Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives
Abstract
:1. Introduction
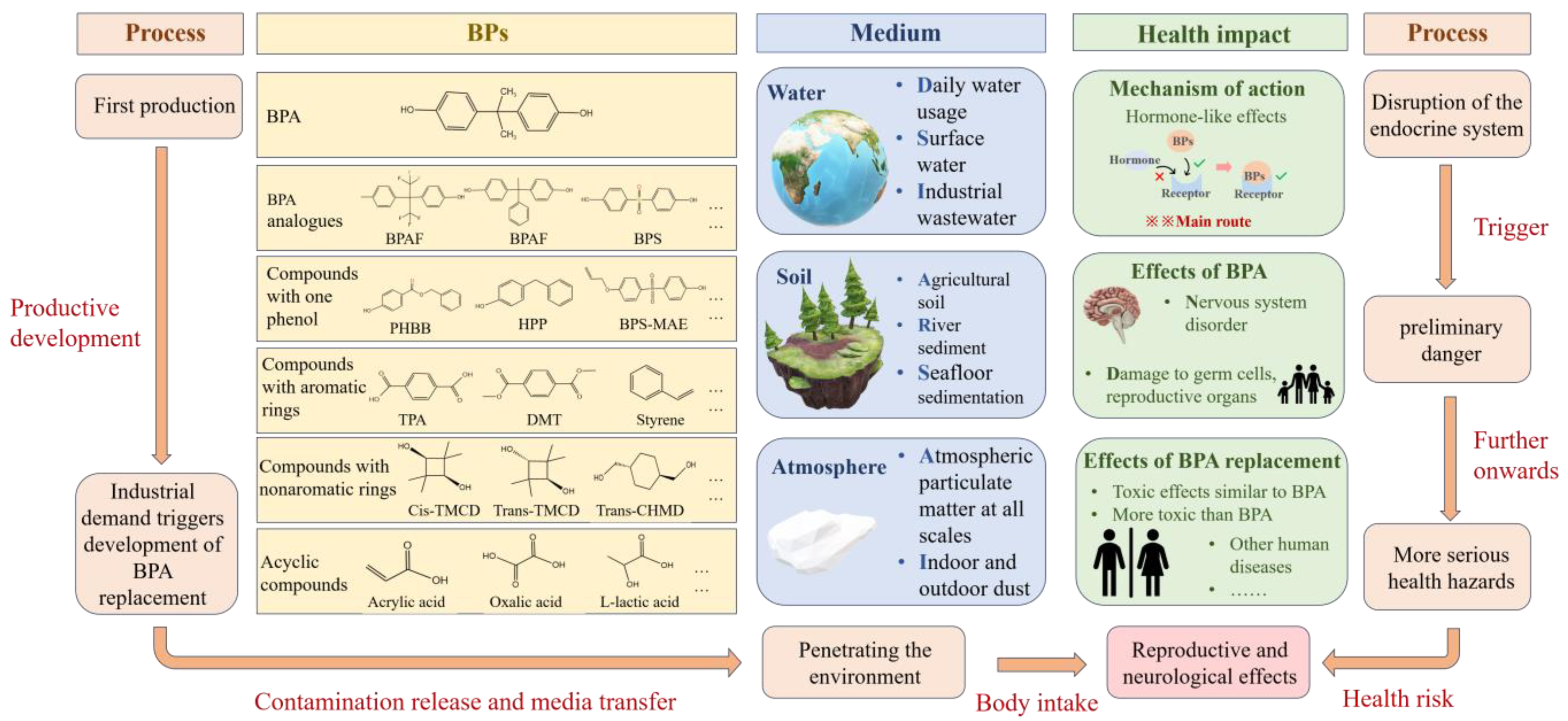
2. Pollution Characteristics
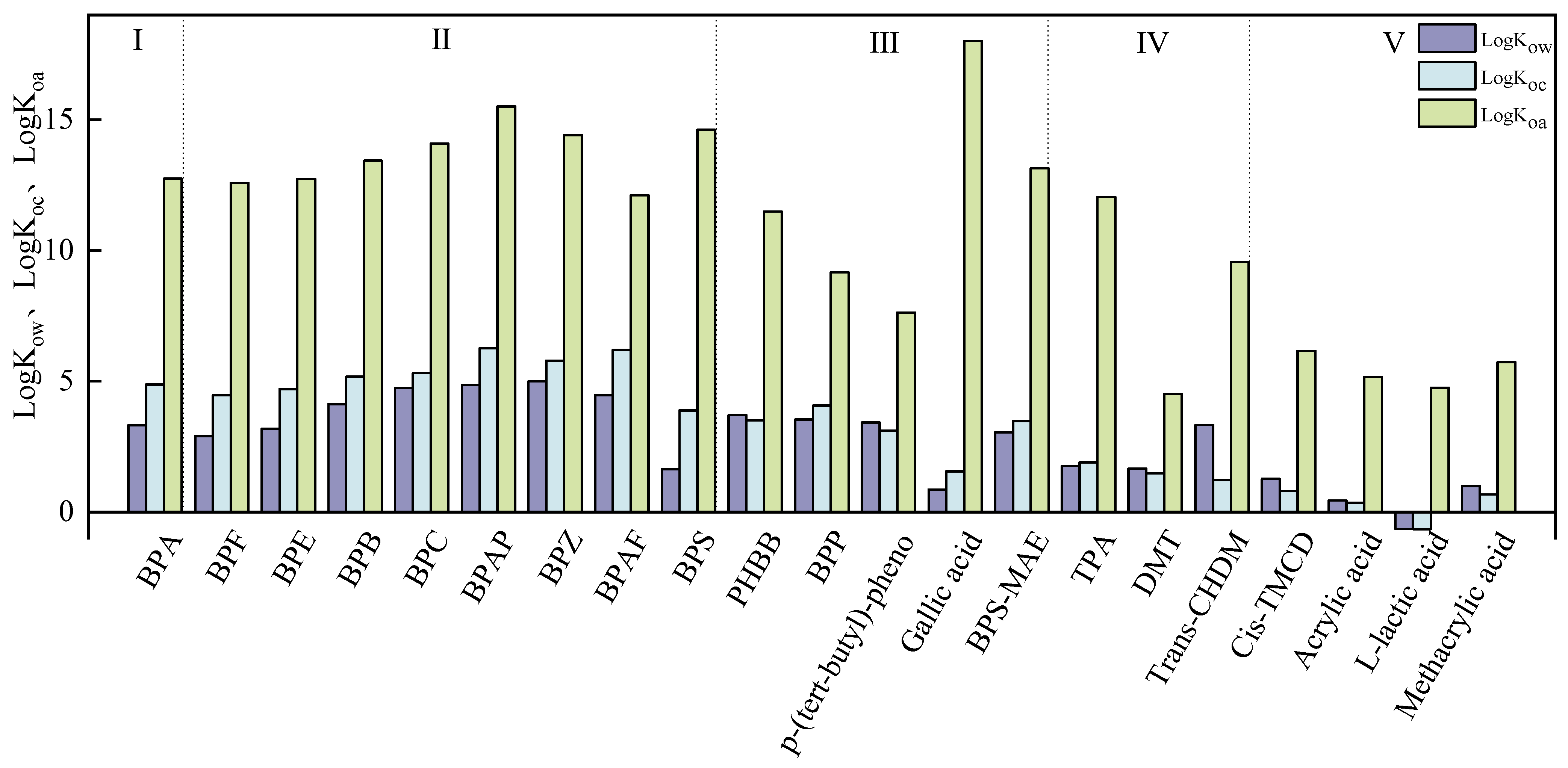
2.1. Physical Properties
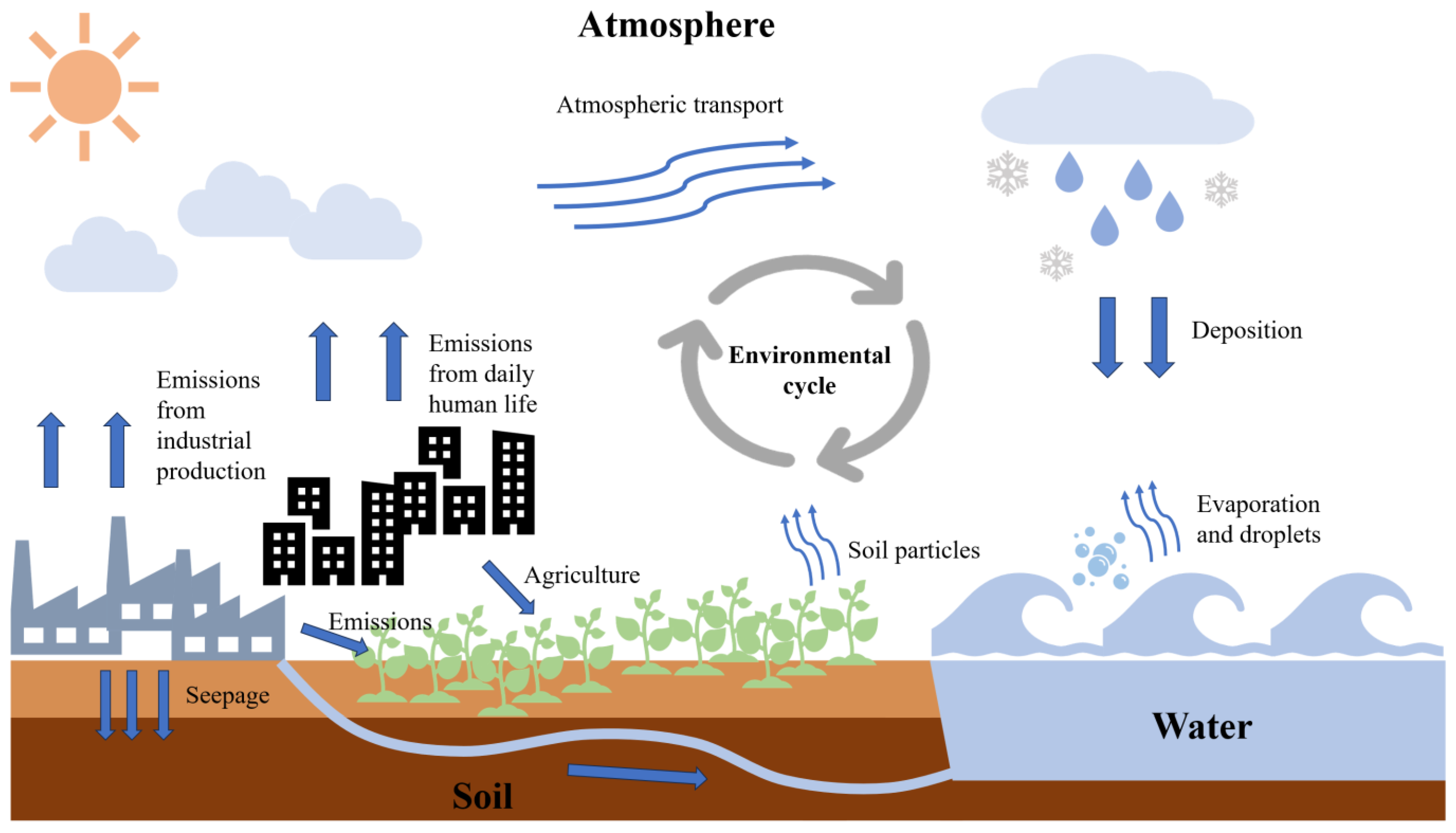
2.2. Environmental Transmission

3. Analytical Methods and Detection Techniques
| Detection Settings | Detectable Substances | Optimization Means | Detection Media | Advantage | Disadvantage | Recovery Rate (%) | Detection Limit | Quantification Limit | Linear Range | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Ultra-high-performance liquid chromatography–tandem mass spectrometry | BPA, BPS, BPF, BPAF, BPAP, BPZ, etc. | - | Air, drinking water, human urine, biological samples, soil, leachate | High sensitivity, good recovery rate, and good repeatability | - | 67.6~119 | 0.007–1.5 µg/L | 0.5 µg/kg | 0.1~100 µg/mL | [5,52,54,60] |
