IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. SARS-CoV-2-Specific Replication Assay
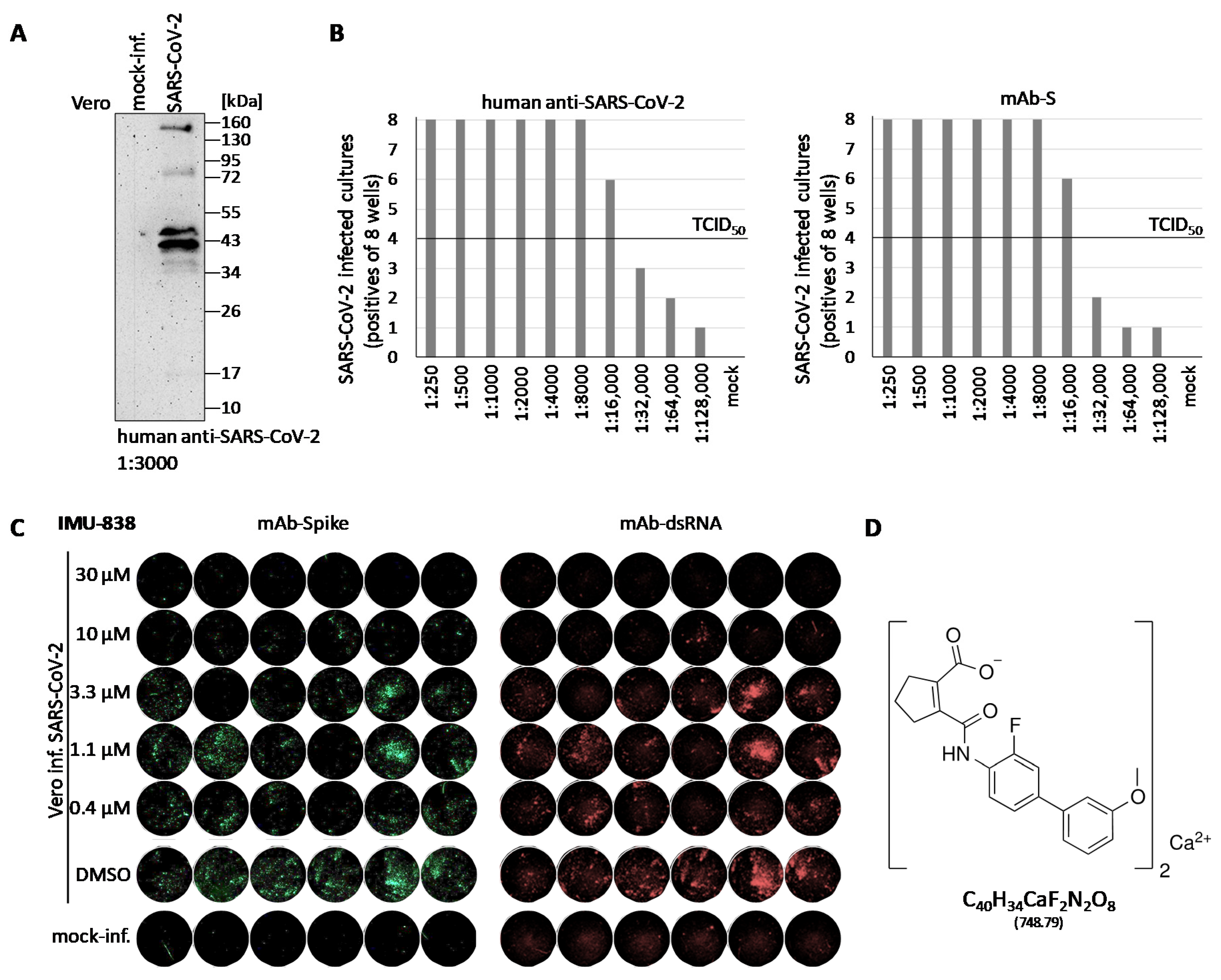
2.3. RT-qPCR for the Detection of Extracellular SARS-CoV-2
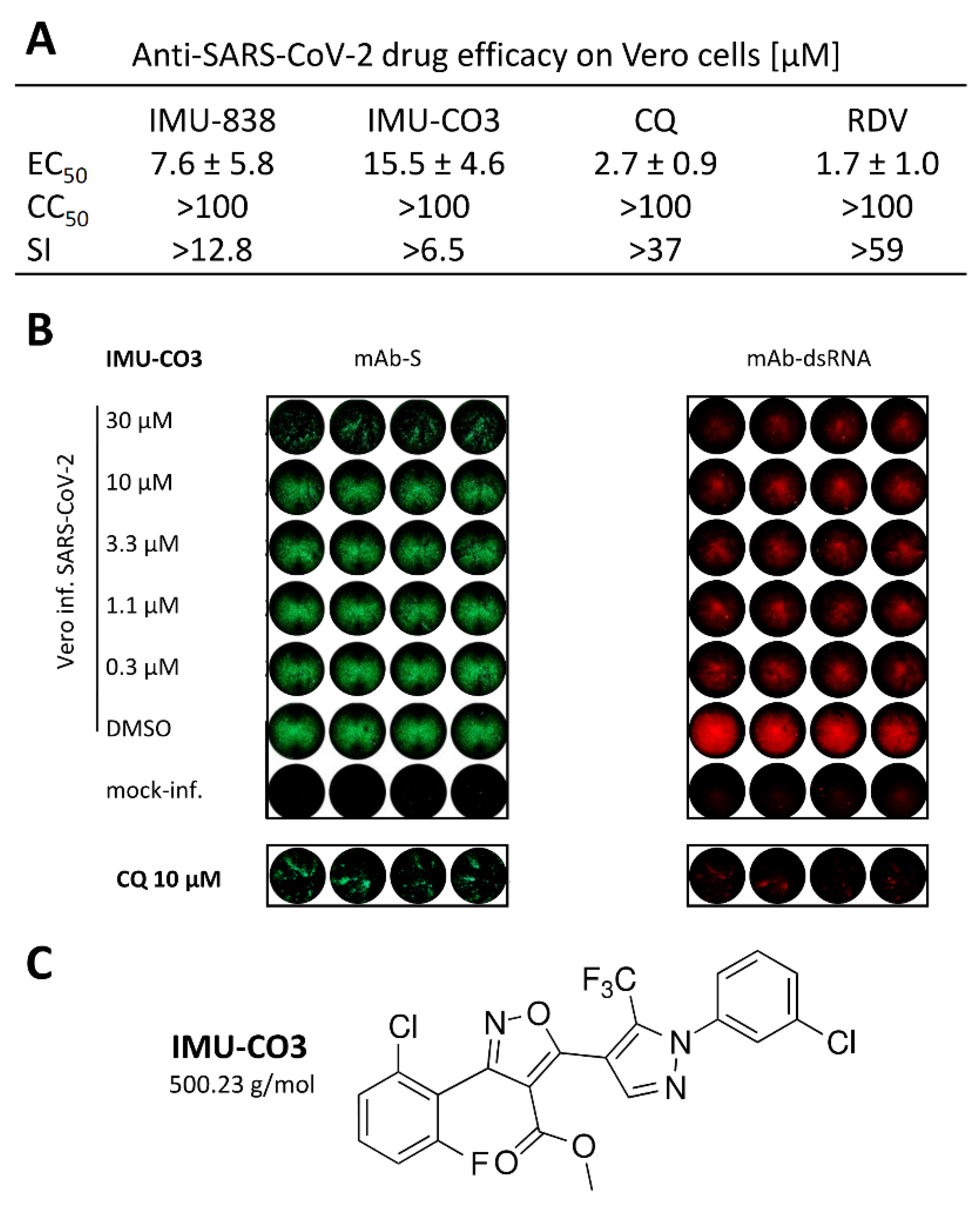
2.4. In-Cell Immunostaining for the Detection of Intracellular SARS-CoV-2
2.5. Western Blot Analysis
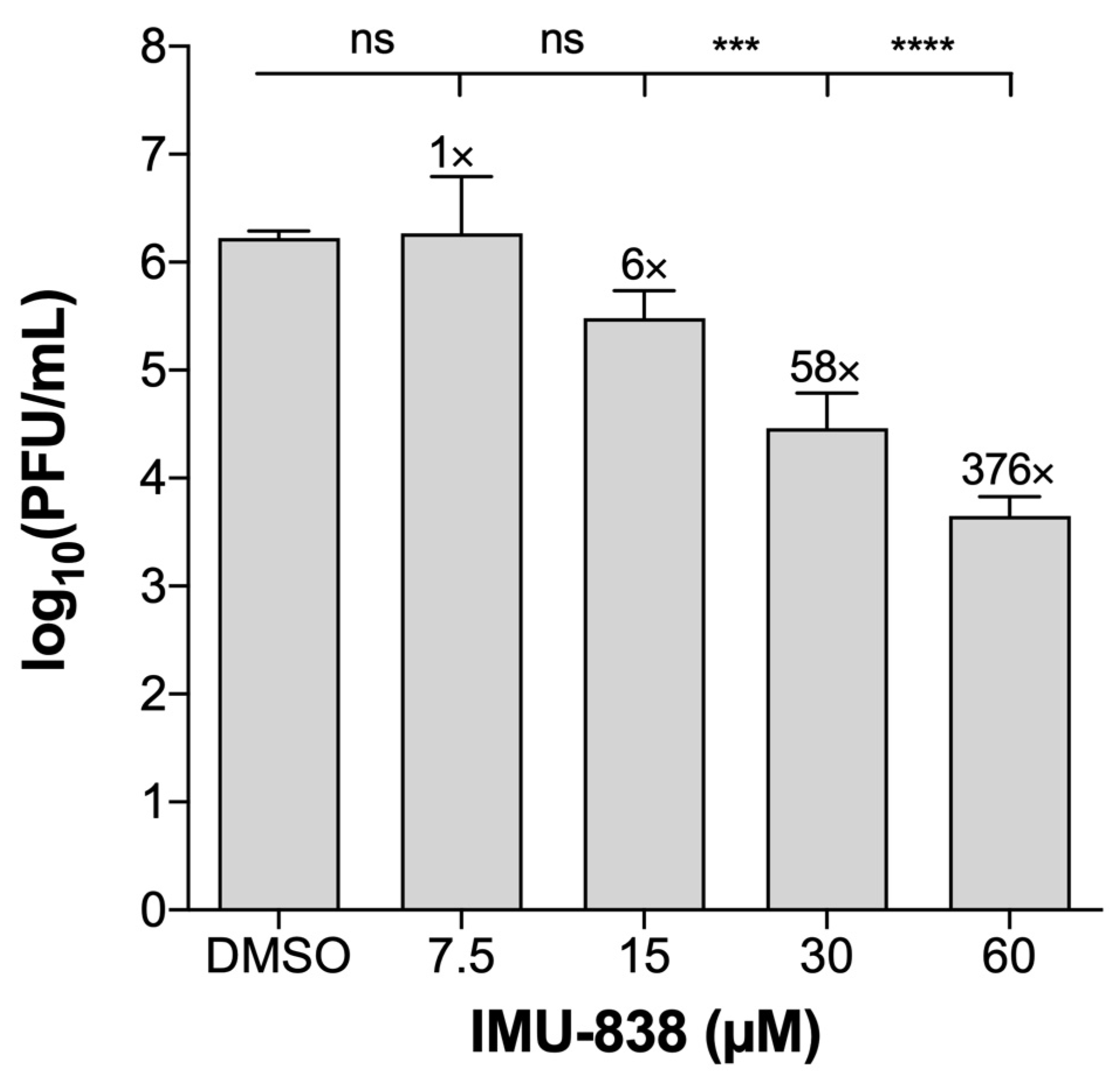
2.6. Viral Plaque and Yield Reduction Assays
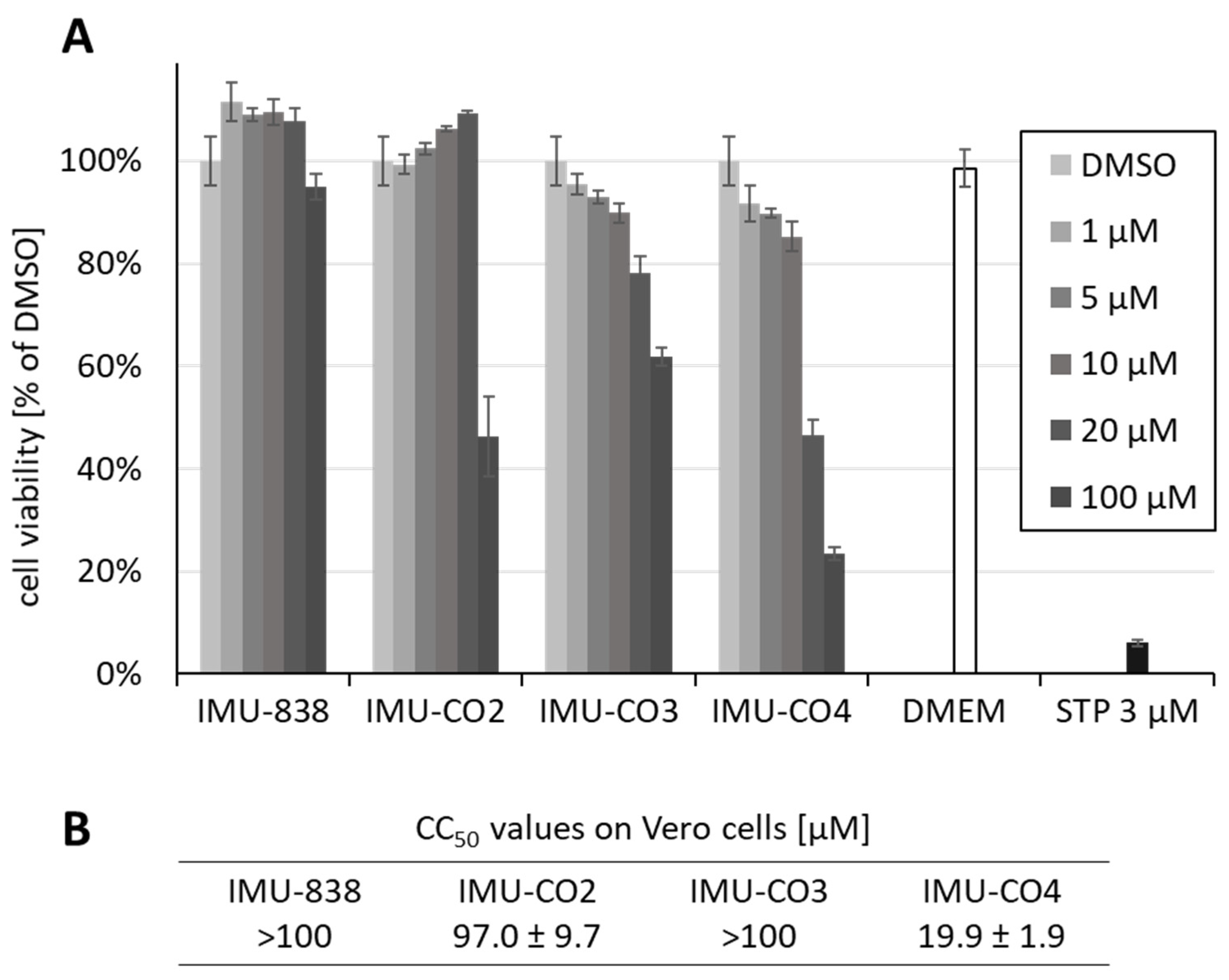
