Modulating Effects of L-Arginine and Tribulus terrestris Extract on Fipronil-Induced Interference in the Male Reproductive System of Rats: Antioxidant Potential, Androgen Receptors, and Nitric Oxide Synthase Interplay
Abstract
1. Introduction
2. Materials and Methods
2.1. Tribulus Terrestris Extract Preparation
2.1.1. Plant Extraction
2.1.2. LC-MS/MS Analysis for Compound Characterization
- Instruments and Acquisition Method
- 2.
- LC-MS Data Processing
2.2. Animals
2.3. Experimental Design
2.4. Body Weights
2.5. Male Rat Sexual Behavioral Test
2.6. Sample Collection
2.7. Hormonal and Biochemical Analysis
2.8. Histopathology
2.9. Immunohistochemistry for PCNA Detection
2.10. Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. LC-MS/MS Analysis of TT
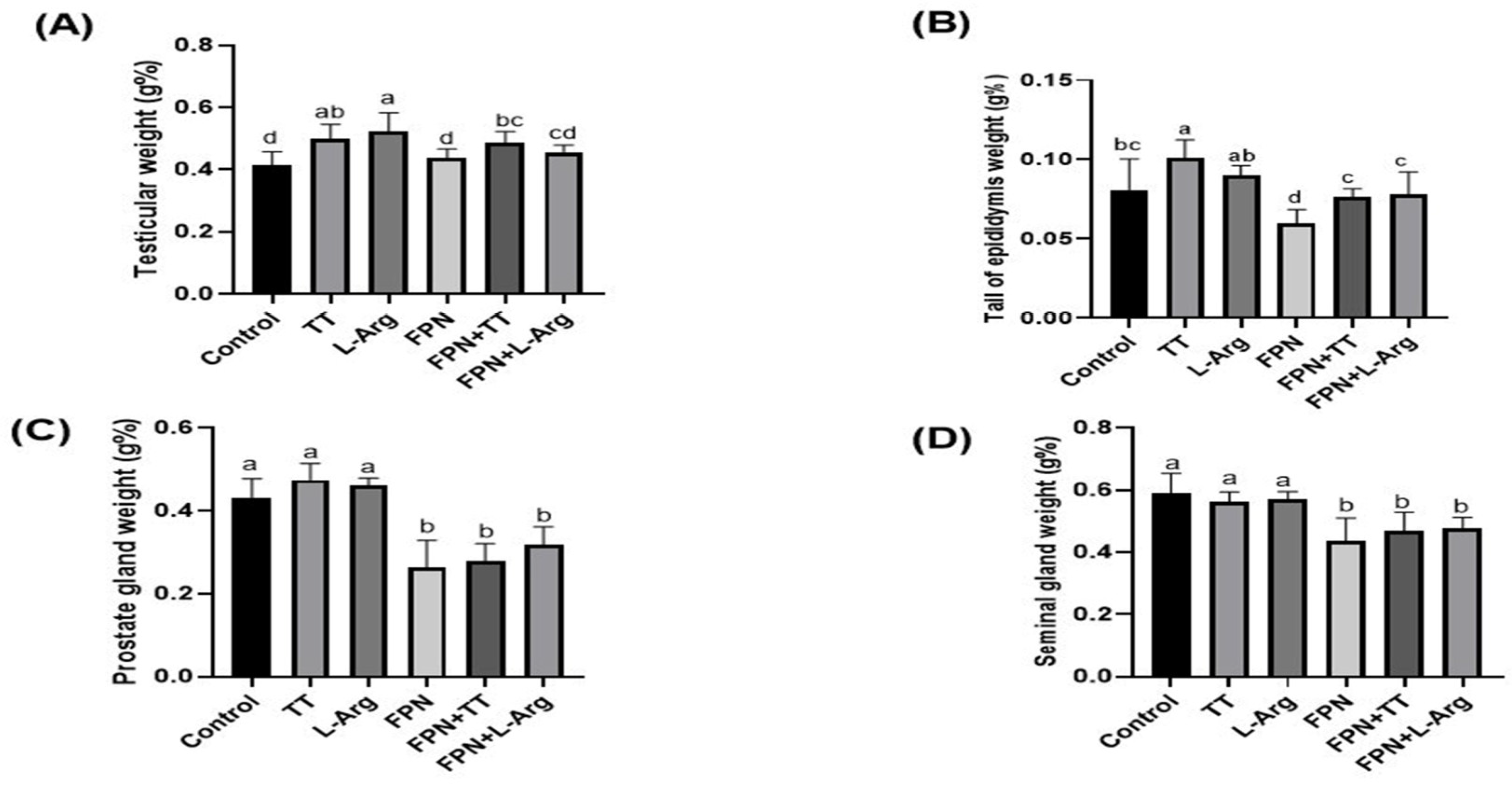
3.2. Body and Relative Sex Organ Weights
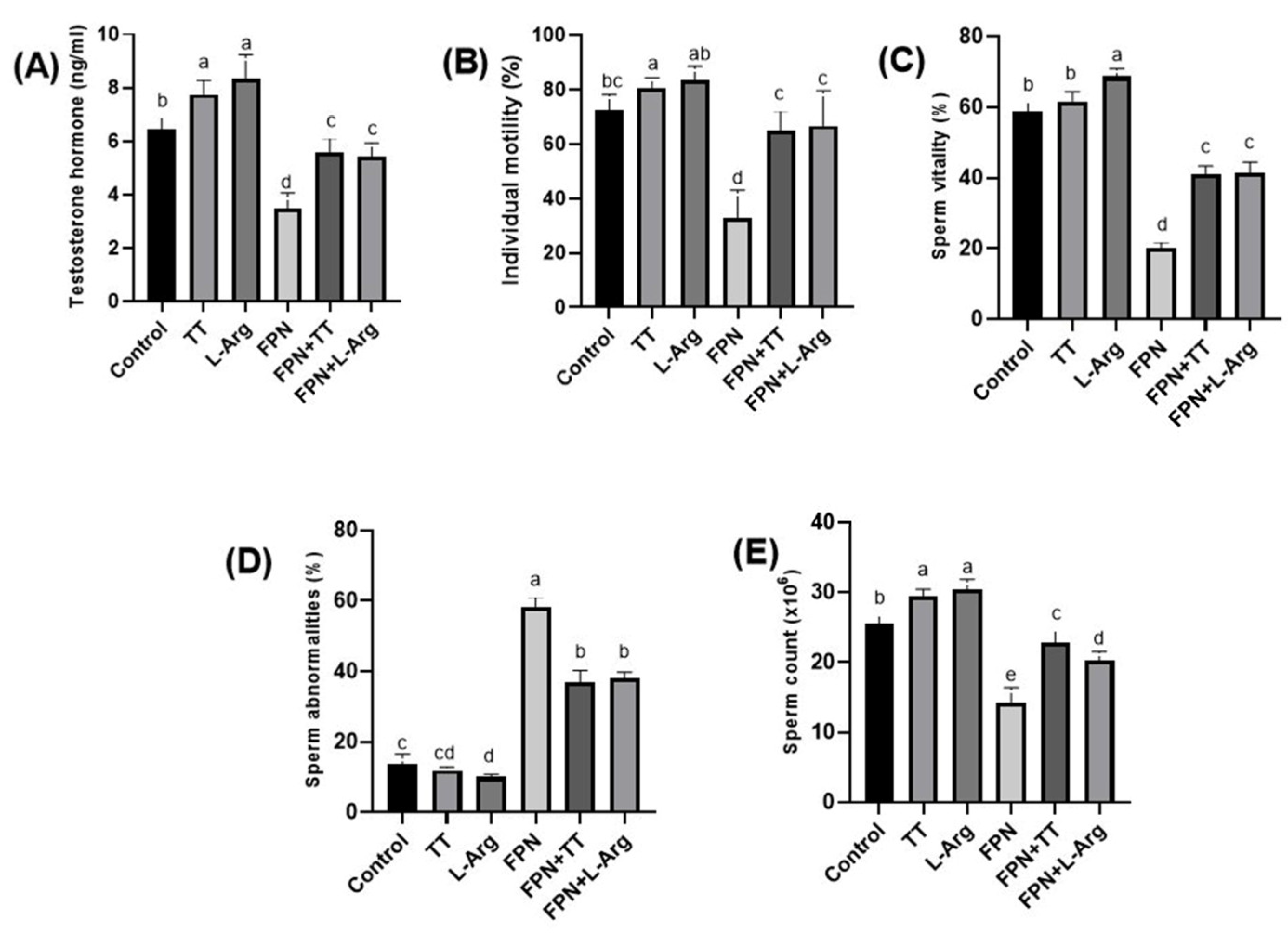
3.3. Testosterone Hormone and Semen Criteria
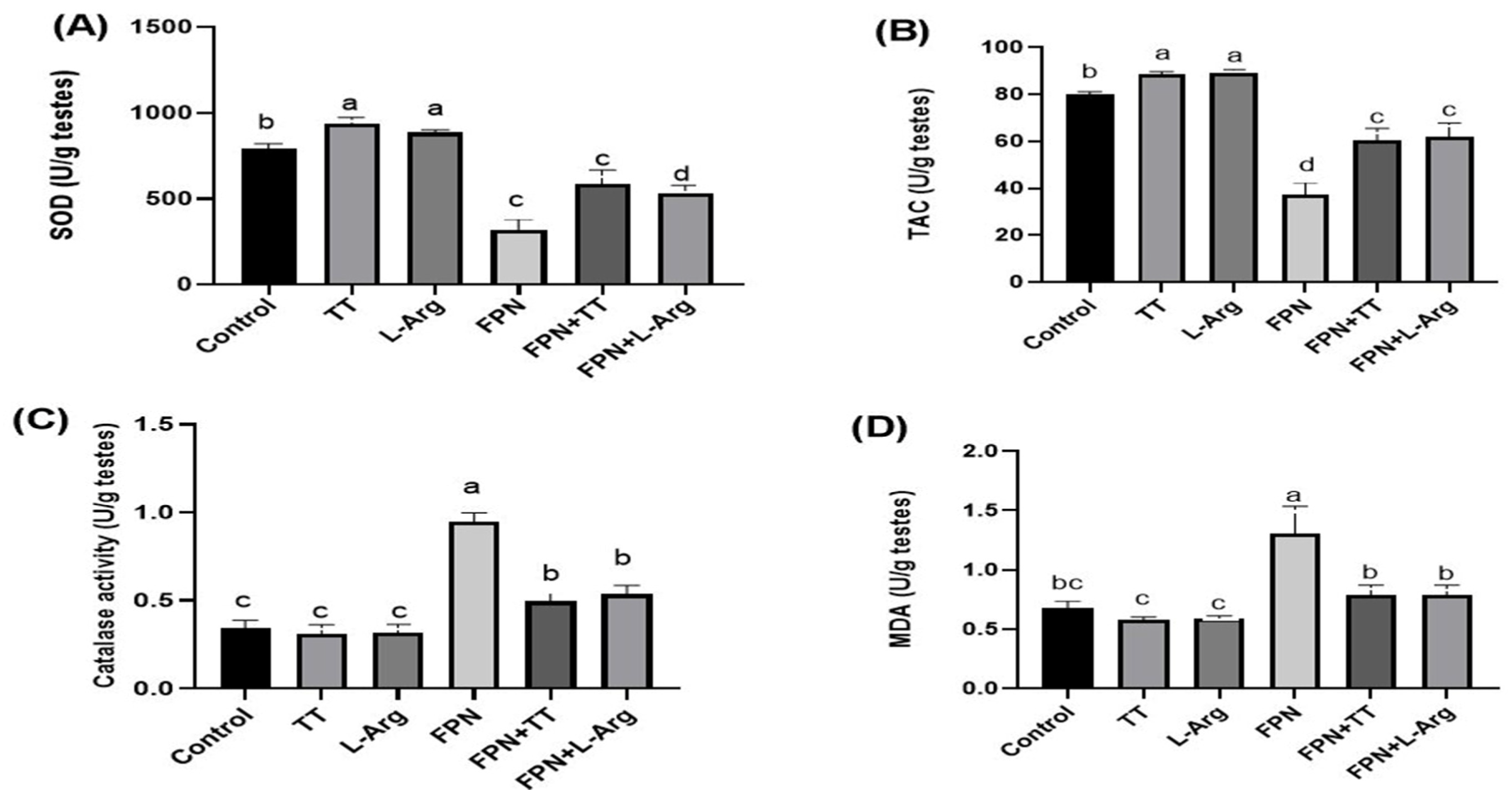
3.4. Oxidant and Antioxidant Markers
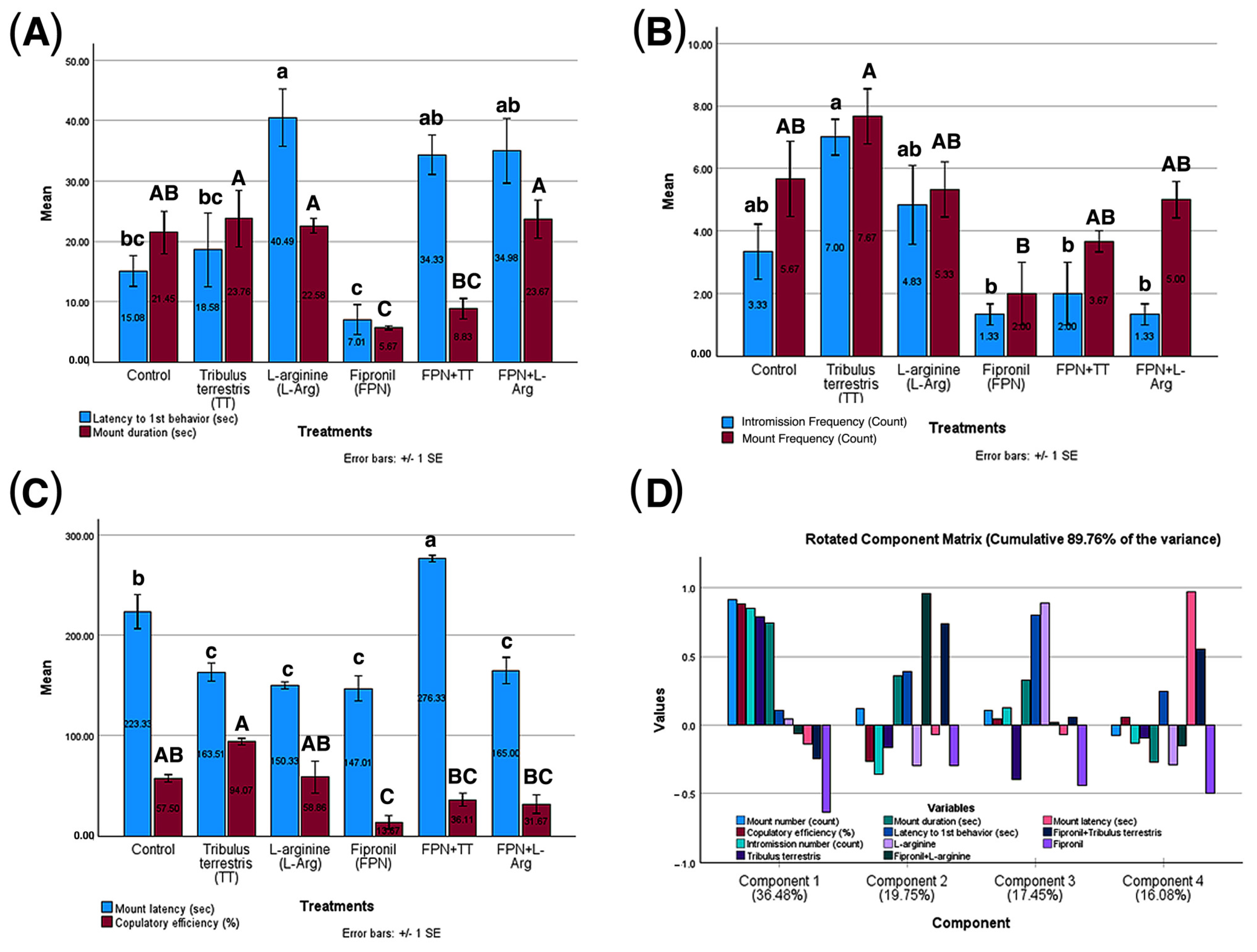
3.5. Results of Male Rats’ Sexual Behavior in Different Treatment Groups
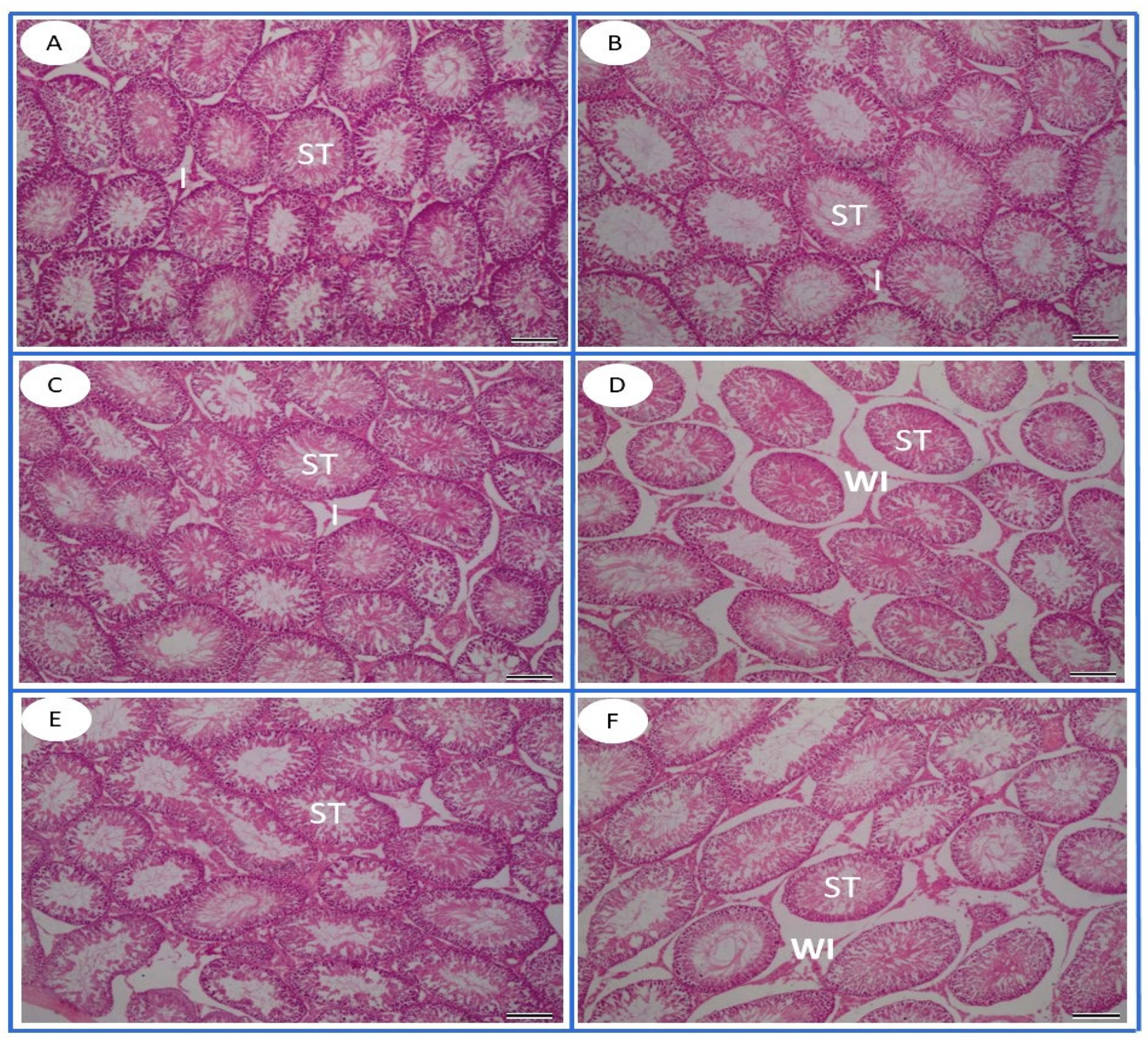
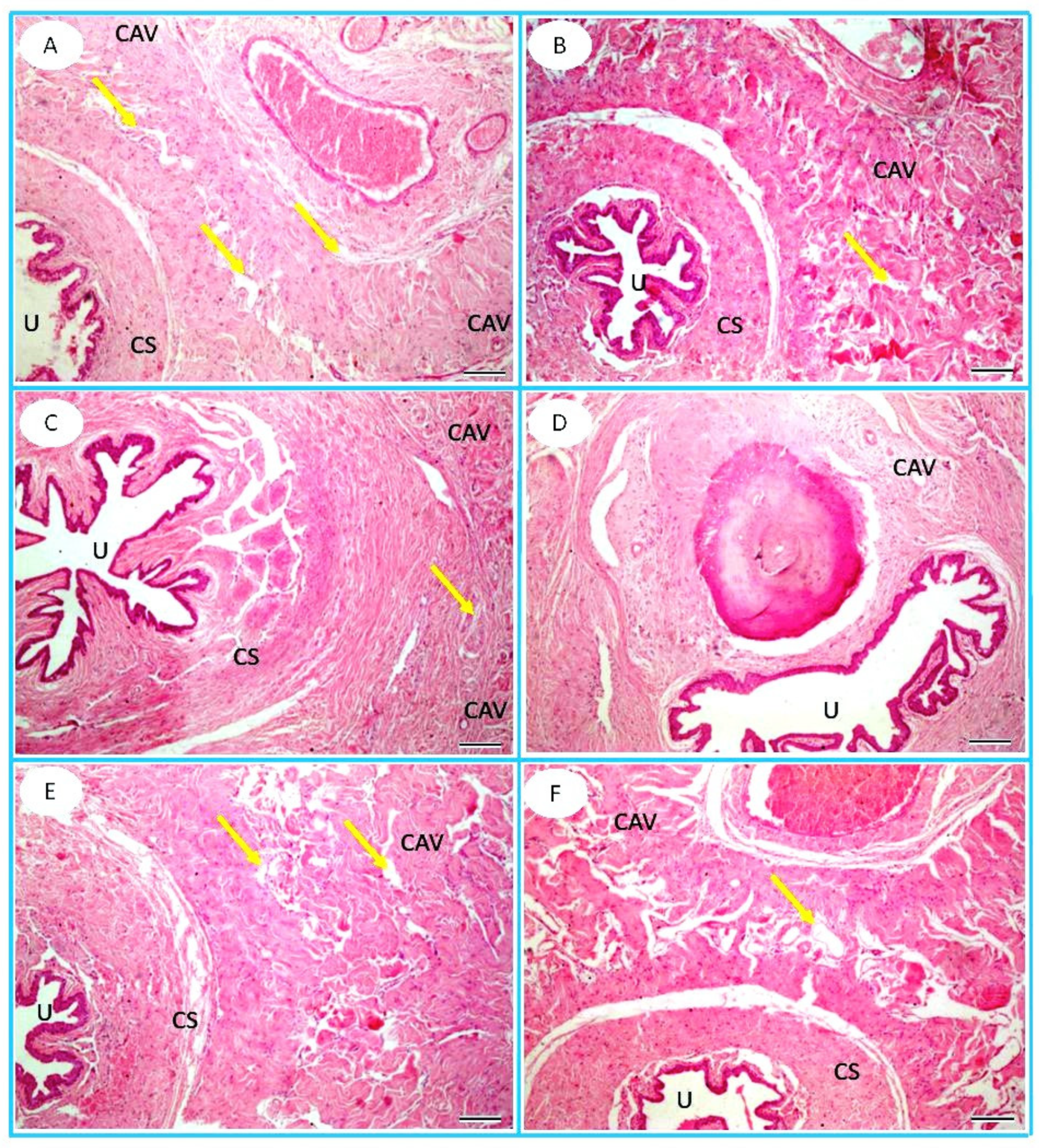
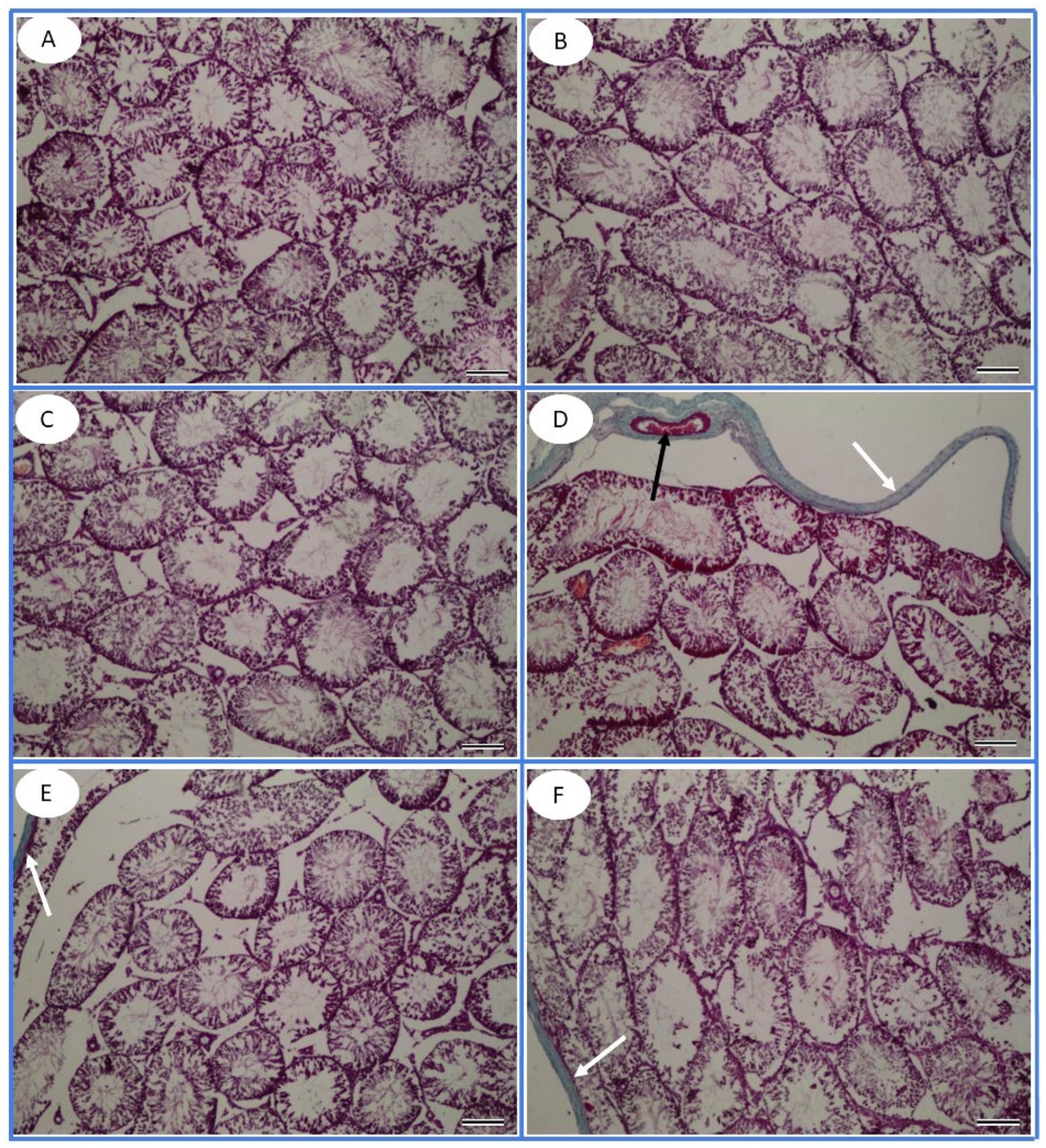
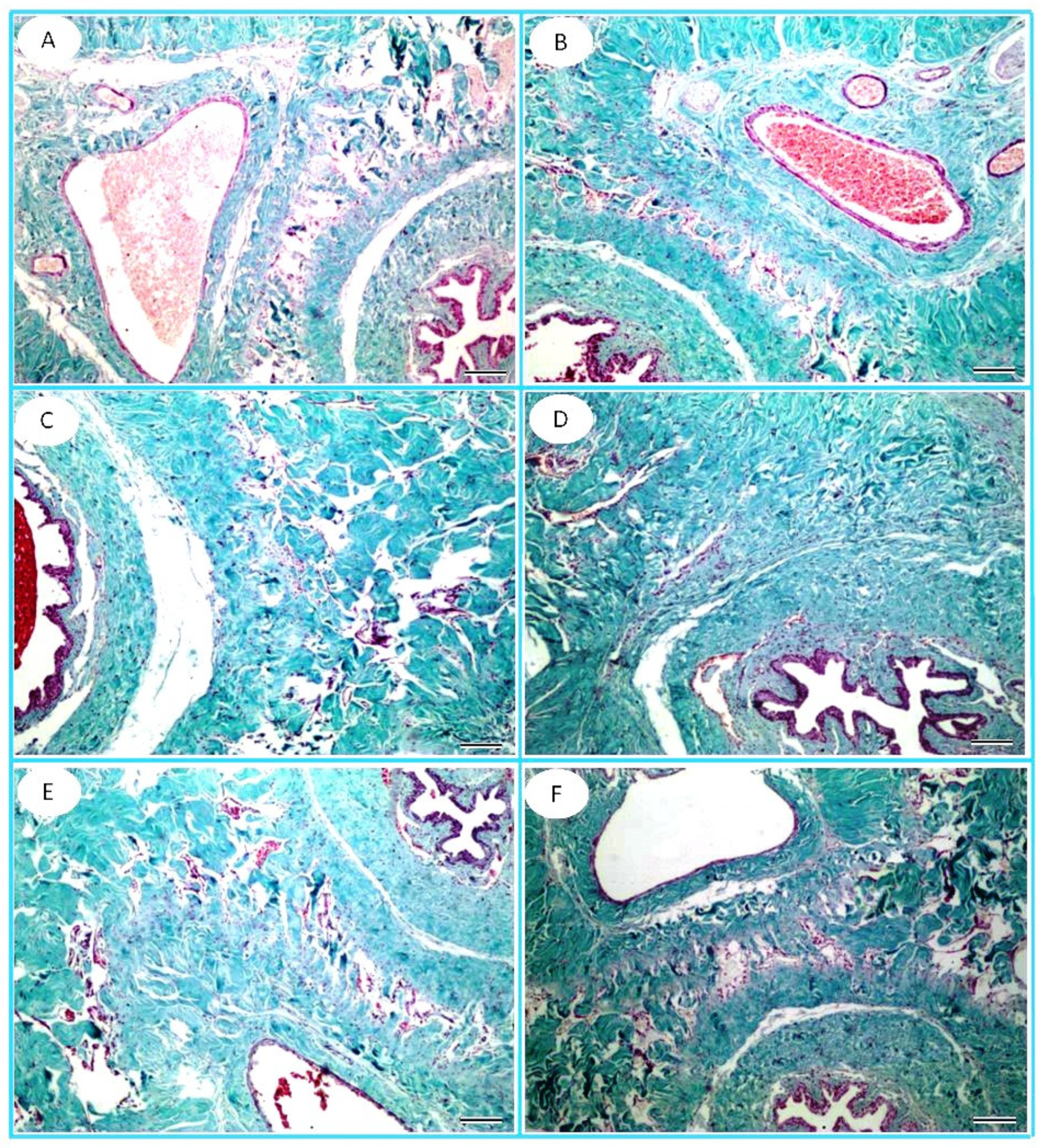
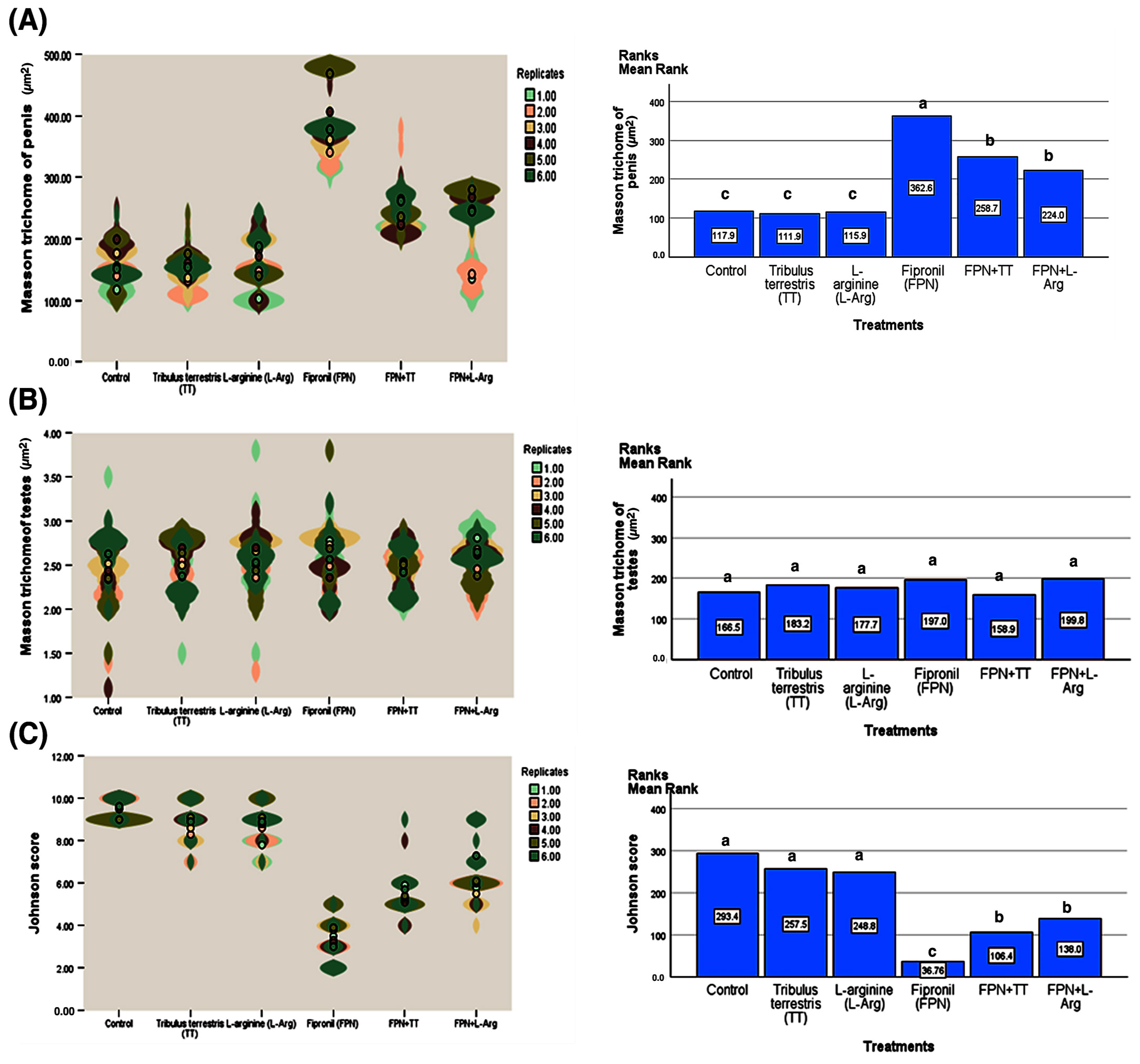
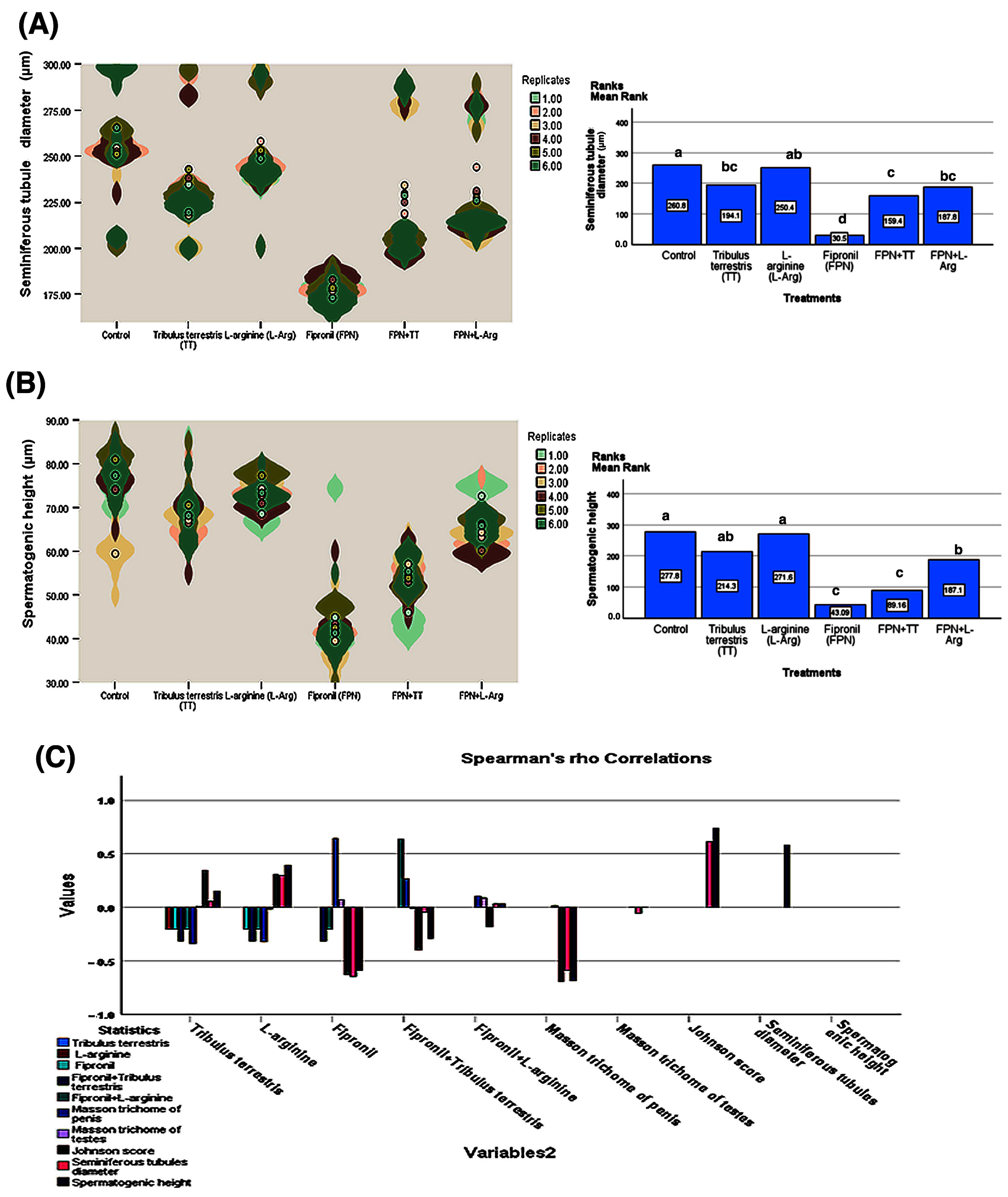
3.6. Histopathological Observation
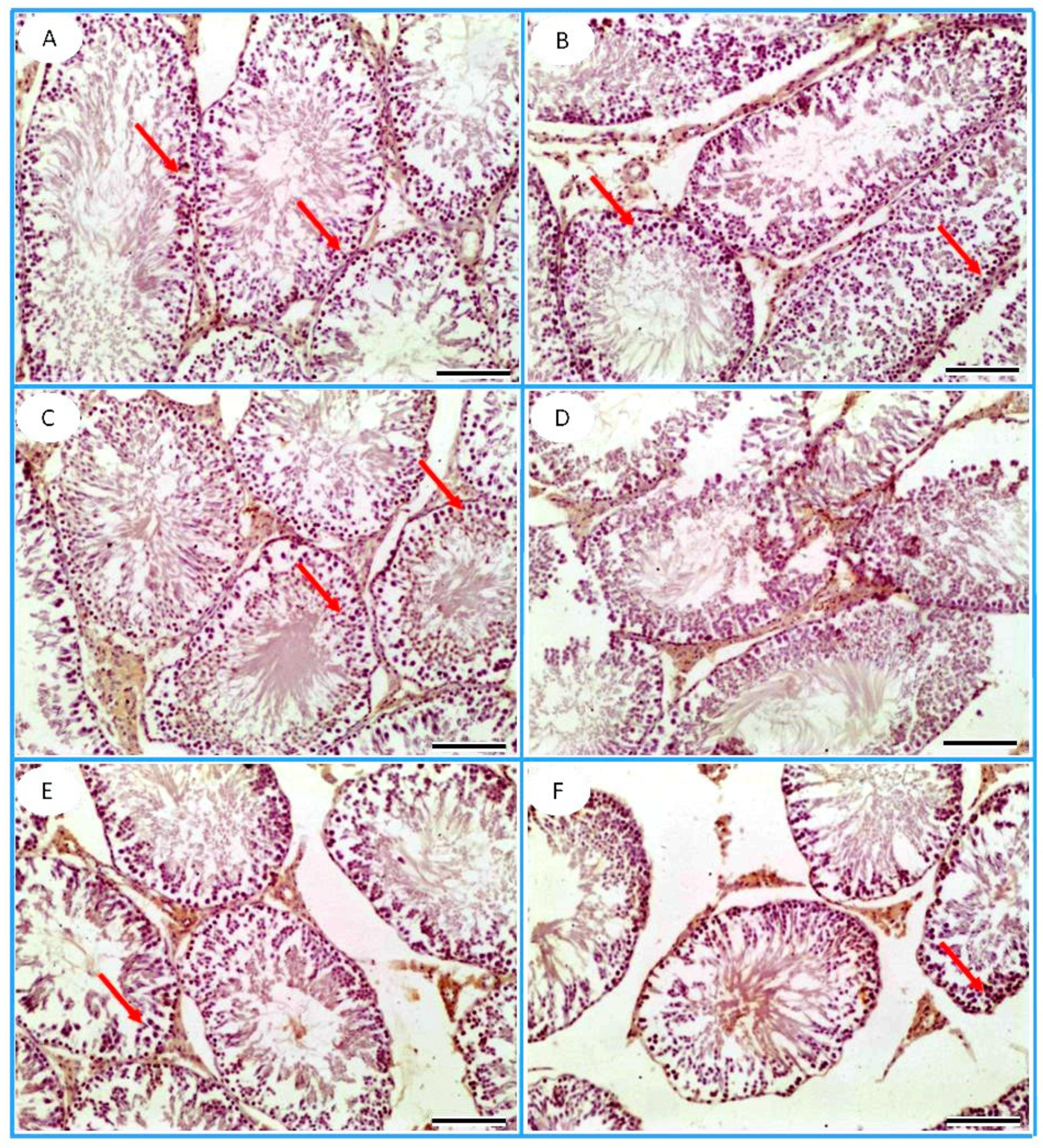
3.7. Immunohistochemistry
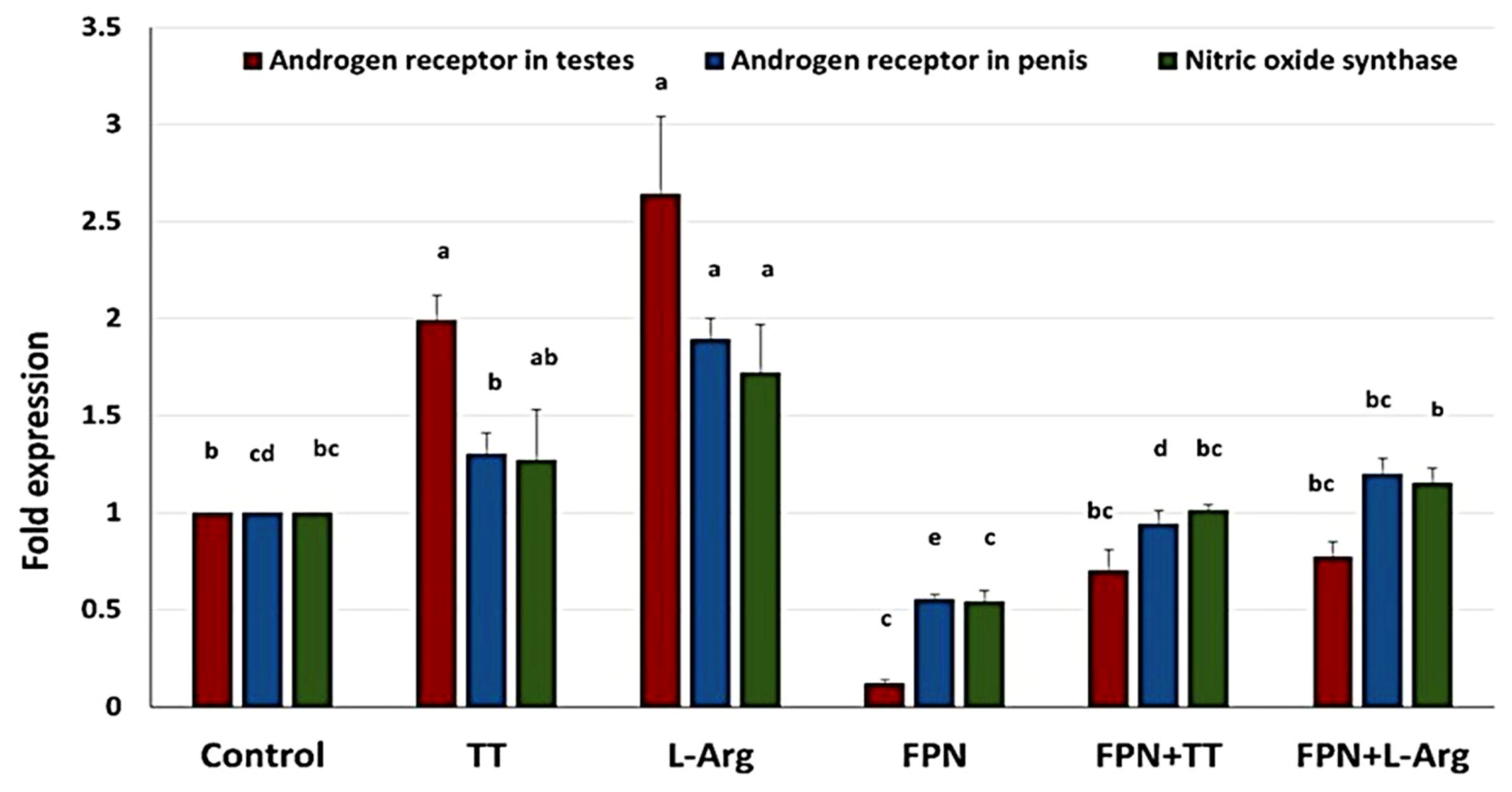
3.8. Quantitative Real-Time PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TT | Tribulus terrestris |
| L-Arg | L-Arginine |
| FPN | Fipronil |
| AR | Androgen Receptor |
| NOS | Nitric Oxide Synthase |
| FBW | Final Body Weight |
| WG | Weight Gain |
| SOD | Superoxide Dismutase |
| TAC | Total Antioxidant Capacity |
| MDA | Malondialdehyde |
| PCNA | Proliferating Cell Nuclear Antigen |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| H&E | Hematoxylin and Eosin |
| LH | Luteinizing Hormone |
| NO | Nitric Oxide |
References
