The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand
Abstract
:1. Introduction
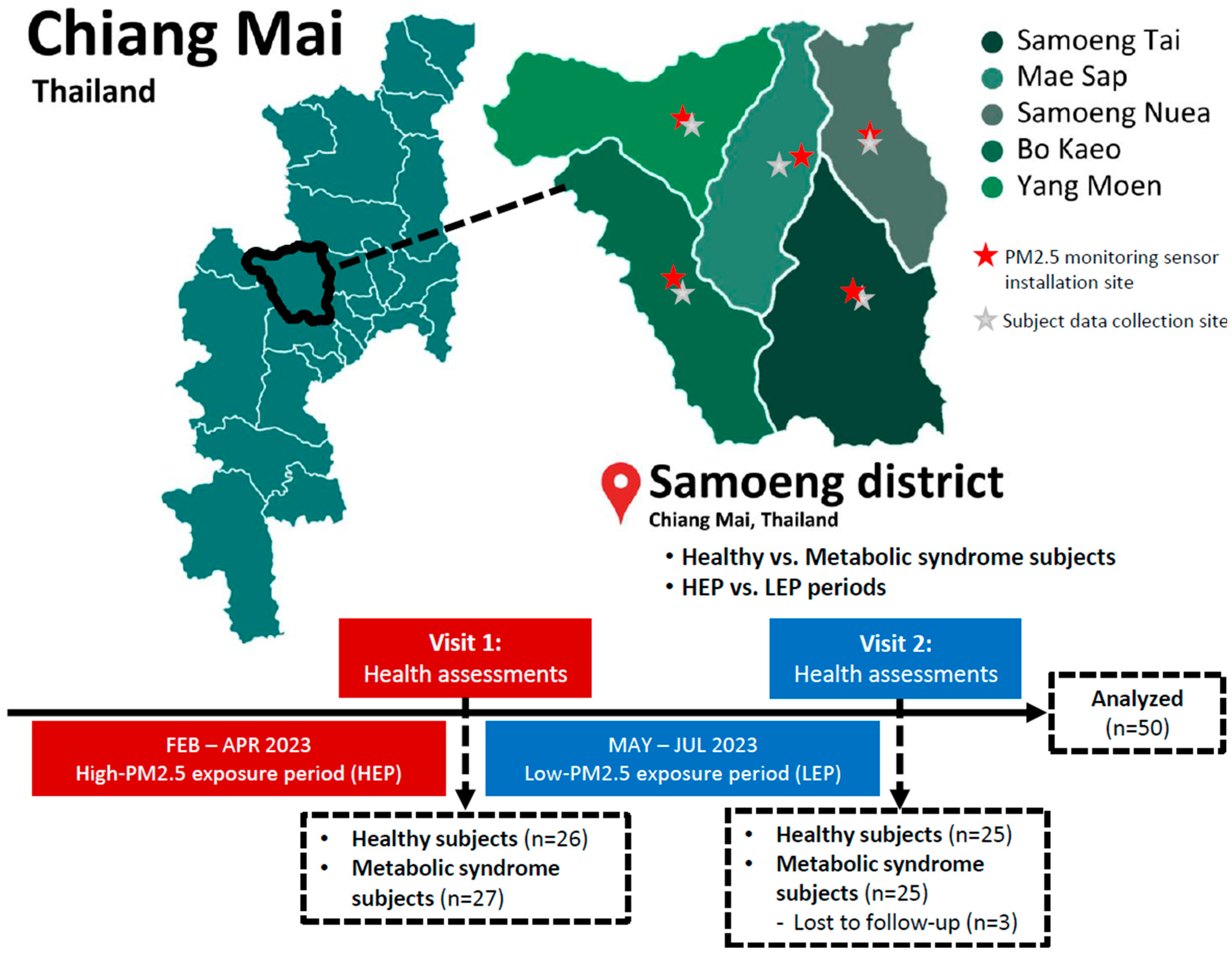
2. Materials and Methods
2.1. Air Quality Monitoring and PM2.5 Measurement
2.2. Ethics Statement
2.3. Study Subjects
2.4. Study Design
2.5. Laboratory Procedures
2.6. Dietary Intake Data
2.7. Statistical Analysis
2.8. Artificial Intelligence (AI) for Research
3. Results
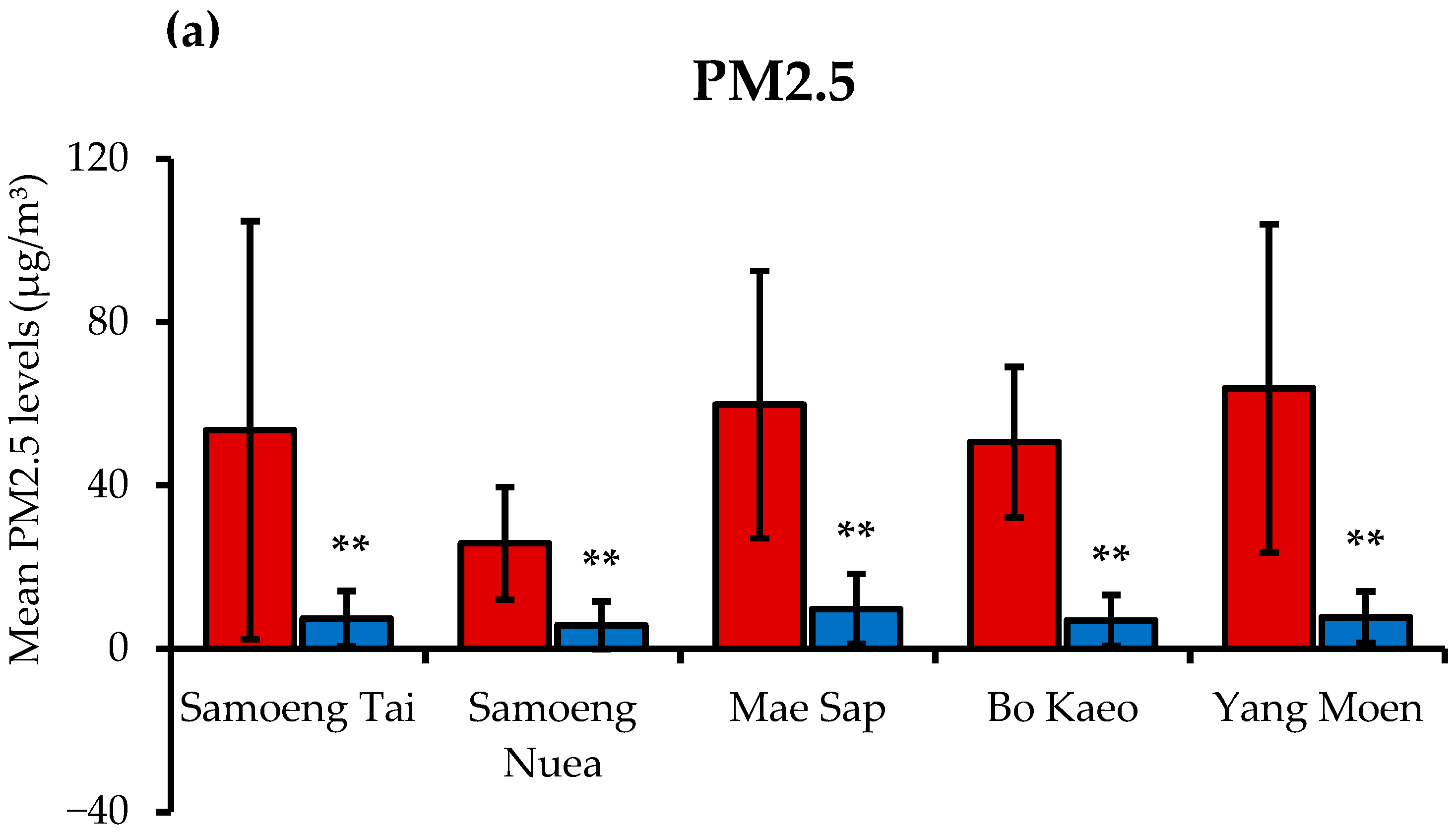
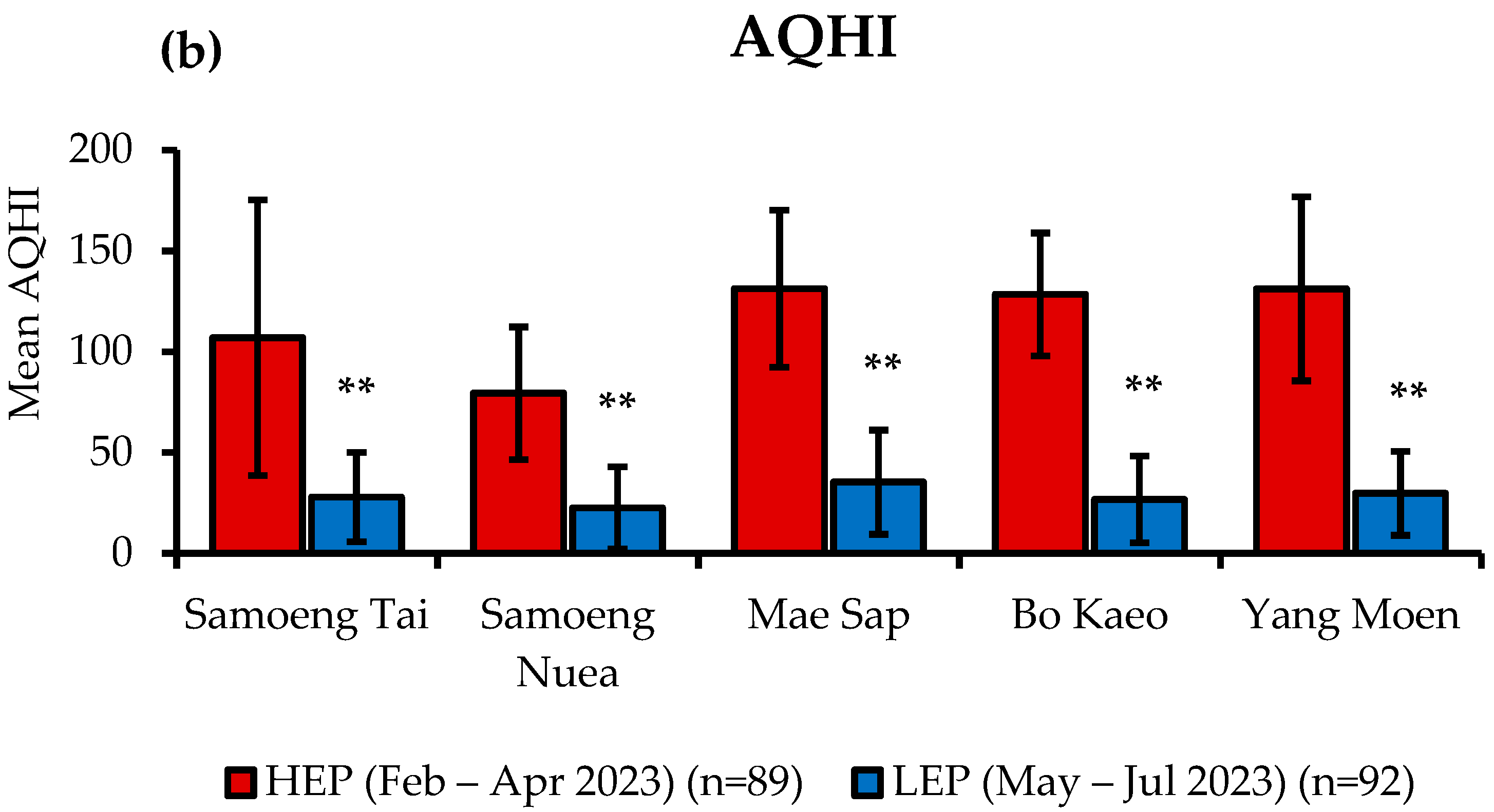
3.1. PM2.5 Levels and Air Quality Health Index (AQHI) in Samoeng District
3.2. The Demographic Data of Subjects
3.3. Effects of PM2.5 Exposure on Physical and Blood Biochemical Parameters
3.4. Energy and Nutrient Intakes
3.4.1. Distribution of Energy Percentages from Macronutrients
3.4.2. Average Daily Energy and Nutrient Consumption
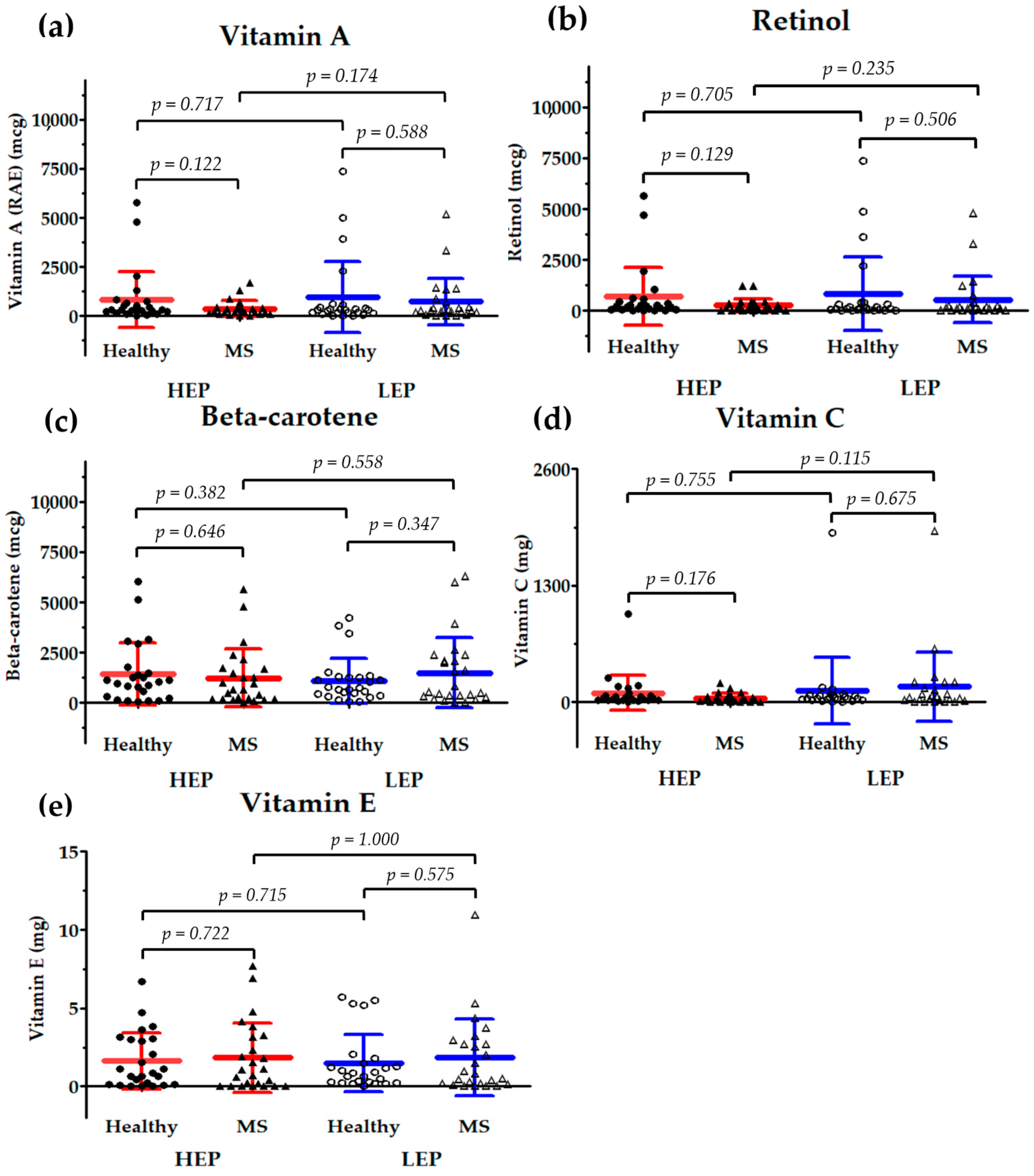
3.4.3. Dietary Intake of Antioxidant Vitamins
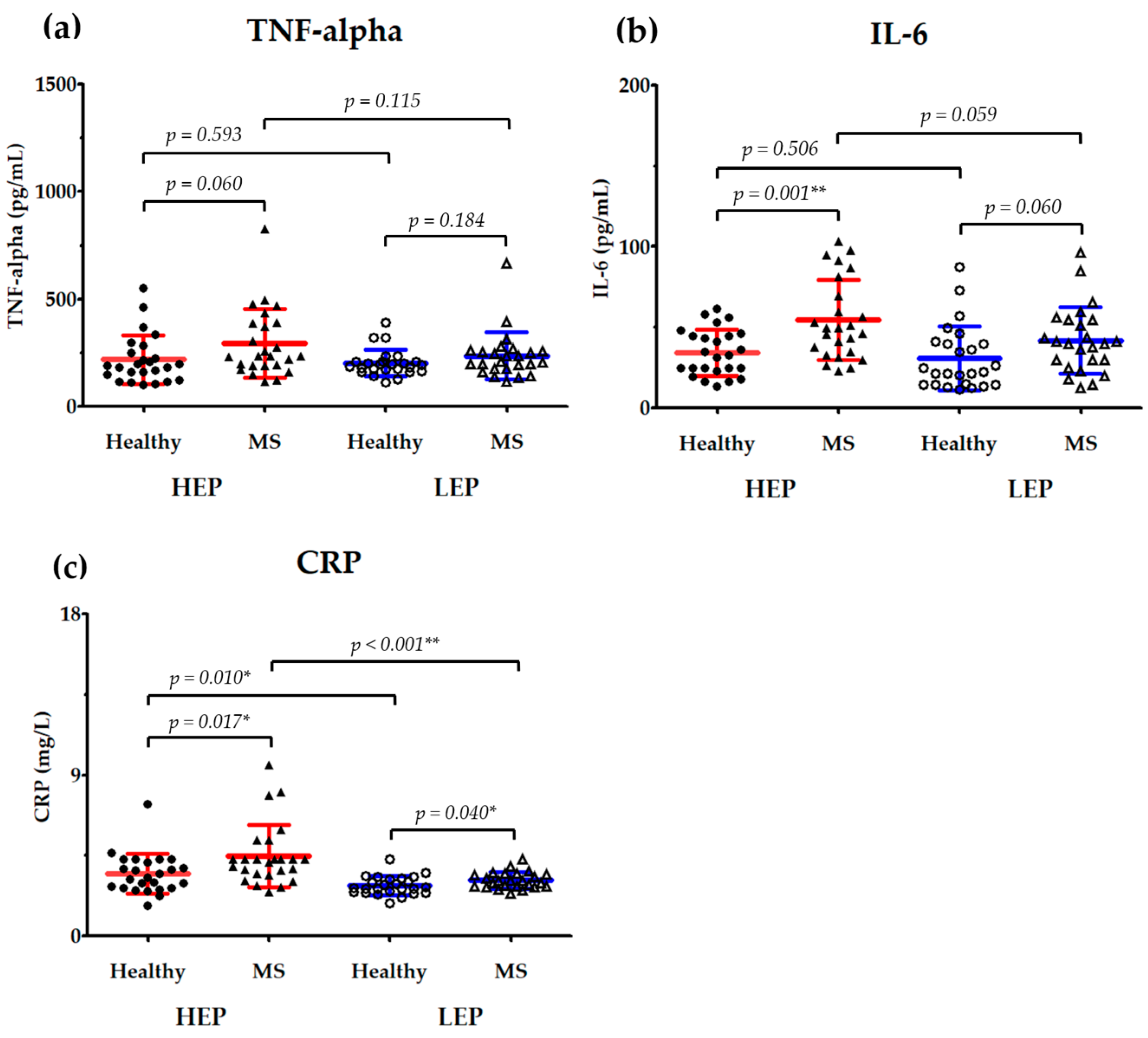
3.5. Effects of PM2.5 Exposure on Serum Inflammatory Markers
3.6. Correlation Between Nutrient Intake and Serum Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-epi-PGF2α | 8-epi-prostaglandin F2α |
| NTAQHI | Northern Thailand Air Quality Health Index |
| SREBP1 | sterol regulatory element-binding protein 1 |
| ELISA | enzyme-linked immunosorbent assay |
| RIHES | Research Institute for Health Sciences |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| SOCS3 | suppressor of cytokine signaling 3 |
| STAT3 | signal transducer and activator of transcription 3 |
| ICAM-1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| AQHI | Air Quality Health Index |
| CVDs | cardiovascular diseases |
| GLUT | glucose transporter |
| NCDs | non-communicable diseases |
| PAHs | polycyclic aromatic hydrocarbons |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| TNF-α | tumor necrosis factor-alpha |
| JAK1 | Janus kinase 1 |
| 1-OHP | 1-hydroxypyrene |
| ALT | alanine aminotransferase |
| AQI | Air Quality Index |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| CRP | C-reactive protein |
| DBP | diastolic blood pressure |
| FBG | fasting blood glucose |
| HEP | high-PM2.5 exposure period |
| LEP | low-PM2.5 exposure period |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| VAT | visceral adipose tissue |
| WHO | World Health Organization |
| IL-6 | interleukin-6 |
| NF-κB | nuclear factor-kappa B |
| PM2.5 | fine particulate matter |
| Cu | copper |
| HC | hip circumference |
| HR | heart rate |
| MS | metabolic syndrome |
| SD | standard deviation |
| TC | total cholesterol |
| TG | triglyceride |
| WC | waist circumference |
| Zn | zinc |
References
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef]
- Chanda, F.; Lin, K.-X.; Chaurembo, A.I.; Huang, J.-Y.; Zhang, H.-J.; Deng, W.-H.; Xu, Y.-J.; Li, Y.; Fu, L.-D.; Cui, H.-D.; et al. PM2.5-mediated cardiovascular disease in aging: Cardiometabolic risks, molecular mechanisms and potential interventions. Sci. Total Environ. 2024, 954, 176255. [Google Scholar] [CrossRef]