2.7. Neutral Red Assay (NRA)
2.8. Methods for Comparative Antiviral Analysis of Additional Human Pathogenic Viruses
2.9. Statistical Analysis
3. Results
3.1. Establishment of a Cell-Culture-Based SARS-CoV-2-Specific Replication Assay
3.2. Assessment of the Anti-SARS-CoV-2 Activity of IMU-838
3.3. Additional Assessment of the Anti-SARS-CoV-2 Activity of DHODH Inhibitor Back-up Compounds
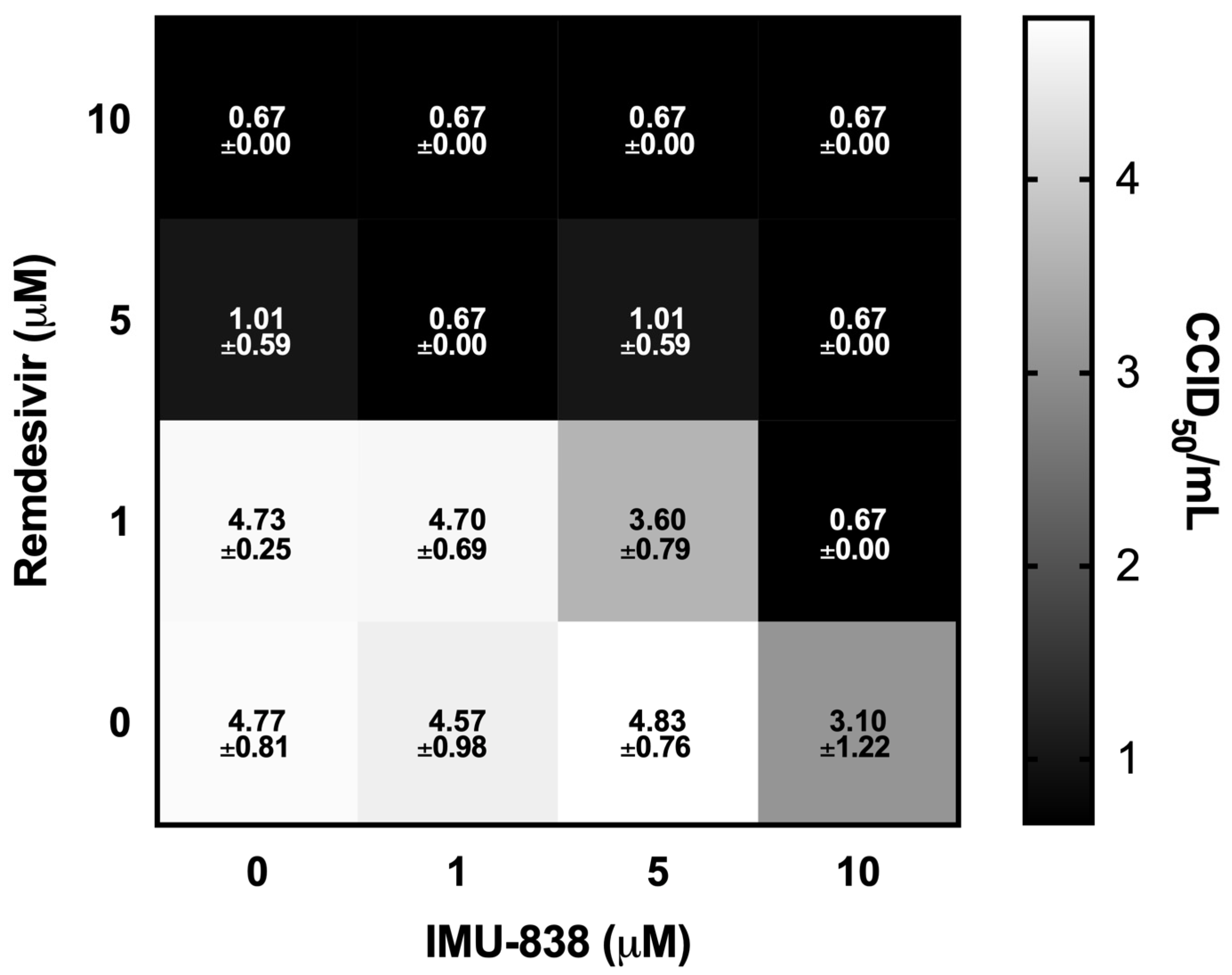
3.4. Combinatorial and Broad-Spectrum Aspects of IMU-838 Antiviral Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muehler, A.; Kohlhof, H.; Groeppel, M.; Vitt, D. Safety, tolerability and pharmacokinetics of Vidofludimus calcium (IMU-838) after single and multiple ascending oral doses in healthy male subjects. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Muehler, A.; Kohlhof, H.; Groeppel, M.; Vitt, D. The selective oral immunomodulator Vidofludimus in patients with active rheumatoid arthritis: Safety results from the COMPONENT study. Drugs R&D 2019, 19, 351–366. [Google Scholar] [CrossRef]
- Immunic, Inc. Reports Positive Top-line Data from Phase 2 EMPhASIS Trial of IMU-838 in Patients with Relapsing-Remitting Multiple Sclerosis. Available online: https://www.immunic-therapeutics.com/2020/08/02/immunic-inc-reports-positive-top-line-data-from-phase-2-emphasis-trial-of-imu-838-in-patients-with-relapsing-remitting-multiple-sclerosis/ (accessed on 4 November 2020).