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ. 2024, 915, 170113. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.K.; Ali, A.A.; Abdelrazek, H.M.; Aldayel, T.S.; Abdel-Daim, M.M.; El-Menyawy, M.A.I. Neurotoxic effect of fipronil in male Wistar rats: Ameliorative effect of L-arginine and L-carnitine. Biology 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-F.; Cheng, Y.-H.; Tung, C.-W.; Wang, C.-C. Fipronil disturbs the antigen-specific immune responses and GABAergic gene expression in the ovalbumin-immunized BALB/c mice. BMC Vet. Res. 2024, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, A.S.; Truong, T.; Goh, K.S.; Spurlock, F.; Tjeerdema, R.S. Environmental fate and toxicology of fipronil. J. Pestic. Sci. 2007, 32, 189–199. [Google Scholar] [CrossRef]
- Aajoud, A.; Ravanel, P.; Tissut, M. Fipronil metabolism and dissipation in a simplified aquatic ecosystem. J. Agric. Food Chem. 2003, 51, 1347–1352. [Google Scholar] [CrossRef]
- De Barros, A.L.; Bae, J.H.; Borges, C.S.; Rosa, J.L.; Cavariani, M.M.; Silva, P.V.; Pinheiro, P.F.; Anselmo-Franci, J.A.; Arena, A.C. Perinatal exposure to insecticide fipronil: Effects on the reproductive system in male rats. Reprod. Fertil. Dev. 2017, 29, 1130–1143. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Chakraborty, S.; Choudhury, A.P.; Das, A.; Jha, N.K.; Slama, P.; Nath, M.; Massanyi, P.; Ruokolainen, J.; Kesari, K.K. Environmental factors-induced oxidative stress: Hormonal and molecular pathway disruptions in hypogonadism and erectile dysfunction. J. Antioxid. 2021, 10, 837. [Google Scholar] [CrossRef]
- Tohamy, H.G.; El-Kazaz, S.E.; Alotaibi, S.S.; Ibrahiem, H.S.; Shukry, M.; Dawood, M.A. Ameliorative effects of boswellic acid on fipronil-induced toxicity: Antioxidant state, apoptotic markers, and testicular steroidogenic expression in male rats. Animals 2021, 11, 1302. [Google Scholar] [CrossRef]
- Uwamahoro, C.; Jo, J.-H.; Jang, S.-I.; Jung, E.-J.; Lee, W.-J.; Bae, J.-W.; Kwon, W.-S. Assessing the Risks of Pesticide Exposure: Implications for Endocrine Disruption and Male Fertility. Int. J. Mol. Sci. 2024, 25, 6945. [Google Scholar] [CrossRef]