- Li, W.; Sun, B.; Li, H.; An, Z.; Li, J.; Jiang, J.; Song, J.; Wu, W. Association between short-term exposure to PM2.5 and nasal microbiota dysbiosis, inflammation and oxidative stress: A panel study of healthy young adults. Ecotoxicol. Environ. Saf. 2023, 262, 115156. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Yang, Y.; Yang, L.; Wu, Q.; Sun, H.; An, Z.; Li, J.; Wu, H.; Song, J.; et al. PM2.5 exposure upregulates pro-inflammatory protein expression in human microglial cells via oxidant stress and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116386. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Lee, T.-L.; Chen, Y.-C.; Liang, C.-J.; Wang, S.-H.; Lue, J.-H.; Tsai, J.-S.; Lee, S.-W.; Chen, S.-H.; Yang, Y.-F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef]
- Elbarbary, M.; Oganesyan, A.; Honda, T.; Morgan, G.; Guo, Y.; Guo, Y.; Negin, J. Systemic Inflammation (C-Reactive Protein) in Older Chinese Adults Is Associated with Long-Term Exposure to Ambient Air Pollution. Int. J. Environ. Res. Public Health 2021, 18, 3258. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Duan, Z.; Nie, J.; Li, X.; Yu, W.; Niu, Z.; Yan, Y. Association between long-term exposure to PM2.5 chemical components and metabolic syndrome in middle-aged and older adults. Front. Public Health 2024, 12, 1462548. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Shi, L.; Jiang, J.; Wan, X.; Wang, Y.; Ma, Y.; Dong, Y.; Zou, Z.; Ma, J. Long-term effects of ambient PM2.5 constituents on metabolic syndrome in Chinese children and adolescents. Environ. Res. 2023, 220, 115238. [Google Scholar] [CrossRef]
- Han, S.; Zhang, F.; Yu, H.; Wei, J.; Xue, L.; Duan, Z.; Niu, Z. Systemic inflammation accelerates the adverse effects of air pollution on metabolic syndrome: Findings from the China health and Retirement Longitudinal Study (CHARLS). Environ. Res. 2022, 215, 114340. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Jiang, S.; Du, X.; Zeng, X.; Zhang, J.; Song, L.; Zhou, J.; Kan, H.; Sun, Q.; Xie, Y.; et al. AMPK activation attenuates inflammatory response to reduce ambient PM2.5-induced metabolic disorders in healthy and diabetic mice. Ecotoxicol. Environ. Saf. 2019, 179, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, T.; Liu, C.; Ma, D.; Wang, J.; Liu, M.; Ran, J.; Wang, X.; Deng, X. PM2.5 induced liver lipid metabolic disorders in C57BL/6J mice. Front. Endocrinol. 2023, 14, 1212291. [Google Scholar] [CrossRef] [PubMed]
- Campolim, C.M.; Weissmann, L.; Ferreira, C.K.d.O.; Zordão, O.P.; Dornellas, A.P.S.; Castro, G.; Zanotto, T.M.; Boico, V.F.; Quaresma, P.G.; Lima, R.P.; et al. Short-term exposure to air pollution (PM2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via Tlr4/Ikbke in mice. Sci. Rep. 2020, 10, 10160. [Google Scholar] [CrossRef]
- Mendes, F.d.C.; Paciência, I.; Rufo, J.C.; Silva, D.; Cunha, P.; Farraia, M.; Delgado, L.; Garcia-Larsen, V.; Severo, M.; Moreira, A.; et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr. Allergy Immunol. 2020, 31, 290–296. [Google Scholar] [CrossRef]
- Ju, K.; Li, C.; Pan, J. Causal evidence of healthy diets, long-term PM2.5 exposure, and kidney function decline in adults: An observational study. J. Clean. Prod. 2023, 433, 139609. [Google Scholar] [CrossRef]