| High-performance liquid chromatography | BPA, BPS, BPF, BPAF, and BPAP | - | Vegetable oil, drinking water, human urine | High sensitivity and good repeatability | Difficult to achieve rapid on-site detection | 90.8~103.2 | 0.007 mg/kg | 13 µg/kg | 0.003~0.7 µg/mL | [52,53,58,60] |
| Liquid chromatography–mass spectrometry tandem method | BPA, BPS, BPF, BPAP, etc. | Salting-out-assisted liquid–liquid extraction | Milk powder | Rapid test | - | 80.8–118.1 | 0.15–0.75 µg/kg | 0.5–2.5 µg/kg | - | [61] |
| Fourier change infrared spectroscopy | BPA | An amine-functionalized poly (N-vinylpyrrolidone divinylbenzene) adsorbent was prepared for the detection of BPA | Water | Low cost, good functional degree, strong selective adsorption ability, and less environmental pollution | - | 99.65 | 0.5 mg/kg | - | 0.5~3.0 µg/mL | [55] |
| Liquid chromatography–tandem triple quadrupole mass spectrometry | BPA | N-hexane/acetone (4:1) ultrasonic extraction three times | Dust | - | 84.5~100 | 0.002–0.018 mg/kg | - | - | [51] | |
| Photoelectric chemical sensor method without bias voltage | BPA | Self-powered molecularly imprinted photo/photochemical sensing based on CdSe/ZnS QD/HOF heterojunction | - | High sensitivity and high selectivity | - | - | - | - | - | [57] |