- Rotimi, D.E.; Acho, M.A.; Falana, B.M.; Olaolu, T.D.; Mgbojikwe, I.; Ojo, O.A.; Adeyemi, O.S. Oxidative Stress-induced Hormonal Disruption in Male Reproduction. Reprod. Sci. 2024, 31, 2943–2956. [Google Scholar] [CrossRef]
- Sayed, M.M.; Abd el-Rady, N.M.; Gomaa, W.M.; Hosny, A.; Gomaa, A.M. Antioxidant, antiapoptotic, and antifibrotic abilities of L-Arginine ameliorate the testicular dysfunction in diabetic rats. Tissue Cell 2023, 82, 102036. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, Q.; Li, W.; Liu, M.; Li, Q.; Chen, Q. Efficacy of L-arginine and Pycnogenol® in the treatment of male erectile dysfunction: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1211720. [Google Scholar] [CrossRef]
- Husein, R.H.; Ahmed, M.O.; Muhammed, S.M. Effects of L-Arginine, vitamin E and their combinations on sperms morphology in albino male mice. J. Al-Nahrain Univ. 2011, 14, 137–143. [Google Scholar] [CrossRef]
- Aydin, S.; Inci, O.; Alagöl, B. The role of arginine, indomethacin and kallikrein in the treatment of oligoasthenospermia. Int. Urol. Nephrol. 1995, 27, 199–202. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Upadhyay, V.R.; Roy, A.; Pandita, S.; Raval, K.; Patoliya, P.; Ramesh, V.; Dewry, R.K.; Yadav, H.P.; Mohanty, T.; Bhakat, M. Optimized addition of nitric oxide compounds in semen extender improves post-thaw seminal attributes of Murrah buffaloes. Trop. Anim. Health Prod. 2023, 55, 47. [Google Scholar] [CrossRef]
- Menafra, D.; de Angelis, C.; Garifalos, F.; Mazzella, M.; Galdiero, G.; Piscopo, M.; Castoro, M.; Verde, N.; Pivonello, C.; Simeoli, C. Long-term high-dose l-arginine supplementation in patients with vasculogenic erectile dysfunction: A multicentre, double-blind, randomized, placebo-controlled clinical trial. J. Endocrinol. Investig. 2022, 45, 941–961. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Marmitt, D.J.; Cheng, Q.; Sun, W. Natural antioxidants of the underutilized and neglected plant species of Asia and South America. Lett. Drug Des. Discov. 2023, 20, 1512–1537. [Google Scholar] [CrossRef]
- Georgieva, T.; Hristov, K. Antioxidant Capacity of Extracts from Bulgarian Medicinal Plants. Tradit. Mod. Vet. Med. 2023, 8, 26–33. [Google Scholar]
