TD-ESI-MS/MS for High-Throughput Screening of 13 Common Drugs and 4 Etomidate Analogs in Hair: Method Validation and Forensic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Solution
2.3. Sample Preparation
2.4. Instrumentation Conditions
2.5. Method Validation
3. Results and Discussion
3.1. Validation of the TD-ESI-MS/MS Method for Qualitative Screening
3.2. Confirmatory Validation Using UPLC-MS/MS
3.3. Practical Forensic Applications
3.3.1. A Case Study: Concurrent Screening and Confirmation
3.3.2. Large-Scale Performance Evaluation
3.4. Methodological Advantages and Forensic Implications
3.5. Limitations of This Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Drug Report 2024. Available online: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2024.html (accessed on 23 March 2025).
- Uhm, J.; Hong, S.; Han, E. The need to monitor emerging issues in etomidate usage: The misuse or abuse potential. Forensic Sci. Med. Pathol. 2024, 20, 249–260. [Google Scholar] [CrossRef]
- Li, J.; Ling, J.; Cai, Z.; Liao, Y.; Xiang, P.; Liu, W.; Ding, Y. Rapid and sensitive detection of etomidate based on functionalized copper nanoclusters fluorescent probe. Forensic Sci. Int. 2024, 361, 112136. [Google Scholar] [CrossRef]
- Smedra, A.; Wochna, K.; Kazmierski, D.; Berent, J. Suicide committed by a paramedic using a cocktail of drugs: Morphine, etomidate, diazepam and rocuronium. Case report and review of literature. Leg. Med. 2021, 52, 101915. [Google Scholar] [CrossRef]
- Forman, S.A. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011, 114, 695–707. [Google Scholar] [CrossRef]
- Zellner, T.; Eyer, F.; Rabe, C.; Geith, S.; Haberl, B.; Schmoll, S. Recreational Drug Overdose—Clinical Value of Toxicological Analysis. Toxics 2024, 12, 662. [Google Scholar] [CrossRef]
- Jung, Y.-K.; You, S.Y.; Kim, S.-Y.; Kim, J.Y.; Paeng, K.-J. Simultaneous Determination of Etomidate and Its Major Metabolite, Etomidate Acid, in Urine Using Dilute and Shoot Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 4459. [Google Scholar] [CrossRef]