| Electrochemical method of carbon nanotube composites based on cobalt nanoparticles/nitrogen doping | BPA | Electrochemical method of carbon nanotube composites based on cobalt nanoparticles/nitrogen doping | - | High selectivity, good stability, and good reproducibility | - | 98.4~104.6 | 5.0 nmol/L | 0.005 µmol/L | 0.01~20 (µmol/L) | [56] |
| Magnesium phytate-modified electrode method | BPA | Magnesium phytate-based modified electrode | - | High sensitivity, wide linear range, good repeatability, low electrode cost, and good stability | - | 92.5~101.5 | 0.1 µmol/L | - | 0.8~50 (µmol/L) | [58] |
| Gold nanoparticle–aptamer electrochemical sensing method | BPA | Gold nanoparticles were prepared on the surface of a glassy carbon electrode via potentiostatic deposition | Water | High sensitivity, wide linear range, good repeatability, low electrode cost, and good stability | - | 87.4~110.0 | 10−9 mg/kg | 10−9 µg/kg | 10−9~5 × 10−6 µg/mL | [59] |
| Solid-phase extraction combined with liquid chromatography–mass spectrometry | BPA | The pH value of the sample is 2 5 mL pure methanol and 5 mL dichloromethane solution | Water | - | 74.41~111.2 | 0.0001~0.0033 µg/L | - | 0.1~100 µg/L | [62] |
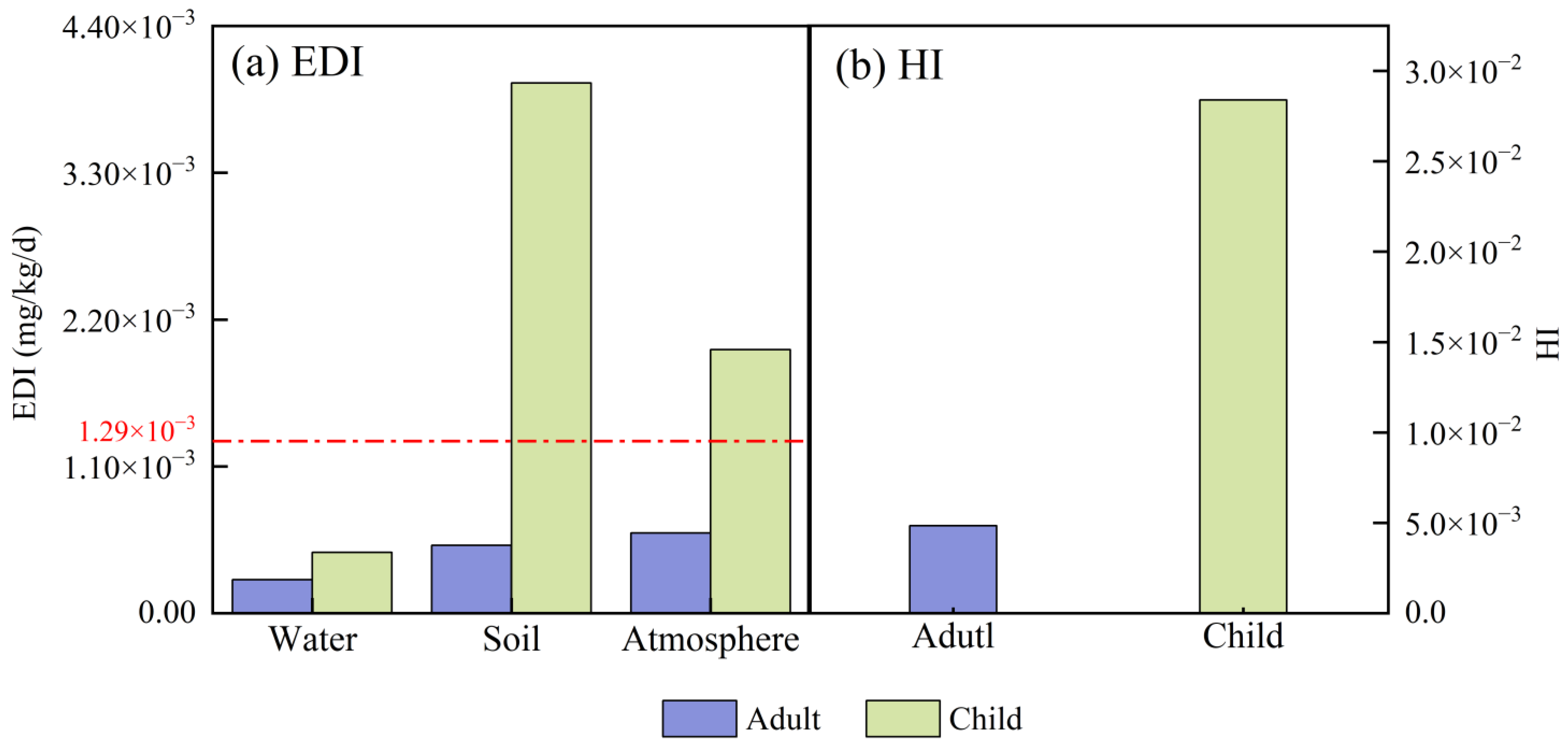
4. Potential Hazards and Risk Assessment
5. Suggestions for Future Prevention and Control Countermeasures
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.; Wang, S.; Yu, P.; Wang, D.; Hu, B.; Zheng, P.; Zhang, M. A Bibliometric Analysis of Emerging Contaminants (ECs) (2001−2021): Evolution of Hotspots and Research Trends. Sci. Total Environ. 2024, 907, 168116. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P. Overview of Air Pollution and Endocrine Disorders. Int. J. Gen. Med. 2018, 11, 191–207. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Miao, M.; Li, D.; Wang, Z.; Li, R.; Liang, H.; Yuan, W. Association of Bisphenol A Exposure with LINE-1 Hydroxymethylation in Human Semen. Int. J. Environ. Res. Public Health 