- Singh, J.; Kaur, J.; Kaur, M.; Rana, A.; Rasane, P.; Kaur, S. Tribulus terrestris: Pharmacological and Nutraceutical Potential. In Harvesting Food from Weeds; Wiley: Hoboken, NJ, USA, 2023; pp. 79–112. [Google Scholar]
- Saeed, M.; Munawar, M.; Bi, J.B.; Ahmed, S.; Ahmad, M.Z.; Kamboh, A.A.; Arain, M.A.; Naveed, M.; Chen, H. Promising phytopharmacology, nutritional potential, health benefits, and traditional usage of Tribulus terrestris L. herb. Heliyon 2024, 10, e25549. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, J.; Dai, Y.; Tang, W.; Cao, H.; Chen, J. Iron indices and hemogram in renal anemia and the improvement with Tribulus terrestris green-formulated silver nanoparticles applied on rat model. Open Chem. 2024, 22, 20230212. [Google Scholar] [CrossRef]
- Tyagi, P.; Prasad, M.; Mathur, S.; Ranjan, R. Diosgenin biosynthesis investigation in medicinal herb (Tribulus terrestris) by transcriptome analysis. Gene 2024, 893, 147937. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, H.; Liu, J. Traditional Chinese medical therapy for erectile dysfunction. Transl. Androl. Urol. 2017, 6, 192. [Google Scholar] [CrossRef]
- Iqbal, T.; Altaf, S. Treatment of Male Infertility Disorders via Tribulus Terrestris and Multivitamins. Biol. Times 2023, 2, 12–13. [Google Scholar]
- Gauthaman, K.; Ganesan, A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction—An evaluation using primates, rabbit and rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
- Shah, S.M.Z.; Ramzan, M.; Khan, M.N.; Shadab, H.; Usman, M.; Rahman, S.; Ali, A.; Uddin, J.; Asmari, M.; Musharraf, S.G. Untargeted screening of plant metabolites based on data-independent and data-dependent acquisition modes using LC-ESI-QTOF-MS: Tribulus terrestris L. as a case study. Arab. J. Chem. 2023, 16, 104978. [Google Scholar] [CrossRef]