- Hasan, M.; Hofstetter, R.; Fassauer, G.M.; Link, A.; Siegmund, W.; Oswald, S. Quantitative chiral and achiral determination of ketamine and its metabolites by LC-MS/MS in human serum, urine and fecal samples. J. Pharm. Biomed. Anal. 2017, 139, 87–97. [Google Scholar] [CrossRef]
- Feltmann, K.; Hauspie, B.; Dirkx, N.; Elgán, T.H.; Beck, O.; Van Havere, T.; Gripenberg, J. Prevalence and Misreporting of Illicit Drug Use among Electronic Dance Music Festivals Attendees: A Comparative Study between Sweden and Belgium. Toxics 2024, 12, 635. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, L.; Zhao, J.; Qian, X.; Qiang, H.; Xiang, P.; Yan, H. Metabolic Profile of Etomidate and Its Three Analogs in Zebrafish, Human Liver Microsomes, Human Urine and Hair Samples Using UHPLC-Q Exactive Orbitrap-HRMS. Drug Test. Anal. 2025, 1–13. [Google Scholar] [CrossRef]
- Xu, S.; Ma, B.; Li, J.; Su, W.; Xu, T.; Zhang, M. Europium Nanoparticles-Based Fluorescence Immunochromatographic Detection of Three Abused Drugs in Hair. Toxics 2023, 11, 417. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, Z.C.F.; Ho, J.Y.M.; Yip, A.W.S.; Cheung, J.K.H.; Ho, K.K.L.; Duan, R.; Tsim, K.W.K. Surveillance of drug abuse in Hong Kong by hair analysis using LC-MS/MS. Drug Test. Anal. 2018, 10, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuo, Y.; Tang, X.; Qiang, H.; Liu, W.; Wu, H.; Xiang, P.; Duan, G.; Shen, M. Segmental analysis of antidepressant and antipsychotic drugs in the hair of schizophrenic patients. Drug Test. Anal. 2020, 12, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-Y.; Chen, Y.-L.; Chen, W.-C.; Chen, B.-H.; Huang, Y.-L. Rapid detection of illegal colorants on traditional Chinese pastries through mass spectrometry with an interchangeable thermal desorption electrospray ionization source. Food Chem. 2018, 252, 189–197. [Google Scholar] [CrossRef]
- Duvivier, W.F.; van Beek, T.A.; Pennings, E.J.M.; Nielen, M.W.F. Rapid analysis of Δ-9-tetrahydrocannabinol in hair using direct analysis in real time ambient ionization orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Feigel, B.; Adamowicz, P.; Wybraniec, S. Recent advances in analysis of new psychoactive substances by means of liquid chromatography coupled with low-resolution tandem mass spectrometry. Anal. Bioanal. Chem. 2024, 416, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.; Salomone, A.; Gerace, E.; Pirro, V. Role of LC-MS/MS in hair testing for the determination of common drugs of abuse and other psychoactive drugs. Bioanalysis 2013, 5, 1919–1938. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Zhao, K.; Wang, Y.; Liu, J.; Gu, J.; Bai, H.; Hasegawa, K.; Wurita, A. Detection and quantification of etomidate and metomidate in human hairs by ultraperformance liquid chromatography with triple quadrupole mass spectrometry (UPLC-MS/MS). Forensic Toxicol. 2024, 42, 232–241. [Google Scholar] [CrossRef]
- Park, Y.J.; Cho, E.; Kim, S.H.; Lee, H.; Jegal, H.; Park, M.; Choe, S.; Sim, Y.E.; Baek, S.H.; Kim, K.M.; et al. Determination of etomidate and etomidate acid in hair using liquid chromatography-tandem mass spectrometry. J. Forensic Sci. 2022, 67, 2479–2486. [Google Scholar] [CrossRef]
- Yum, H.; Jeong, S.; Jang, M.; Moon, S.; Kang, M.; Kim, B.; Kim, D.; Choe, S.; Yang, W.; Kim, J.; et al. Fast and reliable analysis of veterinary metomidate and etomidate in human blood samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in a postmortem case. J. Forensic Sci. 2021, 66, 2532–2538. [Google Scholar] [CrossRef]
- Poetzsch, M.; Steuer, A.E.; Roemmelt, A.T.; Baumgartner, M.R.; Kraemer, T. Single Hair Analysis of Small Molecules Using MALDI-Triple Quadrupole MS Imaging and LC-MS/MS: Investigations on Opportunities and Pitfalls. Anal. Chem. 2014, 86, 11758–11765. [Google Scholar] [CrossRef]
- Wei, Q.; Su, F.H. Determination of Nine Fentanyl Drugs in Hair Samples by GC-MS/MS and LC-MS/MS. ACS Omega 2022, 7, 19176–19182. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Golpe, M.; de-Castro-Rios, A.; Cruz, A.; Lopez-Rivadulla, M.; Lendoiro, E. Determination and Distribution of Cannabinoids in Nail and Hair Samples. J. Anal. Toxicol. 2021, 45, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Standard Practices for Method Validation in Forensic Toxicology. Available online: https://www.aafs.org/sites/default/files/media/documents/036_Std_e1.pdf (accessed on 23 March 2025).