- Klotz, L.; Eschborn, M.; Lindner, M.; Liebmann, M.; Herold, M.; Janoschka, C.; Torres Garrido, B.; Schulte-Mecklenbeck, A.; Gross, C.C.; Breuer, J.; et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, A.; Franti, M.; Pusateri Keaney, E.; Kuhen, K.; Seepersaud, M.; Radetich, B.; Shao, J.; Honda, A.; Dewhurst, J.; Balabanis, K.; et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV). Proc. Natl. Acad. Sci. USA 2011, 108, 6739–6744. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.N.; Lai, K.K.; Dai, J.; Kok, K.H.; Chen, H.; Chan, K.H.; Yuen, K.Y.; Kao, R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017, 98, 946–954. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef]
- Lucas-Hourani, M.; Dauzonne, D.; Jorda, P.; Cousin, G.; Lupan, A.; Helynck, O.; Caignard, G.; Janvier, G.; André-Leroux, G.; Khiar, S.; et al. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog. 2013, 9, e1003678. [Google Scholar] [CrossRef]
- Luthra, P.; Naidoo, J.; Pietzsch, C.A.; De, S.; Khadka, S.; Anantpadma, M.; Williams, C.G.; Edwards, M.R.; Davey, R.A.; Bukreyev, A.; et al. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antivir. Res. 2018, 158, 288–302. [Google Scholar] [CrossRef]
- Marschall, M.; Niemann, I.; Kosulin, K.; Bootz, A.; Wagner, S.; Dobner, T.; Herz, T.; Kramer, B.; Leban, J.; Vitt, D.; et al. Assessment of drug candidates for broad-spectrum antiviral therapy targeting cellular pyrimidine biosynthesis. Antivir. Res. 2013, 100, 640–648. [Google Scholar] [CrossRef]
- Martin, S.; Chiramel, A.I.; Schmidt, M.L.; Chen, Y.C.; Whitt, N.; Watt, A.; Dunham, E.C.; Shifflett, K.; Traeger, S.; Leske, A.; et al. A genome-wide siRNA screen identifies a druggable host pathway essential for the Ebola virus life cycle. Genome Med. 2018, 10, 58. [Google Scholar] [CrossRef]
- Waldman, W.J.; Knight, D.A.; Blinder, L.; Shen, J.; Lurain, N.S.; Miller, D.M.; Sedmak, D.D.; Williams, J.W.; Chong, A.S. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology 1999, 42, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Bushell, S.; Qing, M.; Xu, H.Y.; Bonavia, A.; Nunes, S.; Zhou, J.; Poh, M.K.; Florez de Sessions, P.; Niyomrattanakit, P.; et al. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J. Virol. 2011, 85, 6548–6556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Xu, L.; Zhou, X.; Shokrollahi, E.; Felczak, K.; van der Laan, L.J.; Pankiewicz, K.W.; Sprengers, D.; Raat, N.J.; et al. Cross talk between nucleotide synthesis pathways with cellular immunity in constraining hepatitis E virus replication. Antimicrob. Agents Chemother. 2016, 60, 2834–2848. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, L.; Li, S.; Sun, Y.; Ding, M.; Wang, Y.; Zhao, Y.; Wu, Y.; Shang, W.; Jiang, X.; et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell 2020, 11, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Niesar, A.; Wangen, C.; Wild, M.; Grau, B.; Herrmann, L.; Capci, A.; Adrait, A.; Couté, Y.; Tsogoeva, S.B.; et al. Target verification of artesunate-related antiviral drugs: Assessing the role of mitochondrial and regulatory proteins by click chemistry and fluorescence labeling. Antivir. Res. 2020, 180, 104861. [Google Scholar] [CrossRef]
- Wild, M.; Bertzbach, L.D.; Tannig, P.; Wangen, C.; Müller, R.; Herrmann, L.; Fröhlich, T.; Tsogoeva, S.B.; Kaufer, B.B.; Marschall, M.; et al. The trimeric artesunate derivative TF27 exerts strong anti-cytomegaloviral efficacy: Focus on prophylactic efficacy and oral treatment of immunocompetent mice. Antivir. Res. 2020, 178, 104788. [Google Scholar] [CrossRef]
- Svrlanska, A.; Ruhland, A.; Marschall, M.; Reuter, N.; Stamminger, T. Wedelolactone inhibits human cytomegalovirus replication by targeting distinct steps of the viral replication cycle. Antivir. Res. 2020, 174, 104677. [Google Scholar] [CrossRef]