- Huang, K.; Yu, D.; Fang, H.; Ju, L.; Piao, W.; Guo, Q.; Xu, X.; Wei, X.; Yang, Y.; Zhao, L. Association of fine particulate matter and its constituents with hypertension: The modifying effect of dietary patterns. Environ. Health 2023, 22, 55. [Google Scholar] [CrossRef]
- Luu, H.N.; Wen, W.; Li, H.; Dai, Q.; Yang, G.; Cai, Q.; Xiang, Y.B.; Gao, Y.T.; Zheng, W.; Shu, X.O. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid. Redox Signal. 2015, 22, 951–959. [Google Scholar] [CrossRef]
- Horváth, M.; Babinszky, L. Impact of selected antioxidant vitamins (Vitamin A, E and C) and micro minerals (Zn, Se) on the antioxidant status and performance under high environmental temperature in poultry. A review. Acta Agric. Scand. A Anim. Sci. 2018, 68, 152–160. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Yeom, J.H.; Kim, W. Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2021, 22, 11923. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R. Vitamin A as an anti-inflammatory agent. Proc. Nutr. Soc. 2002, 61, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Agay, D.; Anderson, R.A.; Sandre, C.; Bryden, N.A.; Alonso, A.; Roussel, A.M.; Chancerelle, Y. Alterations of antioxidant trace elements (Zn, Se, Cu) and related metallo-enzymes in plasma and tissues following burn injury in rats. Burns 2005, 31, 366–371. [Google Scholar] [CrossRef]
- Tian, C.; Hao, L.; Yi, W.; Ding, S.; Xu, F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 7398453. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Mostafanezhad, M.; Vaddhanaphuti, C.; Evrard, O. Making air pollution legible: Environmental-health data and the naturalization of the smoky season in Northern Thailand. Singap. J. Trop. Geogr. 2025. [Google Scholar] [CrossRef]
- Jarernwong, K.; Gheewala, S.H.; Sampattagul, S. Health Impact Related to Ambient Particulate Matter Exposure as a Spatial Health Risk Map Case Study in Chiang Mai, Thailand. Atmosphere 2023, 14, 261. [Google Scholar] [CrossRef]
- Pirard, C.; Charoenpanwutikul, A. Comprehensive review of the annual haze episode in Northern Thailand. Earth Arxiv 2023. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. Available online: https://iris.who.int/handle/10665/345329 (accessed on 9 May 2023).
- Bowe, B.; Xie, Y.; Yan, Y.; Al-Aly, Z. Burden of Cause-Specific Mortality Associated with PM2.5 Air Pollution in the United States. JAMA Netw. Open 2019, 2, e1915834. [Google Scholar] [CrossRef]
- Karim, N.; Hod, R.; Wahab, M.I.A.; Ahmad, N. Projecting non-communicable diseases attributable to air pollution in the climate change era: A systematic review. BMJ Open 2024, 14, e079826. [Google Scholar] [CrossRef] [PubMed]
- Parasin, N.; Amnuaylojaroen, T. Effect of PM2.5 on burden of mortality from non-communicable diseases in northern Thailand. PeerJ 2024, 12, e18055. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Z.; Guo, Q.; Gan, Y.; Wang, Q.; Liu, J.A. Association between long-term exposure to PM2.5 and hypertension: A systematic review and meta-analysis of observational studies. Environ. Res. 2022, 204, 112352. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhang, B.; Zhao, X.; Ruan, Y.; Lian, H.; Fan, Z. Effect of exposure to PM2.5 on blood pressure: A systematic review and meta-analysis. J. Hypertens. 