- Maas, A.; Maier, C.; Iwersen-Bergmann, S.; Madea, B.; Hess, C. Simultaneous extraction of propofol and propofol glucuronide from hair followed by validated LC-MS/MS analyses. J. Pharm. Biomed. Anal. 2017, 146, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, T.; Tong, A.; Xu, Y.; Wu, F.; Wang, J.; Chen, X. Segmented detection of quetiapine fumarate in hairs by LC-MS/MS. Chin. J. Drug Depend. 2024, 33, 470–474. [Google Scholar]
- Yang, C.; Zhang, H.; Li, Q.; Gao, Z.; Hu, J.; Liu, B.; Deng, Z.; Liao, Y. Detection of drugs and their metabolites in hair by high-speed grinding pretreatment and LC-MS/MS. Chin. J. Anal. Lab. 2019, 38, 604–608. [Google Scholar]
- Zhang, X.; Chen, Y.; Liu, J.; Wang, M.; Dai, Y.; Zhao, K.; Gu, J.; Zhang, H.; Wurita, A.; Hasegawa, K. Identification of a novel imidazole-derived GABA agonist isopropoxate: Simultaneous detection and quantification of imidazole-derived analogs from human hairs in abused cases by LC-MS/MS. Forensic Toxicol. 2024. [Google Scholar] [CrossRef]
- Dolliver, D.S. A supply-based response to a demand-driven problem: A fifteen-year analysis of drug interdiction in Poland. Crime Law Soc. Change 2020, 73, 1–23. [Google Scholar] [CrossRef]
- Wang, C.; Lassi, N. Incentivizing Narcotics Control Through China’s Belt and Road Initiative in South and Southeast Asia. J. Dev. Soc. 2023, 39, 259–288. [Google Scholar] [CrossRef]
- Di Trana, A.; Pichini, S.; Pacifici, R.; Giorgetti, R.; Busardo, F.P. Synthetic Benzimidazole Opioids: The Emerging Health Challenge for European Drug Users. Front. Psychiatry 2022, 13, 858234. [Google Scholar] [CrossRef]
- Liu, L.; Wheeler, S.E.; Venkataramanan, R.; Rymer, J.A.; Pizon, A.F.; Lynch, M.J.; Tamama, K. Newly Emerging Drugs of Abuse and Their Detection Methods An ACLPS Critical Review. Am. J. Clin. Pathol. 2018, 149, 105–116. [Google Scholar] [CrossRef]