- Jacquet, C.; Marschall, M.; Andouard, D.; El Hamel, C.; Chianea, T.; Tsogoeva, S.B.; Hantz, S.; Alain, S. A highly potent trimeric derivative of artesunate shows promising treatment profiles in experimental models for congenital HCMV infection in vitro and ex vivo. Antivir. Res. 2020, 175, 104700. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Conradie, A.M.; Hahn, F.; Wild, M.; Marschall, M.; Kaufer, B.B. Artesunate derivative TF27 inhibits replication and pathogenesis of an oncogenic avian alphaherpesvirus. Antivir. Res. 2019, 171, 104606. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, K.; Hahn, F.; Wangen, C.; Strojan, H.; Müller, R.; Anand, N.; Ali, I.; Bera, K.; Ray, B.; et al. Chemically sulfated polysaccharides from natural sources: Assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019, 136, 521–530. [Google Scholar] [CrossRef]
- Hamilton, S.T.; Hutterer, C.; Egilmezer, E.; Steingruber, M.; Milbradt, J.; Marschall, M.; Rawlinson, W.D. Human cytomegalovirus utilises cellular dual-specificity tyrosine phosphorylation-regulated kinases during placental replication. Placenta 2018, 72–73, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Hutterer, C.; Henry, C.; Hamilton, S.T.; Strojan, H.; Kraut, A.; Schulte, U.; Schütz, M.; Kohrt, S.; Wangen, C.; et al. Novel cytomegalovirus-inhibitory compounds of the class pyrrolopyridines show a complex pattern of target binding that suggests an unusual mechanism of antiviral activity. Antivir. Res. 2018, 159, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Fröhlich, T.; Frank, T.; Bertzbach, L.D.; Kohrt, S.; Kaufer, B.B.; Stamminger, T.; Tsogoeva, S.B.; Marschall, M. Artesunate-derived monomeric, dimeric and trimeric experimental drugs—Their unique mechanistic basis and pronounced antiherpesviral activity. Antivir. Res. 2018, 152, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, T.; Hahn, F.; Belmudes, L.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Couté, Y.; Marschall, M.; Tsogoeva, S.B. Synthesis of artemisinin-derived dimers, trimers and dendrimers: Investigation of their antimalarial and antiviral activities including putative mechanisms of action. Chemistry 2018, 24, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Milbradt, J.; Hamilton, S.; Zaja, M.; Leban, J.; Henry, C.; Vitt, D.; Steingruber, M.; Sonntag, E.; Zeitträger, I.; et al. Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpesviral activity. Antivir. Res. 2017, 143, 113–121. [Google Scholar] [CrossRef]
- Held, F.E.; Guryev, A.A.; Fröhlich, T.; Hampel, F.; Kahnt, A.; Hutterer, C.; Steingruber, M.; Bahsi, H.; von Bojničić-Kninski, C.; Mattes, D.S.; et al. Facile access to potent antiviral quinazoline heterocycles with fluorescence properties via merging metal-free domino reactions. Nat. Commun. 2017, 8, 15071. [Google Scholar] [CrossRef]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Fröhlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeitträger, I.; Wagner, S.; Einsiedel, J.; et al. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-κB) pathway by targeting RelA/p65. Antivir. Res. 2015, 124, 101–109. [Google Scholar] [CrossRef]
- Hutterer, C.; Eickhoff, J.; Milbradt, J.; Korn, K.; Zeitträger, I.; Bahsi, H.; Wagner, S.; Zischinsky, G.; Wolf, A.; Degenhart, C.; et al. A novel CDK7 inhibitor of the Pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrob. Agents Chemother. 2015, 59, 2062–2071. [Google Scholar] [CrossRef]
- Baumgartner, R.; Walloschek, M.; Kralik, M.; Gotschlich, A.; Tasler, S.; Mies, J.; Leban, J. Dual binding mode of a novel series of DHODH inhibitors. J. Med. Chem. 2006, 49, 1239–1247. [Google Scholar] [CrossRef]
- Leban, J.; Kralik, M.; Mies, J.; Gassen, M.; Tentschert, K.; Baumgartner, R. SAR, species specificity, and cellular activity of cyclopentene dicarboxylic acid amides as DHODH inhibitors. Bioorganic Med. Chem. Lett. 2005, 15, 4854–4857. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. Bull. Eur. Mal. Transm. Eur. Commun. Dis. Bull. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, C.; Gilg, A.; Gross, R.; Schutz, D.; Preising, N.; Standker, L.; Jahrsdorfer, B.; Schrezenmeier, H.; Sparrer, K.M.J.; Stamminger, T.; et al. An enzyme-based immunodetection assay to quantify SARS-CoV-2 infection. Antivir. Res. 2020, 181, 104882. [Google Scholar] [CrossRef] [PubMed]