2014, 32, 2130–2141. [Google Scholar] [CrossRef]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and Cardiovascular Health Risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Y.; Zheng, Y.; Di, Q.; Liu, T.; Xiao, J.; Li, X.; Zeng, W.; Cummings-Vaughn, L.A.; Howard, S.W.; et al. Long-Term Effects of Ambient PM2.5 on Hypertension and Blood Pressure and Attributable Risk Among Older Chinese Adults. Hypertension 2017, 69, 806–812. [Google Scholar] [CrossRef]
- Kang, H.S.; Um, S.H.; Seo, Y.S.; An, H.; Lee, K.G.; Hyun, J.J.; Kim, E.S.; Park, S.C.; Keum, B.; Kim, J.H.; et al. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J. Gastroenterol. Hepatol. 2011, 26, 292–299. [Google Scholar] [CrossRef]
- Hosten, A.O. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 193. [Google Scholar]
- Purkins, L.; Love, E.R.; Eve, M.D.; Wooldridge, C.L.; Cowan, C.; Smart, T.S.; Johnson, P.J.; Rapeport, W.G. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br. J. Clin. Pharmacol. 2004, 57, 199–208. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, M.H.; Yang, J.C.; Nien, C.K.; Yang, C.C.; Yeh, Y.H.; Yueh, S.K. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J. Gastroenterol. Hepatol. 2007, 22, 1482–1489. [Google Scholar] [CrossRef]
- Wong, V.M.; Swartz, S.J.; Devaraj, S.; Poyyapakkam, S. Elevated Serum Creatinine: But Is It Renal Failure? Pediatrics 2020, 146, e20192828. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not so Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Hongsibsong, S.; Chuljerm, H.; Parklak, W.; Ounjaijean, S.; Fakfum, P.; Kausar, S.; Kulprachakarn, K. Assessment of urinary oxidative stress biomarkers associated with fine particulate matter (PM2.5) exposure in Chiang Mai, Thailand. PeerJ 2025, 13, e19047. [Google Scholar] [CrossRef]
- Kim, K.; Park, E.Y.; Lee, K.H.; Park, J.D.; Kim, Y.D.; Hong, Y.C. Differential oxidative stress response in young children and the elderly following exposure to PM(2.5). Environ. Health Prev. Med. 2009, 14, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Ma, Y.C.; Lin, J.M.; Chuang, C.Y.; Sung, F.C. Oxidative DNA damage estimated by urinary 8-hydroxydeoxyguanosine and indoor air pollution among non-smoking office employees. Environ. Res. 2007, 103, 331–337. [Google Scholar] [CrossRef]
- Klöslová, Z.; Drímal, M.; Balog, K.; Koppová, K.; Dubajová, J. The Relations between Polycyclic Aromatic Hydrocarbons Exposure and 1-OHP Levels as a Biomarker of the Exposure. Cent. Eur. J. Public Health 2016, 24, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, X.; Li, W.; Zu, Y.; Zhou, F.; Shou, Q.; Ding, Z. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-κB Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Iyer, H.S.; Hart, J.E.; Fiffer, M.R.; Elliott, E.G.; Yanosky, J.D.; Kaufman, J.D.; Puett, R.C.; Laden, F. Impacts of long-term ambient particulate matter and gaseous pollutants on circulating biomarkers of inflammation in male and female health professionals. Environ. Res. 2022, 214, 113810. [Google Scholar] [CrossRef]