- Gad El-Hak, H.N.; Mahmoud, H.S.; Ahmed, E.A.; Elnegris, H.M.; Aldayel, T.S.; Abdelrazek, H.M.; Soliman, M.T.; El-Menyawy, M.A.I. Methanolic Phoenix dactylifera L. extract ameliorates cisplatin-induced hepatic injury in male rats. Nutrients 2022, 14, 1025. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some African plants belonging to Kalanchoe species: Antitrypanosomal, cytotoxic, antitopoisomerase I activities and chemical profiling using ultra-performance liquid chromatography/quadrupole-time-of-flight mass spect. Pharmacogn. Mag. 2021, 17, 6–15. [Google Scholar]
- Attallah, N.G.; Mokhtar, F.A.; Elekhnawy, E.; Heneidy, S.Z.; Ahmed, E.; Magdeldin, S.; Negm, W.A.; El-Kadem, A.H. Mechanistic insights on the in vitro antibacterial activity and in vivo hepatoprotective effects of salvinia auriculata aubl against methotrexate-induced liver injury. Pharmaceuticals 2022, 15, 549. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Abdel Monem, A.R.; Moussa, M.Y.; Abd-Elwahab, S.M.; El-Tanbouly, N.D. Comparative metabolite profiling of four citrus peel cultivars via ultra-performance liquid chromatography coupled with quadrupole-time-of-flight-mass spectrometry and multivariate data analyses. J. Chromatogr. Sci. 2019, 57, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Laboratory Animals; The National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Aldaddou, W.A.; Aljohani, A.S.M.; Ahmed, I.A.; Al-Wabel, N.A.; El-Ashmawy, I.M. Ameliorative effect of methanolic extract of Tribulus terrestris L. on nicotine and lead-induced degeneration of sperm quality in male rats. J. Ethnopharmacol. 2022, 295, 115337. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.; Shukla, G.; Wahi, A.K. Protective effect of L-arginine against necrosis and apoptosis induced by experimental ischemic and reperfusion in rat liver. Saudi J. Gastroenterol. 2009, 15, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Baum, M. Neuroendocrinology of sexual behavior in the male. Behav. Endocrinol. 2002, 2, 153–204. [Google Scholar]
- Huijgens, P.T.; Guarraci, F.A.; Olivier, J.D.A.; Snoeren, E.M.S. Male rat sexual behavior: Insights from inter-copulatory intervals. Behav. Process. 2021, 190, 104458. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. In Histopathology: Methods and Protocols; Humana: New York, NY, USA, 2014; pp. 31–43. [Google Scholar]
