Lead Causes Lipid Droplet Accumulation by Impairing Lysosomal Function and Autophagic Flux in Testicular Sertoli Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Semen Quality Analysis
2.3. Histological Analysis and Immunofluorescent Labeling in Murine Testicular Tissue
2.4. Cell Culture and Treatment
2.5. Cell Viability
2.6. Transmission Electron Microscopy
2.7. Oil Red O (ORO) Staining
2.8. Measurement of ROS
2.9. Measurement of Lysosomal pH
2.10. Acridine Orange (AO) Staining
2.11. LysoTracker Red Staining
2.12. Apoptosis Assay
2.13. Transfection
2.14. Immunocytochemical Analysis
2.15. Real-Time PCR Analysis
2.16. Western Blot Analysis
2.17. Statistical Analysis
3. Results
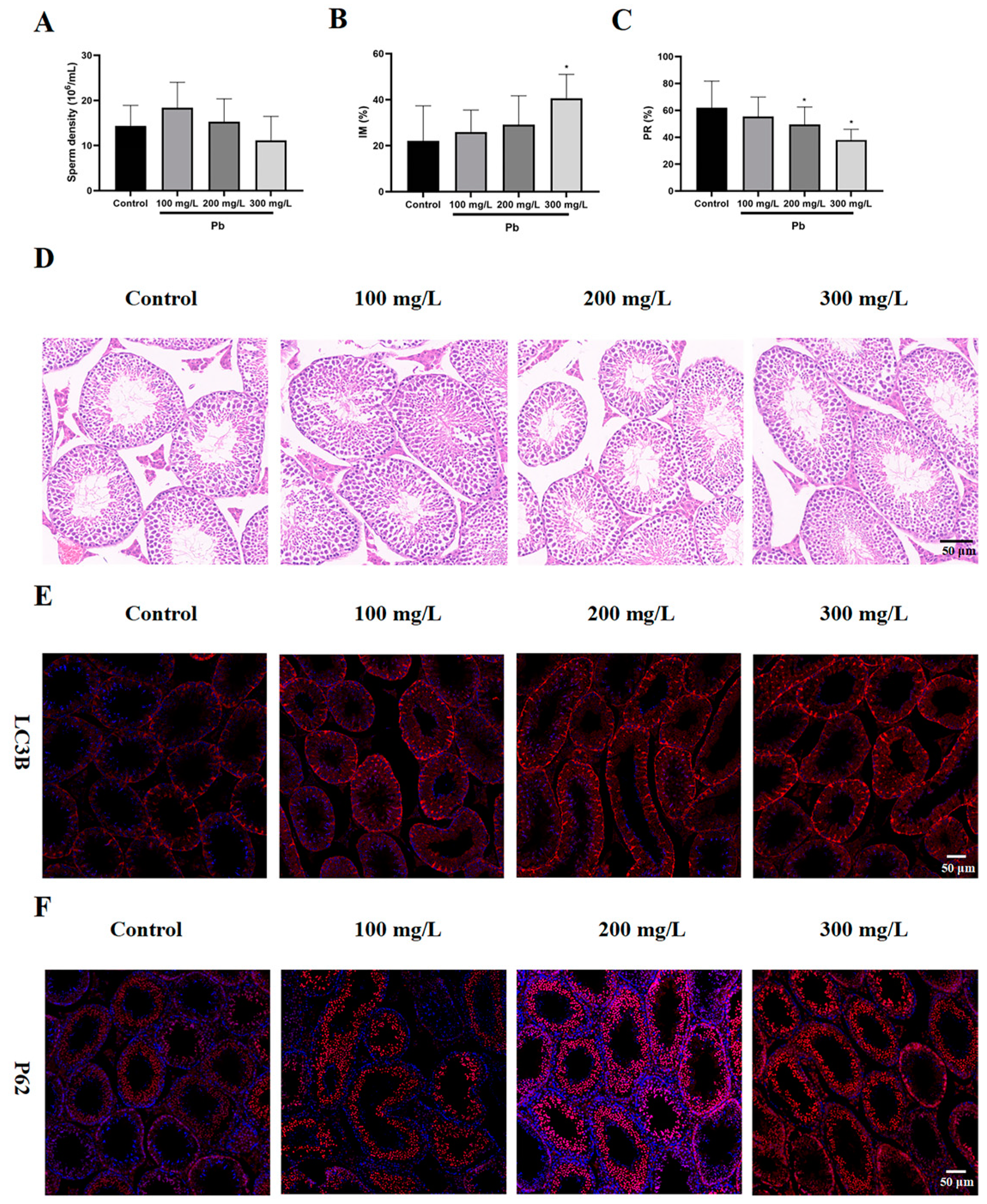
3.1. Lead Exposure Resulted in Reduced Sperm Quality and Increased Autophagy in Mouse Testes
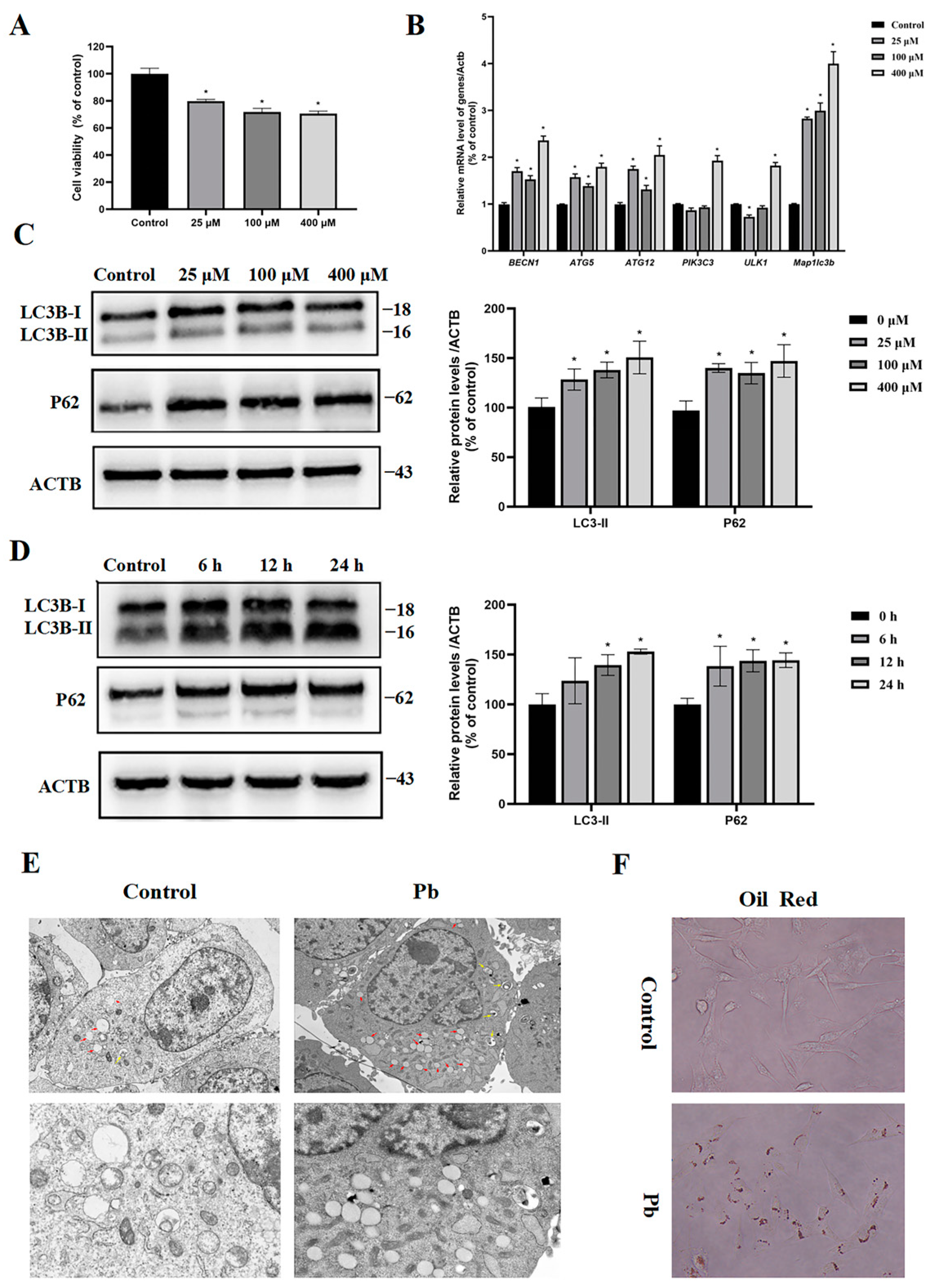
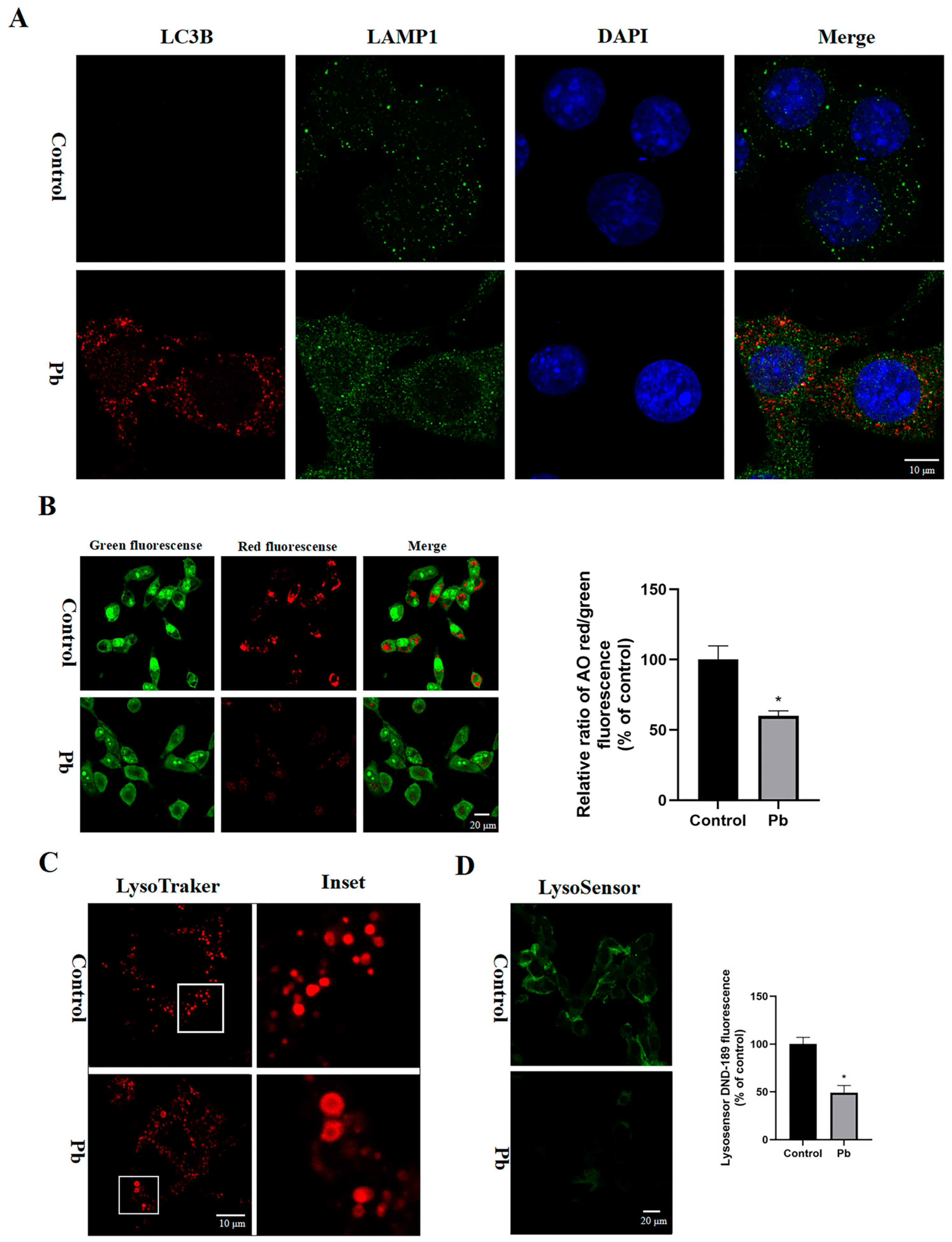
3.2. Pb Blocked Autophagy Flux in TM4 Cells
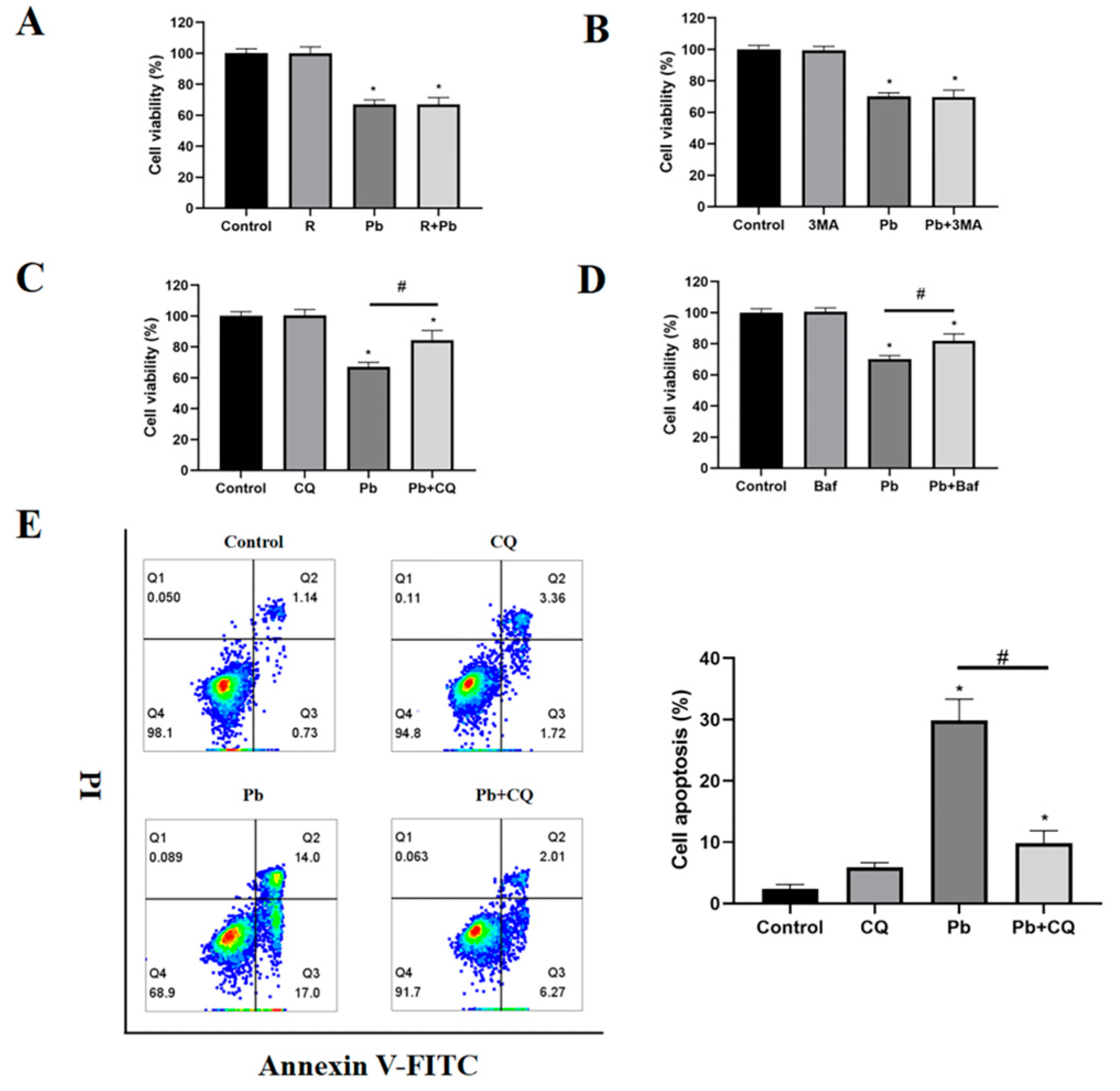
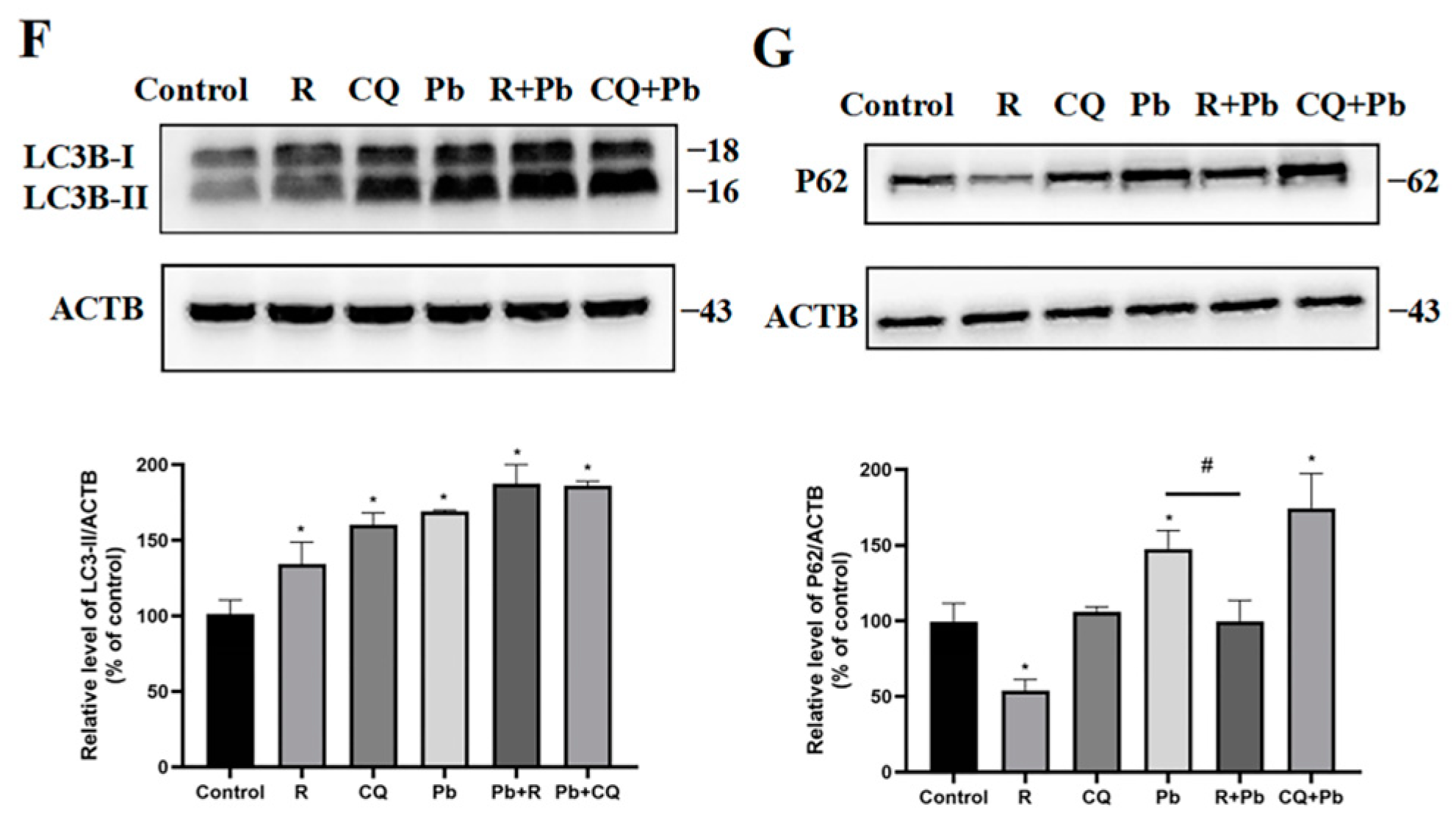
3.3. Preventing the Fusion of Autophagosomes with Lysosomes Reduced Pb-Induced Cell Damage
3.4. The Autophagosomes–Lysosomes Fusion and Lysosomal Function in the TM4 Cells Were Disrupted by Pb
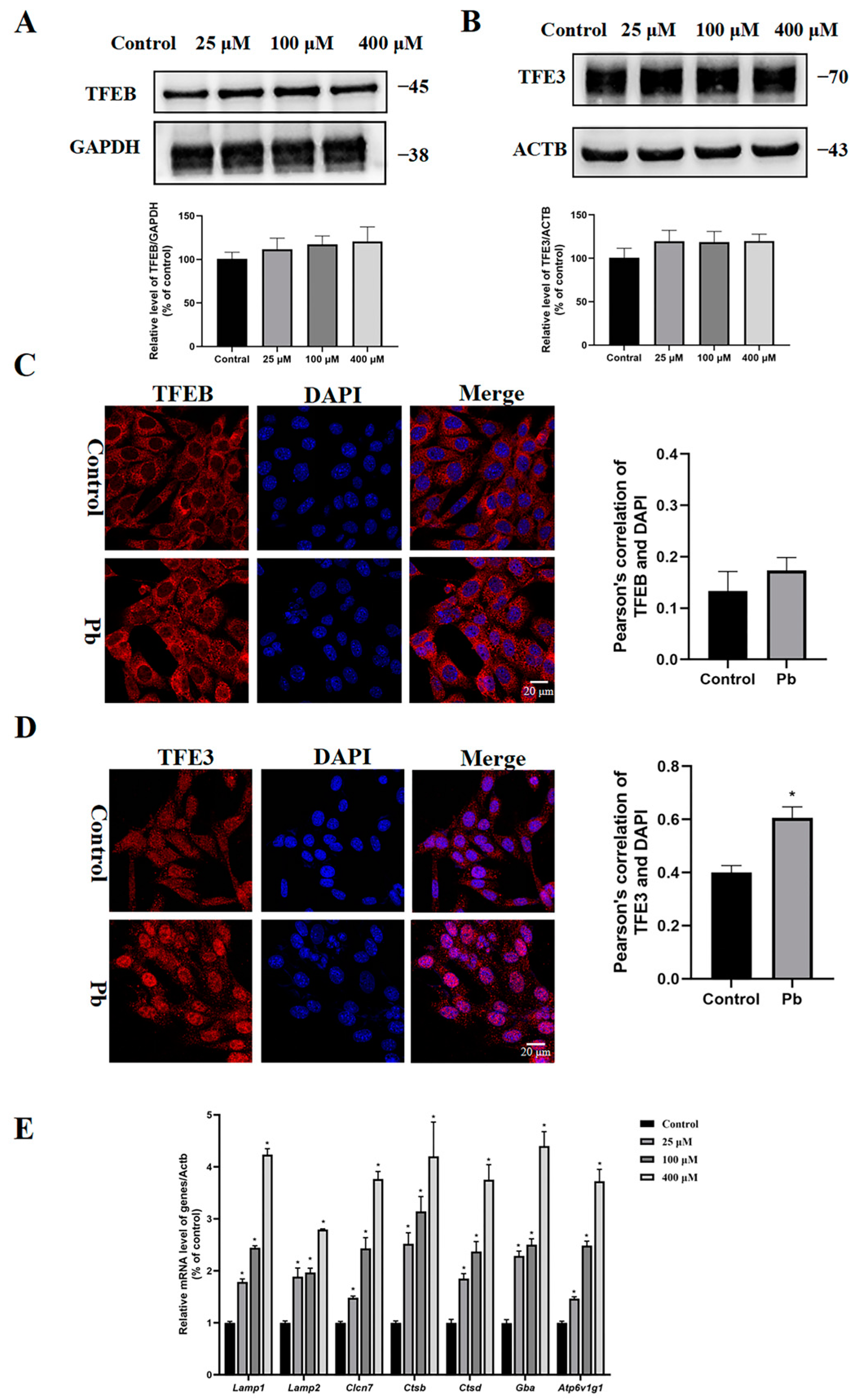
3.5. Pb Treatment Caused Nuclear Translocation of TFE3 Protein
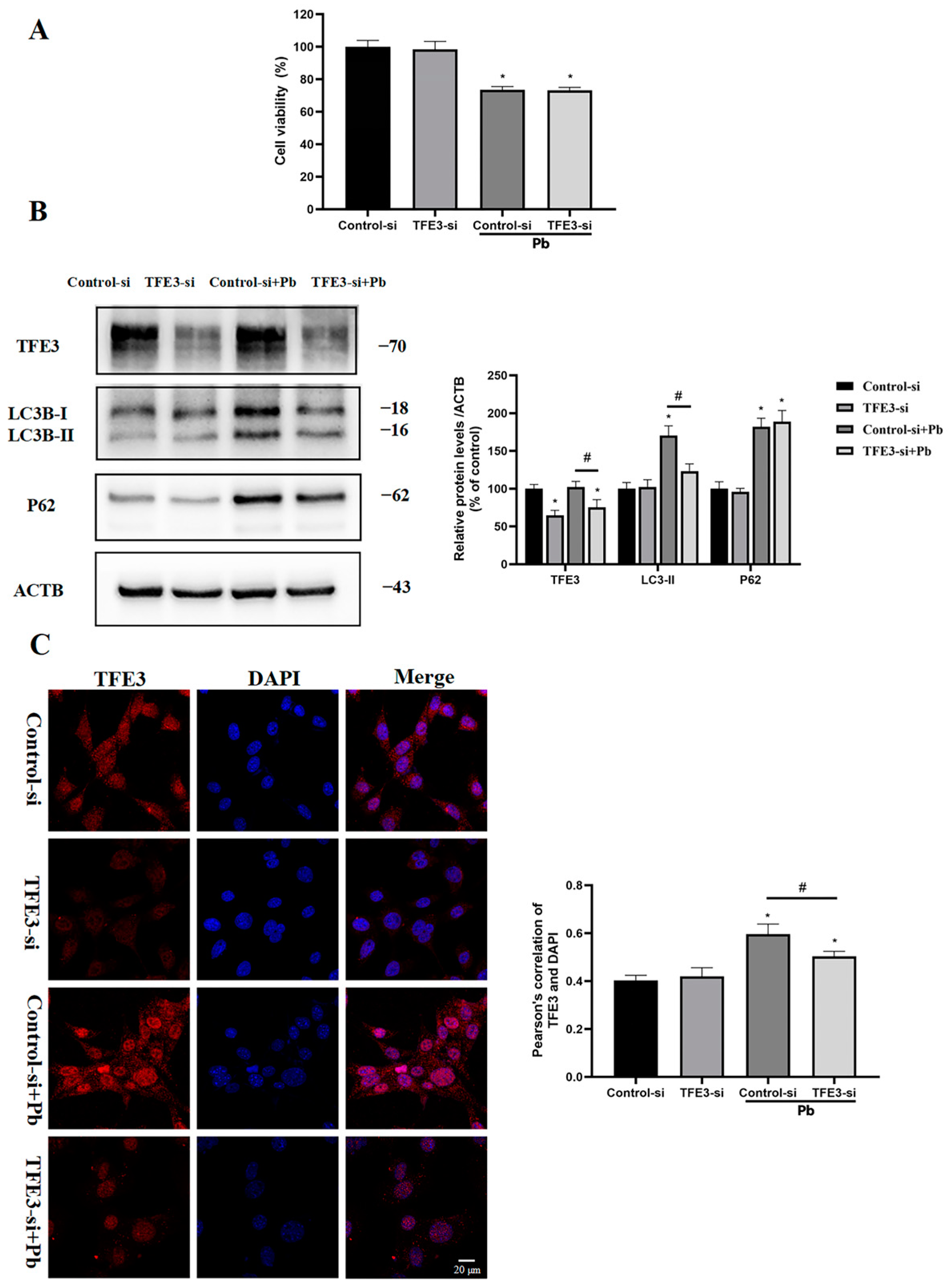
3.6. TFE3 Mediated Pb-Induced Autophagy in TM4 Cells
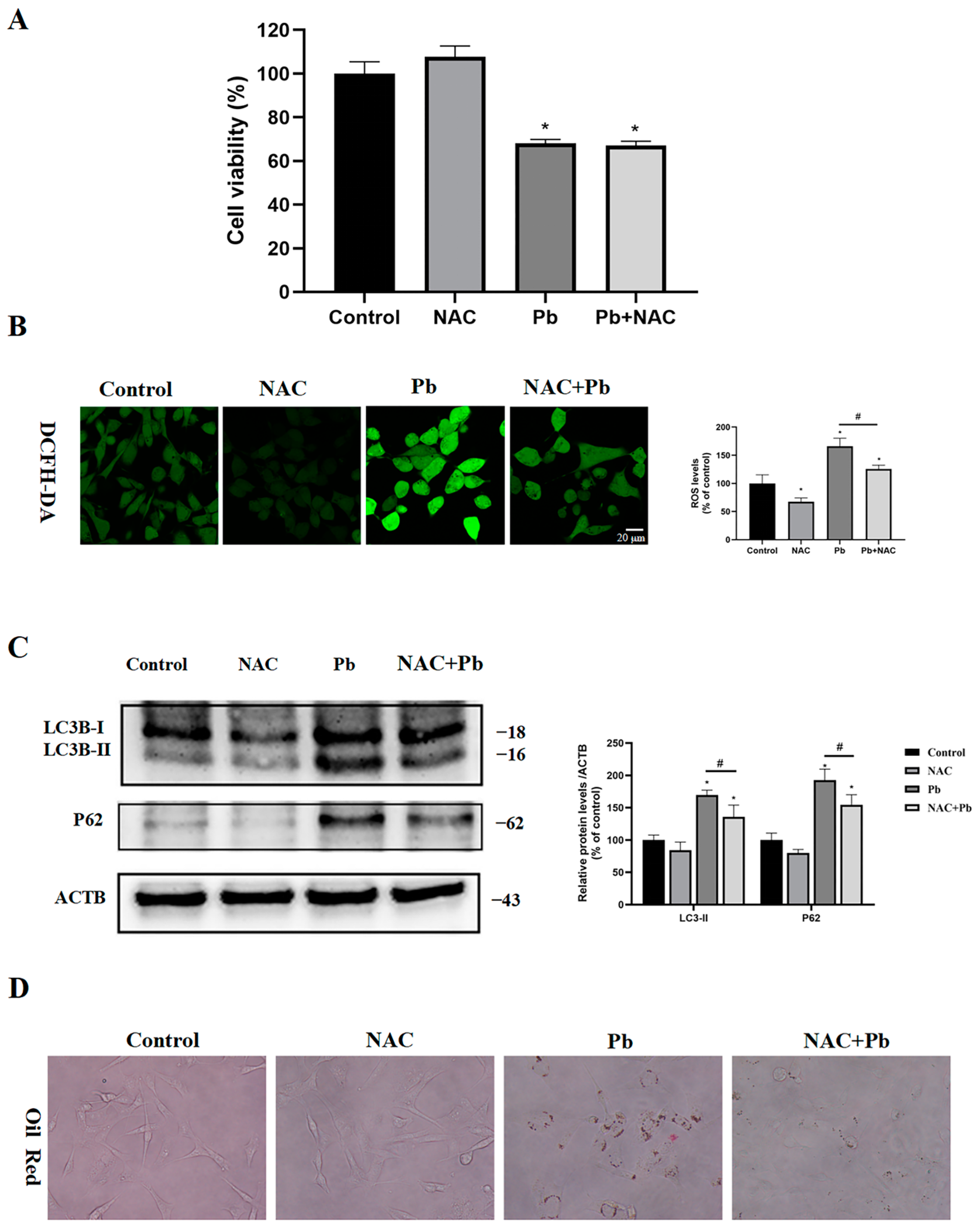