- Sun, B.; Shi, Y.; Li, Y.; Jiang, J.; Liang, S.; Duan, J.; Sun, Z. Short-term PM2.5 exposure induces sustained pulmonary fibrosis development during post-exposure period in rats. J. Hazard. Mater. 2020, 385, 121566. [Google Scholar] [CrossRef]
- Dabass, A.; Talbott, E.O.; Rager, J.R.; Marsh, G.M.; Venkat, A.; Holguin, F.; Sharma, R.K. Systemic inflammatory markers associated with cardiovascular disease and acute and chronic exposure to fine particulate matter air pollution (PM2.5) among US NHANES adults with metabolic syndrome. Environ. Res. 2018, 161, 485–491. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Zeng, Y.; Xu, F.; Zhao, S.; Zhang, L.; An, Z.; Li, H.; Li, J.; Song, J.; et al. Short-term exposure to air pollution on peripheral white blood cells and inflammation biomarkers: A cross-sectional study on rural residents. BMC Public Health 2024, 24, 1702. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li, Q.; Asweto, C.; Feng, L.; Yang, X.; Duan, F.; Duan, J.; Sun, Z. Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicol. Res. 2016, 5, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Long, M.H.; Zhang, C.; Xu, D.Q.; Fu, W.L.; Gan, X.D.; Li, F.; Wang, Q.; Xia, W.; Xu, D.G. PM2.5 aggravates diabetes via the systemically activated IL-6-mediated STAT3/SOCS3 pathway in rats’ liver. Environ. Pollut. 2020, 256, 113342. [Google Scholar] [CrossRef]
- Satheannoppakao, W.; Kasemsup, R.; Inthawong, R.; Chariyalertsak, S.; Sangthong, R.; Taneepanichskul, S.; Putwatana, P.; Kessomboon, P.; Aekplakorn, W. Sodium intake and socio-demographic determinants of the non-compliance with daily sodium intake recommendations: Thai NHES IV. J. Med. Assoc. Thai 2013, 96, S161–S170. [Google Scholar]
- O’Donnell, M.; Mente, A.; Yusuf, S. Evidence relating sodium intake to blood pressure and CVD. Curr. Cardiol. Rep. 2014, 16, 529. [Google Scholar] [CrossRef]
- Zhu, H.; Pollock, N.K.; Kotak, I.; Gutin, B.; Wang, X.; Bhagatwala, J.; Parikh, S.; Harshfield, G.A.; Dong, Y. Dietary sodium, adiposity, and inflammation in healthy adolescents. Pediatrics 2014, 133, e635–e642. [Google Scholar] [CrossRef]
- Costa, A.P.; de Paula, R.C.; Carvalho, G.F.; Araújo, J.P.; Andrade, J.M.; de Almeida, O.L.; de Faria, E.C.; Freitas, W.M.; Coelho, O.R.; Ramires, J.A.; et al. High sodium intake adversely affects oxidative-inflammatory response, cardiac remodelling and mortality after myocardial infarction. Atherosclerosis 2012, 222, 284–291. [Google Scholar] [CrossRef]
- Basdeki, E.D.; Kollias, A.; Mitrou, P.; Tsirimiagkou, C.; Georgakis, M.K.; Chatzigeorgiou, A.; Argyris, A.; Karatzi, K.; Manios, Y.; Sfikakis, P.P.; et al. Does Sodium Intake Induce Systemic Inflammatory Response? A Systematic Review and Meta-Analysis of Randomized Studies in Humans. Nutrients 2021, 13, 2632. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Heo, Y.-R. The association between dietary sodium intake and adiposity, inflammation, and hormone markers: A preliminary study. J. Nutr. Health 2017, 50, 578–584. [Google Scholar] [CrossRef]
- Niero, M.; Bartoli, G.; De Colle, P.; Scarcella, M.; Zanetti, M. Impact of Dietary Fiber on Inflammation and Insulin Resistance in Older Patients: A Narrative Review. Nutrients 2023, 15, 2365. [Google Scholar] [CrossRef]