- Van De Vlekkert, D.; Machado, E.; d’Azzo, A.J.B.-p. Analysis of generalized fibrosis in mouse tissue sections with Masson’s trichrome staining. Bio-protocol 2020, 10, e3629. [Google Scholar] [CrossRef]
- Johnson, F.E.; Farr, S.A.; Mawad, M.; Woo, Y.C.S. Testicular cytotoxicity of intravenous methotrexate in rats. J. Surg. Oncol. 1994, 55, 175–178. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Abusaida, H.; Issa, Y.; Al-Sabaa, A.; Abdelgayed, S. Immunohistochemical expression of proliferating cell nuclear antigen (PCNA) in the parotid salivary glands of male albino rats after long administration of nutmeg. Res. J. Pharm. Biol. Chem. Sci. 2020, 7, 187–199. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Garza-Contreras, J.; Duong, P.; Snyder, B.D.; Schreihofer, D.A.; Cunningham, R.L. Presence of androgen receptor variant in neuronal lipid rafts. eNeuro 2017, 4, ENEURO.0109-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Shehab, N.G.; Omolaoye, T.S.; Du Plessis, S.S.; Rawat, S.S.; Naidoo, N.; Abushawish, K.Y.; Ahmed, A.; Alaa, B.; Ihsan, H.; Abdelhalim, M. Phytochemical Evaluation of Lepidium meyenii, Trigonella foenum-graecum, Spirulina platensis, and Tribulus arabica, and Their Potential Effect on Monosodium Glutamate Induced Male Reproductive Dysfunction in Adult Wistar Rats. Antioxidants 2024, 13, 939. [Google Scholar] [CrossRef]
- Alzahrani, K.J. Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis. Curr. Issues Mol. Biol. 2025, 47, 52. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Howell, S.; Teixeira, F.J. Beyond tribulus (Tribulus terrestris L.): The effects of phytotherapics on testosterone, sperm and prostate parameters. J. Ethnopharmacol. 2019, 235, 392–405. [Google Scholar] [CrossRef]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Rhees, R.W.; Kirk, B.A.; Sephton, S.; Lephart, E.D. Effects of prenatal testosterone on sexual behavior, reproductive morphology and LH secretion in the female rat. Dev. Neurosci. 1997, 19, 430–437. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. E. J. Med. 1993, 329, 2002–2012. [Google Scholar]