- Golladay, M.; Donner, K.; Nechuta, S. Using statewide death certificate data to understand trends and characteristics of polydrug overdose deaths in Tennessee, 2013–2017. Ann. Epidemiol. 2020, 41, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Scholl, L.; Hoots, B.; Seth, P. Nonfatal Drug and Polydrug Overdoses Treated in Emergency Departments-29 States, 2018–2019. Mmwr-Morb. Mortal. Wkly. Rep. 2020, 69, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Stefkova-Mazochova, K.; Danda, H.; Mazoch, V.; Olejnikova-Ladislavova, L.; Sichova, K.; Paskanova, N.; Vagnerova, M.; Jurasek, B.; Rysanek, P.; Sima, M.; et al. The acute effects of methoxphenidine on behaviour and pharmacokinetics profile in animal model. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 137, 111285. [Google Scholar] [CrossRef]
- Vari, M.R.; Ricci, G.; Cavallo, M.; Pichini, S.; Sirignano, A.; Graziano, S. Ketamine: From Prescription Anaesthetic to a New Psychoactive Substance. Curr. Pharm. Des. 2022, 28, 1213–1220. [Google Scholar] [CrossRef]
- Moore, K.; Dargan, P.I.; Wood, D.M.; Measham, F. Do Novel Psychoactive Substances Displace Established Club Drugs, Supplement Them or Act as Drugs of Initiation? The relationship between Mephedrone, Ecstasy and Cocaine. Eur. Addict. Res. 2013, 19, 276–282. [Google Scholar] [CrossRef]
- Palamar, J.J.; Barratt, M.J.; Ferris, J.A.; Winstock, A.R. Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am. J. Addict. 2016, 25, 400–407. [Google Scholar] [CrossRef]
- Cheng, T.; Kerr, T.; Small, W.; Dong, H.; Montaner, J.; Wood, E.; DeBeck, K. High Prevalence of Assisted Injection Among Street-Involved Youth in a Canadian Setting. AIDS Behav. 2016, 20, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Nawi, A.M.; Ismail, R.; Ibrahim, F.; Hassan, M.R.; Manaf, M.R.A.; Amit, N.; Ibrahim, N.; Shafurdin, N.S. Risk and protective factors of drug abuse among adolescents: A systematic review. BMC Public Health 2021, 21, 2088. [Google Scholar] [CrossRef]
- Gao, W.; Schmidt, K.; Enge, S.; Kirschbaum, C. Intra-individual stability of hair endocannabinoid and N-acylethanolamine concentrations. Psychoneuroendocrinology 2021, 133, 105395. [Google Scholar] [CrossRef]
- Kintz, P. Evidence of 2 Populations of Mephedrone Abusers by Hair Testing. Application to 4 Forensic Expertises. Curr. Neuropharmacol. 2017, 15, 658–662. [Google Scholar] [CrossRef]
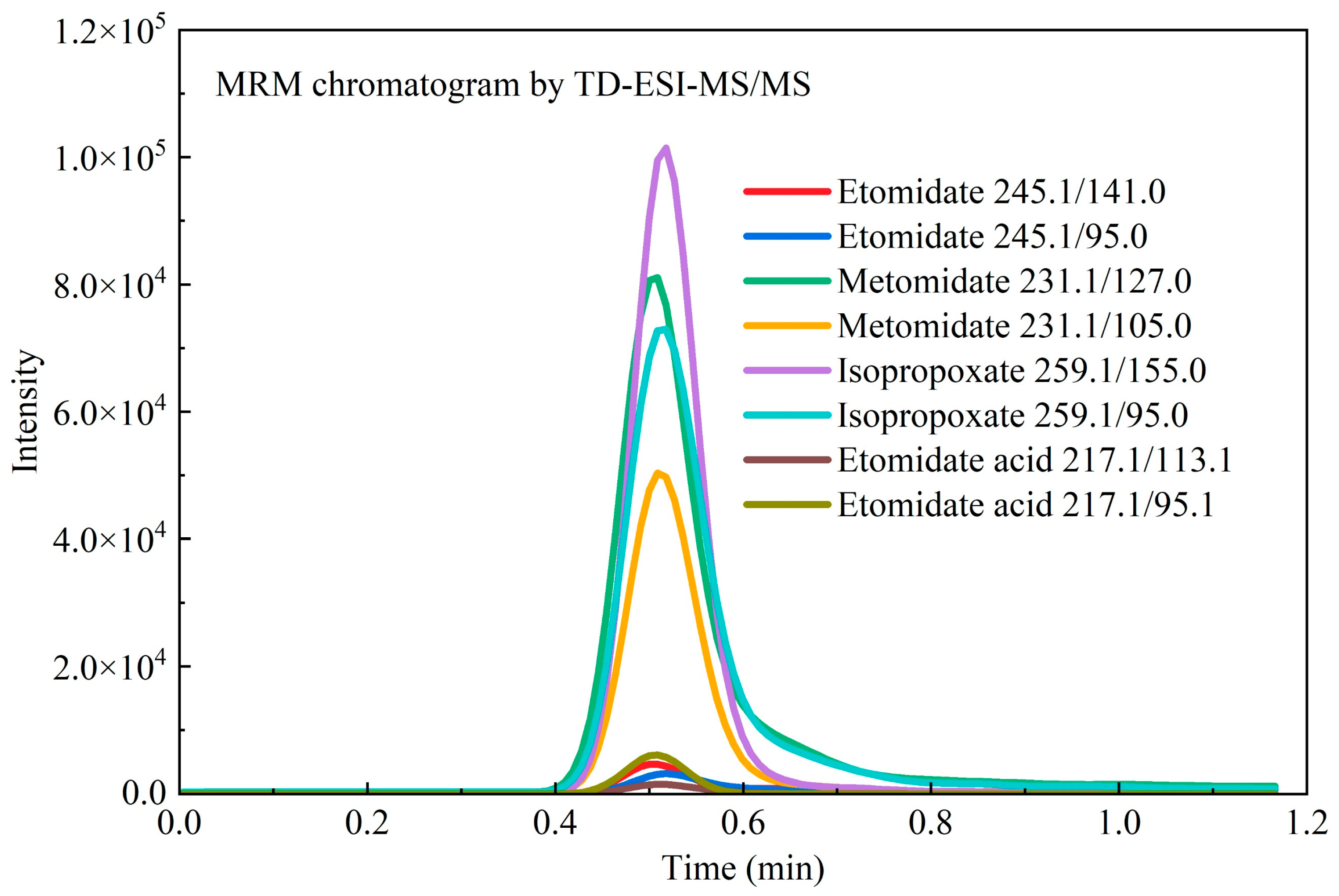