- Su, M.Z.; Lee, S.; Shin, D. Association of Dietary Fiber and Measures of Physical Fitness with High-Sensitivity C-Reactive Protein. Nutrients 2024, 16, 888. [Google Scholar] [CrossRef]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Ma, X.; Cai, M.; Chi, Y.; Sun, C.; Liu, S.; Song, X.; Xu, K. Phytochemical reduces toxicity of PM2.5: A review of research progress. Nutr. Rev. 2024, 82, 654–663. [Google Scholar] [CrossRef]
- Yang, S.; Huang, X.L.; Chen, J.; Mao, L.N.; Liu, X.; Yuan, W.S.; Wu, X.J.; Luo, G.W. Curcumin protects BEAS-2B cells from PM2.5-induced oxidative stress and inflammation by activating NRF2/antioxidant response element pathways. Int. J. Mol. Med. 2021, 47, 45. [Google Scholar] [CrossRef]
| Variables | Healthy (n = 25) | Metabolic Syndrome (n = 25) | Total (n = 50) | p |
|---|---|---|---|---|
| Gender, N (%) | 0.225 | |||
| Male | 10 (40.0) | 6 (24.0) | 16 (32.0) | |
| Female | 15 (60.0) | 19 (76.0) | 34 (68.0) | |
| Age (years), mean ± SD | 48.3 ± 14.3 | 52.2 ± 8.3 | 50.72 ± 11.8 | 0.177 |
| Alcohol consumption, N (%) | 0.140 | |||
| Never | 6 (24.0) | 12 (48.0) | 18 (36.0) | |
| Yes | 19 (76.0) | 13 (52.0) | 32 (64.0) | |
| Smoking, N (%) | 0.411 | |||
| Never smoked | 18 (72.0) | 20 (80.0) | 38 (76.0) | |
| Smoker | 4 (16.0) | 1 (4.0) | 5 (10.0) | |
| Former smoker | 4 (12.0) | 4 (16.0) | 7 (14.0) | |
| Underlying diseases, N (%) | ||||
| Diabetes | 0 | 5 (10.0) | 5 (10.0) | 0.050 |
| Hypertension | 2 (8.0) | 14 (56.0) | 16 (32.0) | 0.001 * |
| Dyslipidemia | 1 (4.0) | 7 (28.0) | 8 (16.0) | 0.049 * |
| Other diseases (gastroesophageal reflux disease, allergy/asthma, herniated disk, thyroid disorder) | 6 (24.0) | 2 (8.0) | 8 (16.0) | 0.446 |
| Variables | HEP | LEP | p 3 | p 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 1 | Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 2 | |||
| Body height (cm) | 156.2 ± 7.6 | 156.6 ± 7.4 | 0.923 | 157.9 ± 7.6 | 156.2 ± 6.5 | 0.313 | 0.390 | 0.758 |
| Body weight (kg) | 59.4 ± 14.4 | 71.5 ± 16.3 | 0.001 ** | 60.0 ± 10.3 | 75.8 ± 22.3 | 0.004 ** | 0.555 | 0.640 |
| Body mass index, BMI (kg/m2) | 24.3 ± 5.0 | 29.1 ± 5.7 | <0.001 ** | 24.0 ± 3.4 | 30.7 ± 6.5 | <0.001 ** | 0.747 | 0.400 |
| Waist circumference, WC (cm) | 79.9 ± 13.7 | 92.1 ± 12.5 | <0.001 ** | 79.9 ± 8.9 | 96.7 ± 15.0 | <0.001 ** | 0.575 | 0.255 |
| Hip circumference, HC (cm) | 93.6 ± 10.5 | 101.9 ± 9.6 | <0.001 ** | 94.0 ± 7.6 | 105.0 ± 13.8 | 0.004 ** | 0.509 | 0.521 |
| Systolic blood pressure, SBP (mmHg) | 134.6 ± 19.8 | 137.9 ± 19.1 | 0.574 | 125.6 ± 19.6 | 130.9 ± 14.3 | 0.289 | 0.056 | 0.256 |
| Diastolic blood pressure, DBP (mmHg) | 83.8 ± 10.6 | 86.1 ± 14.2 | 0.377 | 78.2 ± 10.2 | 83.0 ± 8.2 | 0.116 | 0.057 | 0.245 |
| Heart rate, HR (bpm) | 74.0 ± 9.3 | 72.8 ± 9.6 | 0.534 | 71.0 ± 8.4 | 76.4 ± 0.2 | 0.065 | 0.249 | 0.228 |
| Fasting blood glucose, FBG (mg/dL) | 83.7 ± 7.2 | 114.7 ± 59.6 | 0.004 ** | 86.3 ± 11.7 | 106.5 ± 18.2 | <0.001 ** | 0.612 | 0.245 |
| Total cholesterol, TC (mg/dL) | 206.8 ± 29.8 | 193.0 ± 30.7 | 0.103 | 206.9 ± 40.8 | 209.2 ± 34.2 | 0.934 | 0.921 | 0.240 |
| Triglyceride, TG (mg/dL) | 133.0 ± 78.5 | 191.4 ± 95.1 | 0.003 ** | 126.1 ± 42.7 | 197.8 ± 73.8 | <0.001 ** | 0.640 | 0.438 |