3.7. Inhibition of ROS Partially Restored Autophagic Flux and Reduced Lipid Droplet Accumulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.; Gao, B.; Hao, H.; Zhou, H.; Lu, J.; Sun, K. Lead contamination in sediments in the past 20 years: A challenge for China. Sci. Total Environ. 2018, 640–641, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, M.E.; Afshari, R. Historical documentation of lead toxicity prior to the 20th century in English literature. Hum. Exp. Toxicol. 2018, 37, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.L.; Mínguez-Alarcón, L.; Korrick, S.A.; Lee, M.M.; Plaku-Alakbarova, B.; Burns, J.S.; Smigulina, L.; Dikov, Y.; Abou Ghayda, R.; Hauser, R.; et al. Association of peripubertal blood lead levels with reproductive hormones and semen parameters in a longitudinal cohort of Russian men. Hum. Reprod. 2022, 37, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Fiore, M.; Giacone, F.; Altomare, M.; Asero, P.; Ledda, C.; Romeo, G.; Mongioi, L.M.; Copat, C.; Giuffrida, M.; et al. Exposure to multiple metals/metalloids and human semen quality: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 215, 112165. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yu, J.; Fan, Y.; Zhao, X.; Su, J.; Meng, Y.; Wu, Y.; Uddin, M.B.; Wang, C.; Wang, Z. Low dose lead exposure at the onset of puberty disrupts spermatogenesis-related gene expression and causes abnormal spermatogenesis in mouse. Toxicol. Appl. Pharmacol. 2020, 393, 114942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, C.; Lv, Z.; Jiang, H.; Li, S.; Yu, L.; Zhang, Z. From animal to cell model: Pyroptosis targeted-fibrosis is a novel mechanism of lead-induced testicular toxicity. Food Chem. Toxicol. 