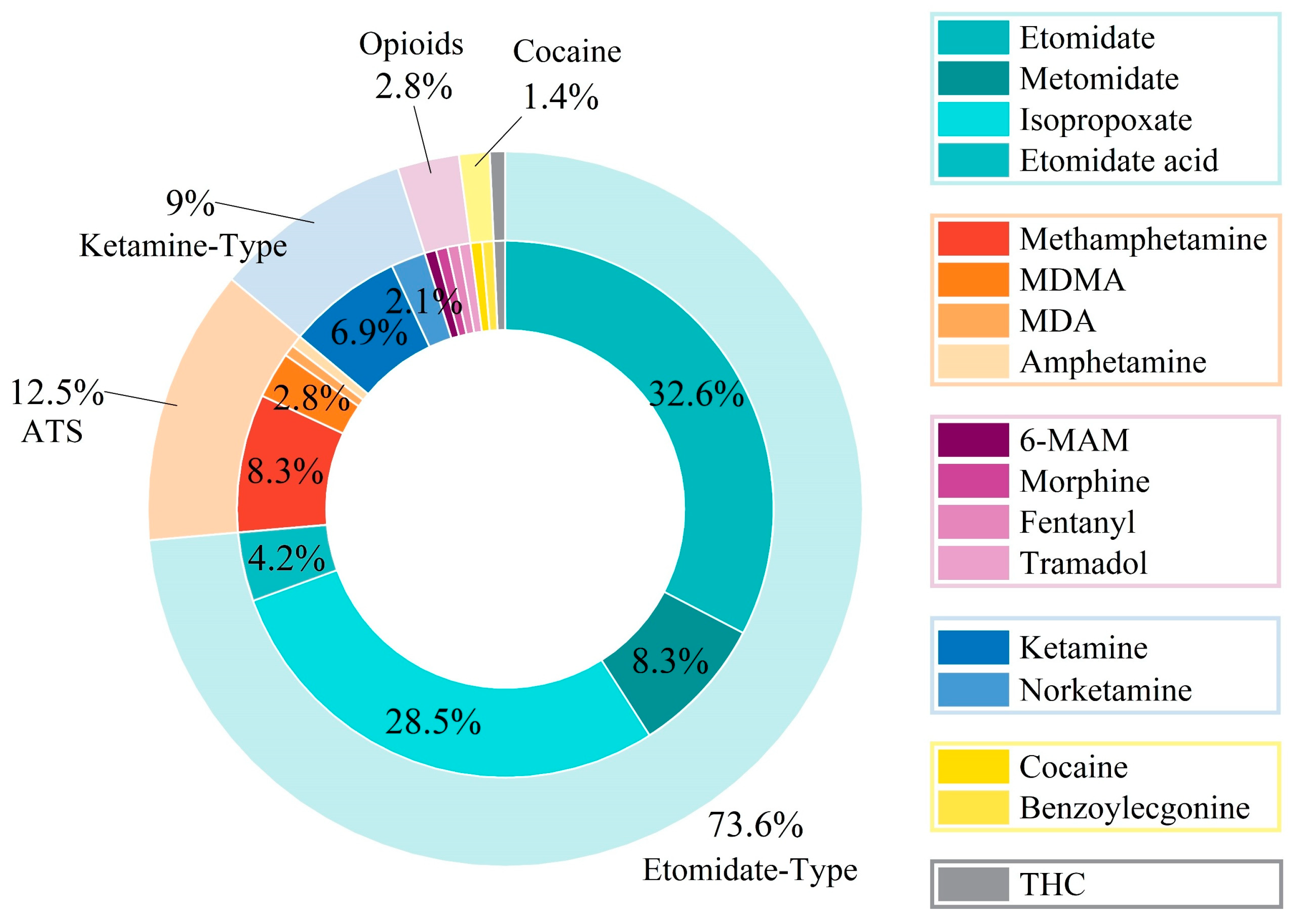
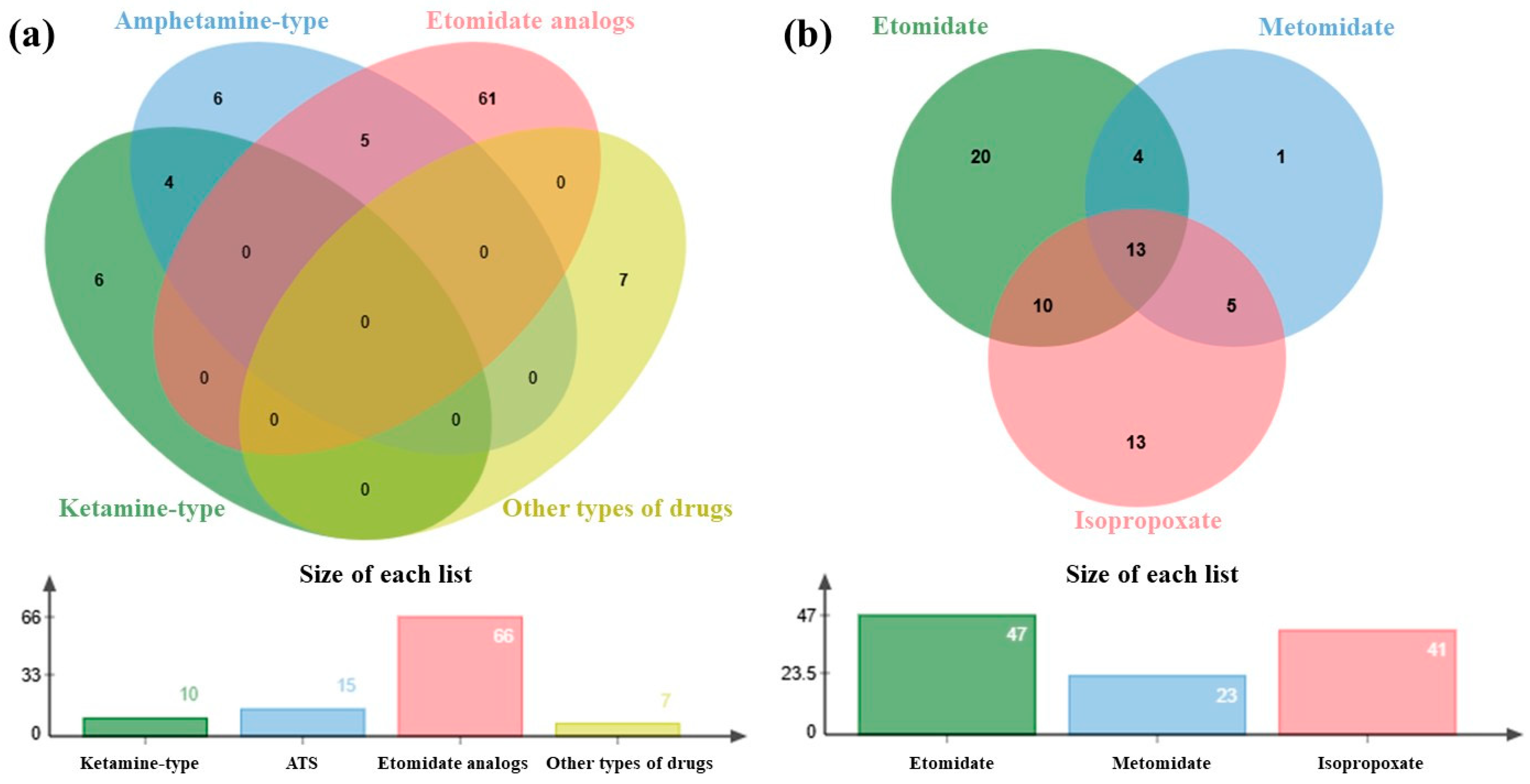
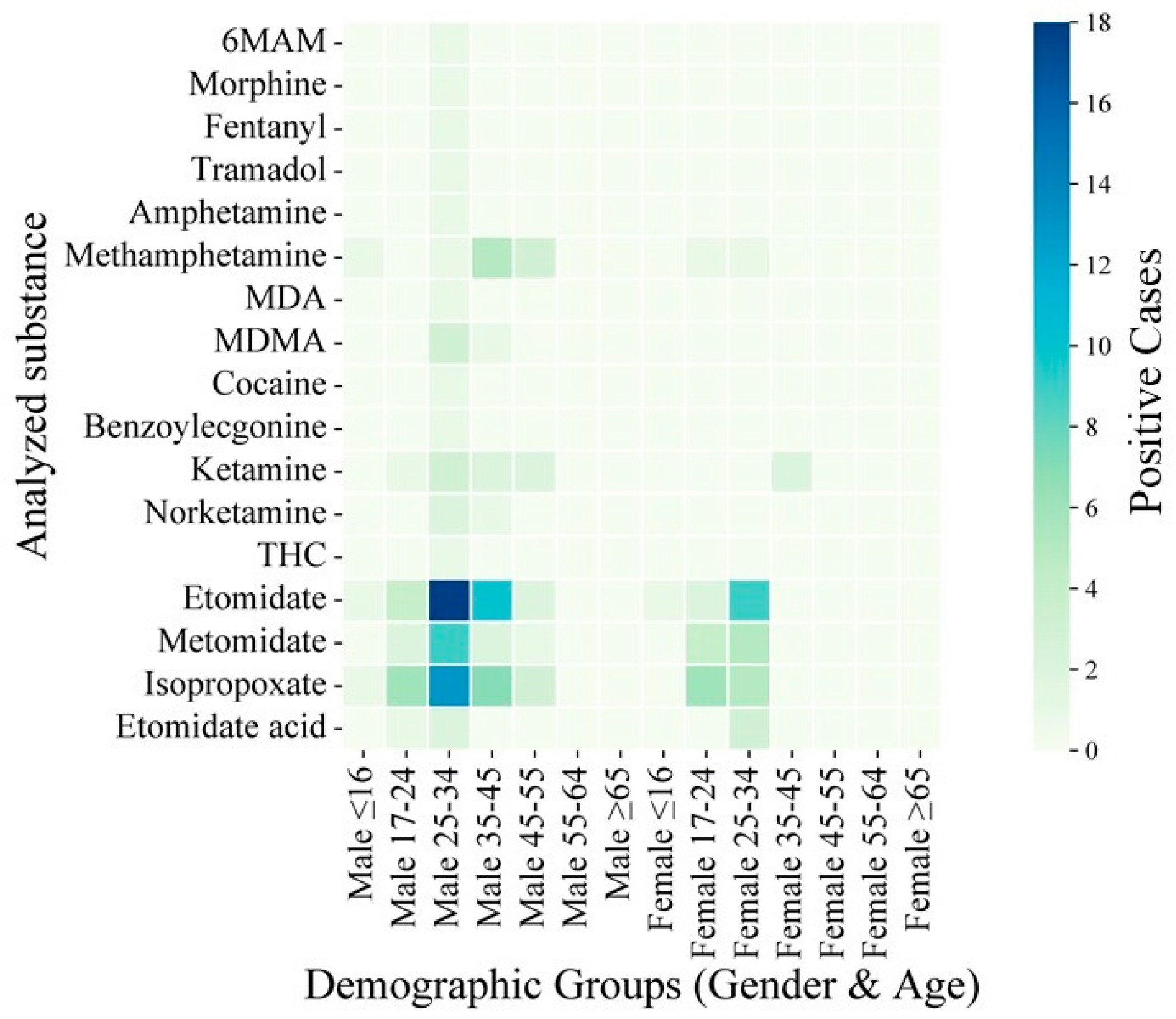
| Compound | LOD (ng/mg) | Concentration (ng/mg) | Inter-Day (n = 6) | Intra-Day (n = 6) | Matrix Effect (%) | ||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | ||||
| 6MAM | 0.1 | 0.5 | 16.2 | 12.6 | 13.3 | 19.3 | 13.3 |
| 2.5 | 6.8 | 6.2 | 9.8 | 12.1 | 9.0 | ||
| 12.5 | 8.3 | 3.0 | 0.3 | 3.8 | 1.9 | ||
| MOR | 0.1 | 0.5 | 15.9 | 13.6 | 14.4 | 14.5 | 15.5 |
| 2.5 | 5.6 | 7.1 | 8.8 | 2.7 | 3.6 | ||
| 12.5 | 10.5 | 3.3 | 1.5 | 9.9 | 2.7 | ||
| FENT | 0.1 | 0.5 | 14.5 | 10.2 | 14.1 | 16.8 | 16.3 |
| 2.5 | 6.2 | 12.4 | 1.6 | 7.7 | 9.7 | ||
| 12.5 | 5.4 | 1.0 | 2.8 | 5.7 | 4.2 | ||
| TRA | 0.2 | 0.5 | 17.1 | 13.9 | 12.3 | 17.3 | 17.3 |
| 2.5 | 8.0 | 5.8 | 6.3 | 1.6 | 2.2 | ||
| 12.5 | 9.9 | 8.9 | 7.0 | 5.5 | 1.8 | ||
| AMP | 0.1 | 0.5 | 4.7 | 11.9 | 10.3 | 16.5 | 11.4 |
| 2.5 | 7.6 | 1.2 | 4.3 | 7.0 | 3.1 | ||
| 12.5 | 8.1 | 2.4 | 5.8 | 6.4 | 0.8 | ||
| METH | 0.1 | 0.5 | 12.2 | 15.8 | 13.7 | 14.8 | 10.2 |
| 2.5 | 11.4 | 8.8 | 4.3 | 10.0 | 1.3 | ||
| 12.5 | 6.8 | 4.1 | 9.5 | 4.7 | 3.2 | ||
| MDA | 0.2 | 0.5 | 12.7 | 15.4 | 12.1 | 17.1 | 12.5 |
| 2.5 | 15.9 | 15.8 | 17.5 | 15.3 | 8.7 | ||
| 12.5 | 6.9 | 8.0 | 3.9 | 5.3 | 7.7 | ||
| MDMA | 0.1 | 0.5 | 15.0 | 17.3 | 10.4 | 10.8 | 16.5 |
| 2.5 | 3.4 | 1.8 | 16.1 | 7.1 | 3.1 | ||
| 12.5 | 7.9 | 8.1 | 9.4 | 1.5 | 4.0 | ||
| COC | 0.1 | 0.5 | 3.1 | 3.0 | 5.2 | 9.9 | 9.9 |
| 2.5 | 8.1 | 5.8 | 6.3 | 5.8 | 1.5 | ||
| 12.5 | 5.1 | 4.1 | 1.3 | 6.8 | 2.1 | ||
| BZE | 0.1 | 0.5 | 10.7 | 8.4 | 16.5 | 18.3 | 10.8 |
| 2.5 | 2.0 | 5.0 | 5.3 | 0.7 | 5.3 | ||
| 12.5 | 9.8 | 0.6 | 0.7 | 1.4 | 3.6 | ||
| KET | 0.1 | 0.5 | 11.9 | 18.1 | 15.3 | 10.4 | 9.9 |
| 2.5 | 7.0 | 5.4 | 10.4 | 9.2 | 7.5 | ||
| 12.5 | 5.8 | 3.6 | 8.3 | 6.9 | 0.9 | ||
| NorKET | 0.1 | 0.5 | 12.5 | 11.3 | 10.8 | 18.8 | 8.1 |
| 2.5 | 10.0 | 8.7 | 7.8 | 6.4 | 2.4 | ||
| 12.5 | 4.9 | 0.4 | 1.9 | 0.1 | 5.2 | ||
| THC | 0.1 | 0.5 | 13.1 | 13.8 | 6.0 | 9.0 | 15.4 |
| 2.5 | 11.7 | 8.6 | 17.8 | 16.8 | 8.9 | ||
| 12.5 | 1.9 | 5.3 | 0.4 | 7.1 | 3.8 | ||
| ETO | 0.1 | 0.5 | 12.6 | 17.7 | 16.0 | 12.8 | 19.6 |