| High-density lipoprotein cholesterol, HDL-C (mg/dL) | 55.2 ± 11.1 | 44.9 ± 10.1 | 0.001 ** | 55.7 ± 13.0 | 48.6 ± 9.3 | 0.034 * | 0.848 | 0.190 |
| Low-density lipoprotein cholesterol, LDL-C (mg/dL) | 122.7 ± 32.0 | 110.3 ± 28.5 | 0.118 | 113.1 ± 33.5 | 114.6 ± 28.0 | 0.967 | 0.283 | 0.728 |
| Alanine aminotransferase, ALT (U/L) | 27.7 ± 17.9 | 26.7 ± 12.4 | 0.560 | 28.5 ± 13.7 | 33.3 ± 14.2 | 0.090 | 0.330 | 0.040 * |
| Aspartate aminotransferase, AST (U/L) | 34.4 ± 11.6 | 34.4 ± 15.4 | 0.466 | 33.8 ± 7.4 | 32.5 ± 10.6 | 0.248 | 0.612 | 0.883 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.404 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.047 * | 0.143 | 0.968 |
| Blood urea nitrogen, BUN (mg/dL) | 13.5 ± 3.6 | 13.5 ± 3.5 | 0.793 | 14.5 ± 3.5 | 13.6 ± 3.1 | 0.400 | 0.217 | 0.852 |
| Macronutrients | HEP | LEP | p 3 | p 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 1 | Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 2 | |||
| Carbohydrate (%) | 56.96 ± 9.23 | 54.24 ± 17.34 | 0.493 | 53.48 ± 16.82 | 53.25 ± 13.94 | 0.960 | 0.357 | 0.745 |
| Protein (%) | 17.44 ± 5.13 | 19.20 ± 11.62 | 0.491 | 18.69 ± 6.10 | 19.68 ± 7.57 | 0.613 | 0.477 | 0.786 |
| Fat (%) | 25.60 ± 8.26 | 26.55 ± 11.70 | 0.741 | 27.83 ± 12.74 | 27.06 ± 9.23 | 0.808 | 0.464 | 0.857 |
| Variables | HEP | LEP | p 3 | p 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 1 | Healthy (Mean ± SD, n = 25) | MS (Mean ± SD, n = 25) | p 2 | |||
| Energy (kcal) | 1704.77 ± 660.21 | 1550.64 ± 684.87 | 0.422 | 1629.87 ± 548.25 | 1567.72 ± 535.05 | 0.687 | 0.625 | 0.909 |
| Carbohydrates (g) | 242.50 ± 103.51 | 214.92 ± 131.01 | 0.413 | 218.18 ± 109.54 | 209.37 ± 86.08 | 0.753 | 0.422 | 0.847 |
| Sugars (g) | 37.28 ± 28.55 | 22.88 ± 25.60 | 0.067 | 31.83 ± 24.20 | 35.30 ± 38.01 | 0.702 | 0.390 | 0.155 |
| Proteins (g) | 72.04 ± 29.73 | 67.42 ± 27.93 | 0.573 | 74.84 ± 31.60 | 74.46 ± 33.10 | 0.967 | 0.725 | 0.355 |
| Fats (g) | 49.62 ± 27.69 | 46.81 ± 29.52 | 0.730 | 50.87 ± 31.73 | 48.04 ± 26.44 | 0.734 | 0.875 | 0.862 |
| Total saturated fatty acids (g) | 12.23 ± 11.38 | 10.20 ± 8.91 | 0.486 | 13.40 ± 10.83 | 12.65 ± 10.35 | 0.803 | 0.721 | 0.416 |
| Cholesterol (mg) | 346.22 ± 275.30 | 321.30 ± 305.16 | 0.763 | 357.70 ± 315.13 | 281.42 ± 221.45 | 0.327 | 0.885 | 0.538 |
| Calcium (mg) | 503.48 ± 431.13 | 415.59 ± 240.97 | 0.378 | 403.41 ± 337.95 | 458.10 ± 453.31 | 0.631 | 0.366 | 0.686 |
| Phosphorus (mg) | 848.08 ± 313.46 | 752.47 ± 344.61 | 0.310 | 855.26 ± 409.06 | 893.73 ± 416.64 | 0.743 | 0.945 | 0.123 |
| Iron (mg) | 12.32 ± 5.33 | 13.36 ± 6.60 | 0.543 | 13.35 ± 6.94 | 14.66 ± 5.77 | 0.470 | 0.514 | 0.389 |
| Potassium (mg) | 1711.90 ± 747.67 | 1370.82 ± 764.01 | 0.117 | 1620.98 ± 746.08 | 1805.51 ± 1190.02 | 0.514 | 0.609 | 0.108 |
| Sodium (mg) | 3417.59 ± 1635.23 | 3307.41 ± 1196.97 | 0.787 | 3494.50 ± 1922.22 | 3546.37 ± 1359.04 | 0.913 | 0.872 | 0.434 |
| Magnesium (mg) | 58.65 ± 47.18 | 45.96 ± 49.92 | 0.360 | 52.34 ± 47.09 | 71.57 ± 66.48 | 0.244 | 0.662 | 0.125 |
| Copper (mg) | 0.86 ± 0.48 | 0.67 ± 0.36 | 0.126 | 0.98 ± 1.06 | 0.94 ± 0.78 | 0.879 | 0.574 | 0.142 |