- Oyovwi, M.O.; Atere, A.D. Exploring the medicinal significance of l-Arginine mediated nitric oxide in preventing health disorders. Eur. J. Med. Chem. Rep. 2024, 12, 100175. [Google Scholar] [CrossRef]
- Kurhaluk, N. The effectiveness of L-arginine in clinical conditions associated with hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A comprehensive review of the phytochemical, pharmacological, and toxicological properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef]
- Pan, J.; Liu, P.; Yu, X.; Zhang, Z.; Liu, J. The adverse role of endocrine disrupting chemicals in the reproductive system. Front. Endocrinol. 2024, 14, 1324993. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.F. Introduction to Environmental Toxicology; CABI: Wallingford, UK, 2020. [Google Scholar]
- Moyad, M.A. Nutraceuticals for Fertility and Erectile Health: A Brief Overview of What Works and What Is Worthless. In Men’s Sexual Health and Fertility A Clinical Guide; Mulhall, J., Hsiao, W., Eds.; Springer: New York, NY, USA, 2014; pp. 183–228. [Google Scholar]
- Saleh, H.; Nassar, A.M.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.; Hassan, S.M.; Saati, A.A.; Hetta, H.F. Chemo-protective potential of cerium oxide nanoparticles against fipronil-induced oxidative stress, apoptosis, inflammation and reproductive dysfunction in male white albino rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Ranjan, R. Comparative study of the pharmacological, phytochemical and biotechnological aspects of Tribulus terrestris Linn. and Pedalium murex Linn: An overview. Acta Ecol. Sin. 2023, 43, 223–233. [Google Scholar] [CrossRef]
- Abdel-Rhman, A.; Morsy, W.; Selim, N.; Abdel-Hady, E.A. L-arginine supplementation attenuates ovarian oxidative stress in female rats subjected to chronic intermittent hypoxia. Physiol. Int. 2023, 110, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 807–816. [Google Scholar] [CrossRef]


| Gene | Sequence (5′–3′) | Annealing Temperature | Amplicon Size (bp) | Accession Number |
|---|---|---|---|---|
| AR [45] | F: 5′-AATGTACAGCCAGTGCGTGA-3′ | 57 | 248 | NM_012502.2 |