| 2.5 | 1.3 | 6.9 | 3.9 | 2.6 | 5.5 | ||
| 12.5 | 7.0 | 7.0 | 8.0 | 5.4 | 3.3 | ||
| METO | 0.1 | 0.5 | 16.4 | 10.1 | 15.6 | 8.0 | 14.4 |
| 2.5 | 6.6 | 9.7 | 0.1 | 13.9 | 9.4 | ||
| 12.5 | 0.5 | 7.1 | 2.4 | 8.4 | 7.1 | ||
| ISP | 0.1 | 0.5 | 11.8 | 10.4 | 14.1 | 17.7 | 19.6 |
| 2.5 | 2.2 | 7.6 | 9.7 | 11.5 | 6.8 | ||
| 12.5 | 4.5 | 9.6 | 6.7 | 6.9 | 5.6 | ||
| ETA | 0.2 | 0.5 | 19.0 | 15.5 | 14.5 | 15.0 | 18.3 |
| 2.5 | 14.7 | 13.6 | 16.7 | 10.8 | 4.7 | ||
| 12.5 | 4.3 | 2.7 | 5.6 | 2.1 | 5.8 | ||
| Compound | LOD (ng/mg) | LLOQ (ng/mg) | Linearity and Range (ng/mg) | Correlation Coefficient | Concentration (ng/mg) | Inter-Day (n = 6) | Intra-Day (n = 6) | ||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | ||||||
| 6MAM | 0.01 | 0.02 | 0.02–2.50 | 0.9959 | 0.10 | 3.5 | 6.6 | 3.7 | 7.6 |
| 1.00 | 5.5 | 3.9 | 6.7 | 5.1 | |||||
| 2.50 | 5.2 | 6.4 | 2.8 | 5.2 | |||||
| MOR | 0.01 | 0.02 | 0.02–5.00 | 0.9988 | 0.10 | 8.1 | 6.1 | 6.8 | 4.8 |
| 1.00 | 1.2 | 1.5 | 1.9 | 4.3 | |||||
| 2.50 | 7.6 | 8.5 | 4.4 | 1.6 | |||||
| FENT | 0.01 | 0.02 | 0.02–5.00 | 0.9953 | 0.10 | 8.5 | 3.3 | 1.3 | 6.9 |
| 1.00 | 1.3 | 2.0 | 5.3 | 1.5 | |||||
| 2.50 | 6.0 | 4.5 | 8.8 | 3.0 | |||||
| TRA | 0.02 | 0.05 | 0.05–5.00 | 0.9962 | 0.10 | 2.0 | 8.5 | 6.1 | 8.5 |
| 1.00 | 6.9 | 3.1 | 5.3 | 5.6 | |||||
| 2.50 | 7.2 | 1.7 | 8.0 | 1.2 | |||||
| AMP | 0.01 | 0.02 | 0.02–5.00 | 0.9986 | 0.10 | 1.3 | 2.1 | 2.5 | 1.5 |
| 1.00 | 8.1 | 5.2 | 5.8 | 2.4 | |||||
| 2.50 | 1.9 | 1.7 | 2.6 | 7.9 | |||||
| METH | 0.01 | 0.02 | 0.02–5.00 | 0.9989 | 0.10 | 7.6 | 6.2 | 1.4 | 9.4 |
| 1.00 | 8.1 | 5.2 | 3.7 | 5.4 | |||||
| 2.50 | 1.4 | 7.9 | 1.1 | 7.1 | |||||
| MDA | 0.02 | 0.05 | 0.05–5.00 | 0.9958 | 0.10 | 5.3 | 6.6 | 5.0 | 5.7 |
| 1.00 | 8.5 | 6.6 | 6.1 | 8.4 | |||||
| 2.50 | 2.2 | 6.2 | 6.9 | 3.6 | |||||
| MDMA | 0.01 | 0.02 | 0.02–5.00 | 0.9960 | 0.10 | 6.1 | 2.7 | 7.5 | 5.7 |
| 1.00 | 3.6 | 1.7 | 4.2 | 4.9 | |||||
| 2.50 | 1.8 | 5.4 | 8.4 | 8.1 | |||||
| COC | 0.01 | 0.02 | 0.02–5.00 | 0.9991 | 0.10 | 6.9 | 8.0 | 8.8 | 4.9 |
| 1.00 | 3.8 | 7.0 | 1.7 | 8.5 | |||||
| 2.50 | 8.9 | 4.2 | 8.5 | 6.0 | |||||
| BZE | 0.01 | 0.02 | 0.02–5.00 | 0.9985 | 0.10 | 5.7 | 4.2 | 3.0 | 7.0 |
