 Percentile (Minimum) | 25 Percentile | 50 Percentile (Median) | 75 Percentile | 100 Percentile (Maximum) | Shapiro–Wilk Normality Test p |
|---|---|---|---|---|---|---|
| BLV PVL | ||||||
| CY415-tax | 0.1 | 15.9 | 119.3 | 347 | 2343.7 | <0.05 |
| RC202-pol | 0.2 | 55.1 | 372.3 | 1037.6 | 5943.2 | <0.05 |
| CoCoMo-LTR | 0.2 | 124.1 | 658.4 | 1681.5 | 10713 | <0.05 |
| Proportion of BLV-infected cells | ||||||
| CY415-tax | <0.0001 | 0.03 | 2.6 | 9.5 | 50 | <0.05 |
| RC202-pol | 0.004 | 2.1 | 9.9 | 26.5 | 128.5 | <0.05 |
| CoCoMo-LTR | 0.003 | 4.7 | 16.9 | 43.5 | 280.4 | <0.05 |
| Type of Reagent | 0 Percentile (Minimum) | 25 Percentile | 50 Percentile (Median) | 75 Percentile | 100 Percentile (Maximum) | Shapiro–Wilk Normality Test p |
|---|---|---|---|---|---|---|
| BLV PVL | ||||||
| CY415-tax | 0.18 | 345.6 | 1016.2 | 2152.8 | 3511.6 | <0.05 |
| RC202-pol | 10.1 | 712.2 | 2255.7 | 4362.7 | 8338.9 | <0.05 |
| CoCoMo-LTR | 6.8 | 501.6 | 2793.1 | 6817.5 | 13845.1 | <0.05 |
| Proportion of BLV-infected cells (%) | ||||||
| CY415-tax | 0.006 | 30.4 | 45 | 76.8 | 127.5 | <0.05 |
| RC202-pol | 0.3 | 67.6 | 99 | 147.3 | 331.2 | <0.05 |
| CoCoMo-LTR | 0.2 | 48.8 | 122.4 | 202.7 | 394.8 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneyama, S.; Kobayashi, S.; Matsunaga, T.; Tonosaki, K.; Leng, D.; Sakai, Y.; Yamada, S.; Kimura, A.; Ichijo, T.; Hikono, H.; et al. Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus. Viruses 2022, 14, 1182. https://doi.org/10.3390/v14061182
Yoneyama S, Kobayashi S, Matsunaga T, Tonosaki K, Leng D, Sakai Y, Yamada S, Kimura A, Ichijo T, Hikono H, et al. Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus. Viruses. 2022; 14(6):1182. https://doi.org/10.3390/v14061182
Chicago/Turabian StyleYoneyama, Syuji, Sota Kobayashi, Towa Matsunaga, Kaoru Tonosaki, Dongze Leng, Yusuke Sakai, Shinji Yamada, Atsushi Kimura, Toshihiro Ichijo, Hirokazu Hikono, and et al. 2022. "Comparative Evaluation of Three Commercial Quantitative Real-Time PCRs Used in Japan for Bovine Leukemia Virus" Viruses 14, no. 6: 1182. https://doi.org/10.3390/v14061182