2023, 178, 113886. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, L.; Wang, Y.; Du, C.; Xu, S.; Zhang, Y.; Wang, C.; Yang, C. The role of PGC-1alpha and MRP1 in lead-induced mitochondrial toxicity in testicular Sertoli cells. Toxicology 2016, 355–356, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Gorga, A.; Rindone, G.M.; Regueira, M.; Pellizzari, E.H.; Camberos, M.C.; Cigorraga, S.B.; Riera, M.F.; Galardo, M.N.; Meroni, S.B. PPARgamma activation regulates lipid droplet formation and lactate production in rat Sertoli cells. Cell Tissue Res. 2017, 369, 611–624. [Google Scholar] [CrossRef]
- Yu, J.; Sun, J.; Fan, Y.; Su, J.; Xie, J.; Wu, Y.; Liu, X.; Wang, C. Exposure to Pb and Cd alters MCT4/CD147 expression and MCT4/CD147-dependent lactate transport in mice Sertoli cells cultured in vitro. Toxicol. In Vitro 2019, 56, 30–40. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Xiong, W.; Chen, Y.; Ma, Q.; Ma, J.; Ge, Y.; Han, D. Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining. Reproduction 2006, 132, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhao, L.; Li, S.; Chen, X.; Ling, X.; Zheng, J.; Yu, K.; Xu, J.; Yao, C.; Han, S.; et al. Targeting dysregulated phago-/auto-lysosomes in Sertoli cells to ameliorate late-onset hypogonadism. Nat. Aging 2024, 4, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Shafai, A.E.; Zohdy, N.; Mulla, K.E.; Hassan, M.; Morad, N. Light and electron microscopic study of the toxic effect of prolonged lead exposure on the seminiferous tubules of albino rats and the possible protective effect of ascorbic acid. Food Chem. Toxicol. 