- Brachs, S.; Lang, C.; Buslei, R.; Purohit, P.; Fürnrohr, B.; Kalbacher, H.; Jäck, H.M.; Mielenz, D. Monoclonal antibodies to discriminate the EF hand containing calcium binding adaptor proteins EFhd1 and EFhd2. Monoclon. Antibodies Immunodiagn. Immunother. 2013, 32, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Schonborn, J.; Oberstrass, J.; Breyel, E.; Tittgen, J.; Schumacher, J.; Lukacs, N. Monoclonal antibodies to double-stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 1991, 19, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Pirici, D.; Mogoanta, L.; Kumar-Singh, S.; Pirici, I.; Margaritescu, C.; Simionescu, C.; Stanescu, R. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J. Histochem. Cytochem. 2009, 57, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Hage, S.; Sonntag, E.; Borst, E.M.; Tannig, P.; Seyler, L.; Bauerle, T.; Bailer, S.M.; Lee, C.P.; Muller, R.; Wangen, C.; et al. Patterns of autologous and nonautologous interactions between core nuclear egress complex (NEC) proteins of alpha-, beta- and gamma-herpesviruses. Viruses 2020, 12, 303. [Google Scholar] [CrossRef]
- Sonntag, E.; Hamilton, S.T.; Bahsi, H.; Wagner, S.; Jonjic, S.; Rawlinson, W.D.; Marschall, M.; Milbradt, J. Cytomegalovirus pUL50 is the multi-interacting determinant of the core nuclear egress complex (NEC) that recruits cellular accessory NEC components. J. Gen. Virol. 2016, 97, 1676–1685. [Google Scholar] [CrossRef]
- Auerochs, S.; Korn, K.; Marschall, M. A reporter system for Epstein-Barr virus (EBV) lytic replication: Anti-EBV activity of the broad anti-herpesviral drug artesunate. J. Virol. Methods 2011, 173, 334–339. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res. 2011, 92, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.; Freitag, M.; Weiler, S.; Sorg, G.; Stamminger, T. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob. Agents Chemother. 2000, 44, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; König, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dörnenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, T.; Lohmann, V.; Kaul, A.; Krieger, N.; Rinck, G.; Rutter, G.; Strand, D.; Bartenschlager, R. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 2002, 76, 4008–4021. [Google Scholar] [CrossRef] [PubMed]
- Buckheit, R.W., Jr.; Swanstrom, R. Characterization of an HIV-1 isolate displaying an apparent absence of virion-associated reverse transcriptase activity. AIDS Res. Hum. Retrovir. 1991, 7, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Gilles, M.; Barral, K.; Nougairede, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef] [PubMed]
- Pruijssers, A.J.; George, A.S.; Schafer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H., 3rd; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef]
- Gendrot, M.; Andreani, J.; Boxberger, M.; Jardot, P.; Fonta, I.; Le Bideau, M.; Duflot, I.; Mosnier, J.; Rolland, C.; Bogreau, H.; et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med. Infect. Dis. 2020, 37, 101873. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cubitt, B.; Cai, Y.; Kuhn, J.H.; Vitt, D.; Kohlhof, H.; de la Torre, J.C. Novel dihydroorotate dehydrogenase inhibitors with potent interferon-independent antiviral activity against mammarenaviruses in vitro. Viruses 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Muehler, A.; Peelen, E.; Kohlhof, H.; Gröppel, M.; Vitt, D. Vidofludimus calcium, a next generation DHODH inhibitor for the treatment of relapsing-remitting multiple sclerosis. Multiple Scler. Relat. Disord. 2020, 43, 102129. [Google Scholar] [CrossRef] [PubMed]