| Selenium (μg) | 49.91 ± 45.23 | 42.10 ± 47.12 | 0.553 | 53.49 ± 47.18 | 45.99 ± 52.42 | 0.597 | 0.760 | 0.698 |
| Zinc (mg) | 5.09 ± 2.00 | 4.68 ± 2.10 | 0.479 | 4.99 ± 2.79 | 4.87 ± 2.39 | 0.869 | 0.856 | 0.724 |
| Vitamin B1 (mg) | 1.34 ± 0.89 | 1.72 ± 3.75 | 0.627 | 1.30 ± 1.05 | 3.92 ± 12.87 | 0.315 | 0.880 | 0.420 |
| Vitamin B2 (mg) | 1.25 ± 0.67 | 0.98 ± 0.39 | 0.086 | 1.14 ± 0.57 | 1.29 ± 1.02 | 0.530 | 0.533 | 0.142 |
| Vitamin B6 (mg) | 0.75 ± 1.11 | 0.40 ± 0.32 | 0.137 | 0.78 ± 0.83 | 0.74 ± 1.08 | 0.883 | 0.897 | 0.098 |
| Vitamin B12 (mg) | 2.27 ± 3.17 | 1.43 ± 1.54 | 0.241 | 3.55 ± 6.62 | 1.75 ± 1.76 | 0.195 | 0.361 | 0.387 |
| Niacin (mg) | 17.46 ± 10.82 | 13.09 ± 7.96 | 0.110 | 15.32 ± 8.30 | 16.08 ± 12.57 | 0.803 | 0.363 | 0.298 |
| Dietary fiber (g) | 13.29 ± 8.89 | 8.79 ± 8.01 | 0.066 | 11.73 ± 9.63 | 14.05 ± 12.56 | 0.468 | 0.521 | 0.037 * |
| Groups | Nutrient Intake | Serum Inflammatory Markers | ||
|---|---|---|---|---|
| TNF-α | IL-6 | CRP | ||
| Healthy | Dietary fiber | −0.242 | −0.290 * | −0.308 * |
| 0.090 | 0.041 | 0.029 | ||
| Vitamin A | −0.052 | −0.308 * | −0.117 | |
| 0.720 | 0.030 | 0.417 | ||
| Retinol | −0.029 | −0.289 * | −0.106 | |
| 0.843 | 0.042 | 0.465 | ||
| β-carotene | −0.237 | −0.248 | −0.098 | |
| 0.098 | 0.082 | 0.497 | ||
| Vitamin C | −0.076 | −0.223 | −0.301 * | |
| 0.600 | 0.120 | 0.033 | ||
| Vitamin E | −0.153 | −0.235 | −0.291 * | |
| 0.288 | 0.101 | 0.040 | ||
| MS | Dietary fiber | −0.182 | −0.322 * | −0.403 ** |
| 0.206 | 0.022 | 0.004 | ||
| Vitamin A | −0.173 | −0.160 | −0.270 | |
| 0.230 | 0.267 | 0.058 | ||
| Retinol | −0.128 | −0.121 | −0.217 | |
| 0.375 | 0.404 | 0.130 | ||
| β-carotene | −0.183 | −0.244 | −0.244 | |
| 0.203 | 0.088 | 0.087 | ||
| Vitamin C | −0.111 | −0.226 | −0.202 | |
| 0.443 | 0.114 | 0.159 | ||
| Vitamin E | −0.090 | −0.108 | −0.231 | |
| 0.535 | 0.457 | 0.107 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parklak, W.; Chuljerm, H.; Kawichai, S.; Fakfum, P.; Jiraya, P.; Kijkuokool, P.; Khiaolaongam, W.; Ngamsang, P.; Ounjaijean, S.; Rerkasem, K.; et al. The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand. Toxics 2025, 13, 325. https://doi.org/10.3390/toxics13050325
Parklak W, Chuljerm H, Kawichai S, Fakfum P, Jiraya P, Kijkuokool P, Khiaolaongam W, Ngamsang P, Ounjaijean S, Rerkasem K, et al. The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand. Toxics. 2025; 13(5):325. https://doi.org/10.3390/toxics13050325
Chicago/Turabian StyleParklak, Wason, Hataichanok Chuljerm, Sawaeng Kawichai, Puriwat Fakfum, Putita Jiraya, Praporn Kijkuokool, Wiritphon Khiaolaongam, Pakaphorn Ngamsang, Sakaewan Ounjaijean, Kittipan Rerkasem, and et al. 2025. "The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand" Toxics 13, no. 5: 325. https://doi.org/10.3390/toxics13050325
APA StyleParklak, W., Chuljerm, H., Kawichai, S., Fakfum, P., Jiraya, P., Kijkuokool, P., Khiaolaongam, W., Ngamsang, P., Ounjaijean, S., Rerkasem, K., & Kulprachakarn, K. (2025). The Impact of Nutrition and Fine Particulate Matter (PM2.5) on Inflammatory Responses in Individuals with Metabolic Syndrome: A Paired Case Study from Chiang Mai, Thailand. Toxics, 13(5), 325. https://doi.org/10.3390/toxics13050325