| 1.00 | 6.2 | 8.3 | 3.4 | 3.4 | |||||
| 2.50 | 4.5 | 2.7 | 6.6 | 8.5 | |||||
| KET | 0.01 | 0.02 | 0.02–5.00 | 0.9968 | 0.10 | 8.6 | 4.4 | 3.5 | 7.2 |
| 1.00 | 7.4 | 6.5 | 2.3 | 4.0 | |||||
| 2.50 | 5.0 | 6.0 | 4.6 | 4.3 | |||||
| NorKET | 0.01 | 0.02 | 0.02–5.00 | 0.9993 | 0.10 | 5.4 | 8.7 | 4.5 | 2.3 |
| 1.00 | 3.6 | 7.4 | 6.5 | 4.4 | |||||
| 2.50 | 3.5 | 5.4 | 3.1 | 5.8 | |||||
| THC | 0.01 | 0.02 | 0.02–5.00 | 0.9976 | 0.10 | 1.6 | 6.2 | 3.5 | 7.7 |
| 1.00 | 8.2 | 8.5 | 5.8 | 6.3 | |||||
| 2.50 | 8.0 | 2.9 | 2.5 | 7.4 | |||||
| ETO | 0.01 | 0.02 | 0.02–5.00 | 0.9959 | 0.10 | 4.6 | 5.0 | 7.3 | 6.1 |
| 1.00 | 4.5 | 7.4 | 3.2 | 2.1 | |||||
| 2.50 | 7.1 | 6.7 | 2.1 | 2.3 | |||||
| METO | 0.01 | 0.02 | 0.02–5.00 | 0.9985 | 0.10 | 5.7 | 4.8 | 4.1 | 7.4 |
| 1.00 | 1.2 | 3.5 | 8.0 | 8.0 | |||||
| 2.50 | 5.8 | 7.8 | 6.3 | 7.2 | |||||
| ISP | 0.01 | 0.02 | 0.02–5.00 | 0.9978 | 0.10 | 5.0 | 2.3 | 5.8 | 5.2 |
| 1.00 | 3.5 | 4.6 | 5.4 | 3.3 | |||||
| 2.50 | 4.4 | 4.9 | 7.4 | 7.6 | |||||
| ETA | 0.02 | 0.05 | 0.05–5.00 | 0.9973 | 0.10 | 2.8 | 4.7 | 8.6 | 3.1 |
| 1.00 | 8.3 | 5.6 | 5.9 | 3.6 | |||||
| 2.50 | 7.9 | 7.0 | 4.9 | 4.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, J.; Zhu, B. TD-ESI-MS/MS for High-Throughput Screening of 13 Common Drugs and 4 Etomidate Analogs in Hair: Method Validation and Forensic Applications. Toxics 2025, 13, 329. https://doi.org/10.3390/toxics13050329
Li M, Li J, Zhu B. TD-ESI-MS/MS for High-Throughput Screening of 13 Common Drugs and 4 Etomidate Analogs in Hair: Method Validation and Forensic Applications. Toxics. 2025; 13(5):329. https://doi.org/10.3390/toxics13050329
Chicago/Turabian StyleLi, Meng, Jinbo Li, and Binling Zhu. 2025. "TD-ESI-MS/MS for High-Throughput Screening of 13 Common Drugs and 4 Etomidate Analogs in Hair: Method Validation and Forensic Applications" Toxics 13, no. 5: 329. https://doi.org/10.3390/toxics13050329
APA StyleLi, M., Li, J., & Zhu, B. (2025). TD-ESI-MS/MS for High-Throughput Screening of 13 Common Drugs and 4 Etomidate Analogs in Hair: Method Validation and Forensic Applications. Toxics, 13(5), 329. https://doi.org/10.3390/toxics13050329