2011, 49, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; Engelbrecht, A.M.; Lockshin, R.A.; Klionsky, D.J.; Zakeri, Z. The variability of autophagy and cell death susceptibility: Unanswered questions. Autophagy 2013, 9, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Wang, H.; Wan, H.; Li, X.; Liu, W.; Chen, Q.; Wang, Y.; Yang, L.; Tang, H.; Zhang, X.; Duan, E.; et al. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 2014, 24, 852–869. [Google Scholar] [CrossRef]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, K.; Ling, X.; Wang, Z.; Zou, P.; Wang, X.; Gao, J.; Yin, L.; Zhang, X.; Liu, J.; et al. DBP-induced endoplasmic reticulum stress in male germ cells causes autophagy, which has a cytoprotective role against apoptosis in vitro and in vivo. Toxicol. Lett. 2016, 245, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, W.; Bian, X.; Yuan, Y.; Gu, J.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol. Lett. 2014, 226, 182–191. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong, A.L.; Patange, S.; Raben, N.; Puertollano, R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, R.; Liu, C.; Ma, R.; Wang, L.; Chen, B.; Li, L.; Niu, J.; Zhao, D.; Mo, F.; et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. Biomed Res. Int. 2017, 2017, 9050754. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, J.; Ran, Y.; Wang, S.; Li, J.; Zhao, Y.; Ran, X.; Lu, B.; Liu, J.; Li, R. Ethylmalonic encephalopathy 1 initiates overactive autophagy in depleted uranium-induced cytotoxicity in the human embryonic kidney 293 cells. J. Biochem. Mol. Toxicol. 2021, 35, e22669. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Dong, W.; Li, Y.; Zheng, X.; Piao, F.; Li, S. Subchronic exposure to lead acetate inhibits spermatogenesis and downregulates the expression of Ddx3y in testis of mice. Reprod. Toxicol. 2013, 42, 242–250. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, C.; Lv, Z.; Li, J.; Jiang, H.; Li, S.; Yu, L.; Zhang, Z. Novel findings from arsenic-lead combined exposure in mouse testicular TM4 Sertoli cells based on transcriptomics. Sci. Total Environ. 2024, 913, 169611. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ye, J.; Yu, J.; Chen, L.; Zhou, L.; Wang, H.; Li, Z.; Wang, C. The accumulation and efflux of lead partly depend on ATP-dependent efflux pump-multidrug resistance protein 1 and glutathione in testis Sertoli cells. Toxicol. Lett. 2014, 226, 277–284. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Wang, K.; Kong, F.; Qiu, Y.; Chen, T.; Fu, J.; Jin, X.; Su, Y.; Gu, Y.; Hu, Z.; Li, J. Autophagy regulation and protein kinase activity of PIK3C3 controls sertoli cell polarity through its negative regulation on SCIN (scinderin). Autophagy 2023, 19, 2934–2957. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Ravel, C.; Rolland, A.D.; Bourmeyster, N.; Jegou, B. MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles. Cells 2021, 10, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, J.; Xie, J.; Liu, D.; Zhang, Z.; Wang, Z.; Wang, C. Genome-wide identification and functional analysis of long non-coding RNAs and mRNAs in male mice testes at the onset of puberty after low dose lead exposure. Toxicol. Appl. Pharmacol. 2021, 422, 115556. [Google Scholar] [CrossRef]
- Ji, X.; Wang, B.; Paudel, Y.N.; Li, Z.; Zhang, S.; Mou, L.; Liu, K.; Jin, M. Protective Effect of Chlorogenic Acid and Its Analogues on Lead-Induced Developmental Neurotoxicity Through Modulating Oxidative Stress and Autophagy. Front. Mol. Biosci. 2021, 8, 655549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, T.; Zhao, F.; Yao, T.; Chen, Y.; Liu, X.; Luo, W.; Chen, J. The Role of α-synuclein and Tau Hyperphosphorylation-Mediated Autophagy and Apoptosis in Lead-induced Learning and Memory Injury. Int. J. Biol. Sci. 2012, 8, 935–944. [Google Scholar] [CrossRef]
- Lv, X.H.; Zhao, D.H.; Cai, S.Z.; Luo, S.Y.; You, T.; Xu, B.L.; Chen, K. Autophagy plays a protective role in cell death of osteoblasts exposure to lead chloride. Toxicol. Lett. 2015, 239, 131–140. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Xu, D.; Ma, Q.; Chen, Y.; Xu, S.; Xia, Q.; Zhang, Y.; Prehn, J.H.M.; Wang, G.; et al. Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy 2020, 16, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Li, M.; Xie, J.; Yang, Z.; Xi, Y.; Yu, Z.; Zhou, Z. Transcription factor E3 protects against cadmium-induced apoptosis by maintaining the lysosomal-mitochondrial axis but not autophagic flux in Neuro-2a cells. Toxicol. Lett. 2018, 295, 335–350. [Google Scholar] [CrossRef]