- Jatana, S.; Homer, C.R.; Madajka, M.; Ponti, A.K.; Kabi, A.; Papay, F.; McDonald, C. Pyrimidine synthesis inhibition enhances cutaneous defenses against antibiotic resistant bacteria through activation of NOD2 signaling. Sci. Rep. 2018, 8, 8708. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Hourani, M.; Dauzonne, D.; Munier-Lehmann, H.; Khiar, S.; Nisole, S.; Dairou, J.; Helynck, O.; Afonso, P.V.; Tangy, F.; Vidalain, P.O. Original chemical series of pyrimidine biosynthesis inhibitors that boost the antiviral interferon response. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Deml, L.; Hofmann, C.; Small, J.S.; Groeppel, M.; Hamm, S.; Lemstra, S.; Leban, J.; Ammendola, A. 4SC-101, a novel immunosuppressive drug, inhibits IL-17 and attenuates colitis in two murine models of inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1763–1777. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, O.P.; Sayyed, S.G.; Kantner, C.; Ryu, M.; Schnurr, M.; Sárdy, M.; Leban, J.; Jankowsky, R.; Ammendola, A.; Doblhofer, R.; et al. 4SC-101, a novel small molecule dihydroorotate dehydrogenase inhibitor, suppresses systemic lupus erythematosus in MRL-(Fas)lpr mice. Am. J. Pathol. 2010, 176, 2840–2847. [Google Scholar] [CrossRef]
- Diedrichs-Möhring, M.; Niesik, S.; Priglinger, C.S.; Thurau, S.R.; Obermayr, F.; Sperl, S.; Wildner, G. Intraocular DHODH-inhibitor PP-001 suppresses relapsing experimental uveitis and cytokine production of human lymphocytes, but not of RPE cells. J. Neuroinflamm. 2018, 15, 54. [Google Scholar] [CrossRef]
- Shen, W.; Ren, X.; Zhu, J.; Xu, Y.; Lin, J.; Li, Y.; Zhao, F.; Zheng, H.; Li, R.; Cui, X.; et al. Discovery of a new structural class of competitive hDHODH inhibitors with in vitro and in vivo anti-inflammatory, immunosuppressive effects. Eur. J. Pharmacol. 2016, 791, 205–212. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5. [Google Scholar] [CrossRef]

| Cells | SARS-CoV-2 Isolate | MOI | Assay | Cmp | EC50 (µM) | CC50 (µM) | SI50 | EC90 (µM) | CC90 (µM) | SI90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Vero 76b | USA_WA1/2020 | 0.002 | VYR | IMU-838 | - | - | - | 6.2 ± 1.9 | >100 e | >16.1 |
| TFNM | - | - | - | 14 | - | - | ||||
| Vero 76c | MUC-IMB-1 | 0.0002 | RT-qPCR | IMU-838 | 10.0 ± 9.0 | 88.1 ± 3.7 | >10.0 | - | - | - |
| Vero B4c | MUC-IMB-1 | 0.0002 | RT-qPCR | IMU-838 | 6.8 ± 3.8 f 6.0 ± 5.0 g | >100 | >14.7 | - | - | - |
| CaCo-2d | MUC-IMB-1 | 0.001 | VYR | IMU-838 | 7.5–15 | >60 e | >4–8 | 15–30 | >60 e | >2–4 |
| Virus a | Family | Cells b | Assay c | Drug d | EC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|---|---|---|---|
| HCMV | Herpesviridae | HFF | GFP-based replication assay | Vido | 7.4 ± 0.1 | >100 | >13.5 |
| GCV | 1.69 ± 0.8 | >15 e | >8.8 | ||||
| HCV | Flaviviridae | Huh7 | Luc reporter assay | Vido | 5.9 (4.5–7.7) | >30 | >5.1 |
| rIFNα-2b f | 0.21 (0.17–0.26) | 2 | >9.5 | ||||
| HIV-1 | Retroviridae | Human PBMC | RT assay | Vido | 2.1 (1.0–4.4) | >100 | >47.5 |
| AZT | 0.38 × 10−3 (0.20 × 10−3– 0.63 × 10−3) | >1 | >2655 | ||||
| p24 ELISA | Vido | 1.3 (0.6–2.7) | >100 | >76.1 | |||
| AZT | 0.27 × 10−3 (0.10 × 10−3– 0.53 × 10−3) | >1 | >3693 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, F.; Wangen, C.; Häge, S.; Peter, A.S.; Dobler, G.; Hurst, B.; Julander, J.; Fuchs, J.; Ruzsics, Z.; Überla, K.; et al. IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro. Viruses 2020, 12, 1394. https://doi.org/10.3390/v12121394
Hahn F, Wangen C, Häge S, Peter AS, Dobler G, Hurst B, Julander J, Fuchs J, Ruzsics Z, Überla K, et al. IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro. Viruses. 2020; 12(12):1394. https://doi.org/10.3390/v12121394
Chicago/Turabian StyleHahn, Friedrich, Christina Wangen, Sigrun Häge, Antonia Sophia Peter, Gerhard Dobler, Brett Hurst, Justin Julander, Jonas Fuchs, Zsolt Ruzsics, Klaus Überla, and et al. 2020. "IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro" Viruses 12, no. 12: 1394. https://doi.org/10.3390/v12121394
APA StyleHahn, F., Wangen, C., Häge, S., Peter, A. S., Dobler, G., Hurst, B., Julander, J., Fuchs, J., Ruzsics, Z., Überla, K., Jäck, H.-M., Ptak, R., Muehler, A., Gröppel, M., Vitt, D., Peelen, E., Kohlhof, H., & Marschall, M. (2020). IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro. Viruses, 12(12), 1394. https://doi.org/10.3390/v12121394