| R: 5′-TTGGTGAGCTGGTAGAAGCG-3′ | ||||
| NOS [46] | F: 5′-CATTGGAAGTGAAGCGTTTCG-3′ | 58 | 95 | L12562 |
| R: 5′-CAGCTGGGCTGTACAAACCTT-3′ | ||||
| β-actin [46] | F: 5′-AAGTCCCTCACCCTCCCAAAAG-3′ | 60 | 98 | V01217 |
| R: 5′-AAGCAATGCTGTCACCTTCCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, D.H.; Bakhashwain, A.A.; Ahmed, E.A.; Al-Abbadi, H.A.; Abdelrazek, H.M.A.; El-Menyawy, M.A.I.; Teleb, W.K.; Tawfik, N.M.; Helal, I.E.; EL-Hak, H.N.G. Modulating Effects of L-Arginine and Tribulus terrestris Extract on Fipronil-Induced Interference in the Male Reproductive System of Rats: Antioxidant Potential, Androgen Receptors, and Nitric Oxide Synthase Interplay. Toxics 2025, 13, 371. https://doi.org/10.3390/toxics13050371
Elsayed DH, Bakhashwain AA, Ahmed EA, Al-Abbadi HA, Abdelrazek HMA, El-Menyawy MAI, Teleb WK, Tawfik NM, Helal IE, EL-Hak HNG. Modulating Effects of L-Arginine and Tribulus terrestris Extract on Fipronil-Induced Interference in the Male Reproductive System of Rats: Antioxidant Potential, Androgen Receptors, and Nitric Oxide Synthase Interplay. Toxics. 2025; 13(5):371. https://doi.org/10.3390/toxics13050371
Chicago/Turabian StyleElsayed, Doaa H., Ahmed A. Bakhashwain, Eman A. Ahmed, Hatim A. Al-Abbadi, Heba M. A. Abdelrazek, Menna Allah I. El-Menyawy, Wafaa K. Teleb, Noran M. Tawfik, Ibrahim E. Helal, and Heba N. Gad EL-Hak. 2025. "Modulating Effects of L-Arginine and Tribulus terrestris Extract on Fipronil-Induced Interference in the Male Reproductive System of Rats: Antioxidant Potential, Androgen Receptors, and Nitric Oxide Synthase Interplay" Toxics 13, no. 5: 371. https://doi.org/10.3390/toxics13050371
APA StyleElsayed, D. H., Bakhashwain, A. A., Ahmed, E. A., Al-Abbadi, H. A., Abdelrazek, H. M. A., El-Menyawy, M. A. I., Teleb, W. K., Tawfik, N. M., Helal, I. E., & EL-Hak, H. N. G. (2025). Modulating Effects of L-Arginine and Tribulus terrestris Extract on Fipronil-Induced Interference in the Male Reproductive System of Rats: Antioxidant Potential, Androgen Receptors, and Nitric Oxide Synthase Interplay. Toxics, 13(5), 371. https://doi.org/10.3390/toxics13050371