- Pi, H.; Li, M.; Zou, L.; Yang, M.; Deng, P.; Fan, T.; Liu, M.; Tian, L.; Tu, M.; Xie, J.; et al. AKT inhibition-mediated dephosphorylation of TFE3 promotes overactive autophagy independent of MTORC1 in cadmium-exposed bone mesenchymal stem cells. Autophagy 2019, 15, 565–582. [Google Scholar] [CrossRef]
- Siddarth, M.; Chawla, D.; Raizada, A.; Wadhwa, N.; Banerjee, B.D.; Sikka, M. Lead-induced DNA damage and cell apoptosis in human renal proximal tubular epithelial cell: Attenuation via N-acetyl cysteine and tannic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22038. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, K.; He, J.; Zhang, G.; Zhang, D.; Chen, F. TFE3 Alleviates Hepatic Steatosis through Autophagy-Induced Lipophagy and PGC1α-Mediated Fatty Acid β-Oxidation. Int. J. Mol. Sci. 2016, 17, 387. [Google Scholar] [CrossRef] [PubMed]
| Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | |
|---|---|---|
| Becn1 | ATGGAGGGGTCTAAGGCGTC | TGGGCTGTGGTAAGTAATGGA |
| Atg5 | TGTGCTTCGAGATGTGTGGTT | ACCAACGTCAAATAGCTGACTC |
| Atg12 | CGGAAGATTCAGAGGTTGTGCT | CAGCCTTCAGCAGGATGTCAA |
| Ulk1 | AAGTTCGAGTTCTCTCGCAAG | ACCTCCAGGTCGTGCTTCT |
| Pik3c3 | GGGCTATACCAAGAGACATGC | CGCCTTGTAGGATGTTCTGACT |
| Map1lc3b | TTATAGAGCGATACAAGGGGGAG | CGCCGTCTGATTATCTTGATGAG |
| Lamp1 | CAGCACTCTTTGAGGTGAAAAAC | CCATTCGCAGTCTCGTAGGTG |
| Lamp2 | TGTATTTGGCTAATGGCTCAGC | TATGGGCACAAGGAAGTTGTC |
| Clcn7 | GACAACAGCGAGAATCAGCTC | CCAATGAGGGCACAGATAACC |
| Ctsb | CAGGCTGGACGCAACTTCTAC | TCACCGAACGCAACCCTTC |
| Ctsd | CGATTATCAGAATCCCTCTGCG | GGTCTTAGGCGATGACTGCAT |
| Gba | GTATGGCCTAAGATTCTGGGC | CTAGGTCACGGGAAATGAAGTC |
| Atp6v1g1 | CCCAGGCTGAAATTGAACAGT | TTCTGGAGGACGGTCATCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Wang, L.; Cui, K.; Zhang, G.; Tan, Y.; Chen, W.; Wang, Y.; Liu, J.; Liu, W.; Zhang, G.; et al. Lead Causes Lipid Droplet Accumulation by Impairing Lysosomal Function and Autophagic Flux in Testicular Sertoli Cells. Toxics 2025, 13, 175. https://doi.org/10.3390/toxics13030175
Guo C, Wang L, Cui K, Zhang G, Tan Y, Chen W, Wang Y, Liu J, Liu W, Zhang G, et al. Lead Causes Lipid Droplet Accumulation by Impairing Lysosomal Function and Autophagic Flux in Testicular Sertoli Cells. Toxics. 2025; 13(3):175. https://doi.org/10.3390/toxics13030175
Chicago/Turabian StyleGuo, Chengwei, Lingqiao Wang, Ke Cui, Guowei Zhang, Yao Tan, Weiyan Chen, Yiqi Wang, Jijun Liu, Wenbin Liu, Guanghui Zhang, and et al. 2025. "Lead Causes Lipid Droplet Accumulation by Impairing Lysosomal Function and Autophagic Flux in Testicular Sertoli Cells" Toxics 13, no. 3: 175. https://doi.org/10.3390/toxics13030175
APA StyleGuo, C., Wang, L., Cui, K., Zhang, G., Tan, Y., Chen, W., Wang, Y., Liu, J., Liu, W., Zhang, G., & Zhou, Z. (2025). Lead Causes Lipid Droplet Accumulation by Impairing Lysosomal Function and Autophagic Flux in Testicular Sertoli Cells. Toxics, 13(3), 175. https://doi.org/10.3390/toxics13030175