2018, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. The Pollution Characteristics and Bioaccumulation of BPs in Multiple Media at Longyearbyen, Arctic. Master’s Thesis, Harbin Institute of Technology, Shenzhen, Guangdong, 2022. [Google Scholar]
- Qiao, Y.; Yan, Z.; Feng, C.; Wang, J.; Bai, Y.; Wu, F. Research Focus Analysis of Endocrine Disrupting Chemicals (EDCs) Based on Bibliometrics. Res. Environ. Sci. 2022, 35, 424–434. [Google Scholar] [CrossRef]
- Schneider, M.; Pons, J.-L.; Labesse, G.; Bourguet, W. In Silico Predictions of Endocrine Disruptors Properties. Endocrinology 2019, 160, 2709–2716. [Google Scholar] [CrossRef]
- Yang, J. Endocrine Disrupting Effects of Typical Bisphenols and their Ecological Risks in the Sediments of Taihu Laike. Master’s Thesis, Chinese Research Academy of Environmental Sciences, Beijing, China, 2024. [Google Scholar]
- Švajger, U.; Dolenc, M.S.; Jeras, M. In Vitro Impact of Bisphenols BPA, BPF, BPAF and 17β-Estradiol (E2) on Human Monocyte-Derived Dendritic Cell Generation, Maturation and Function. Int. Immunopharmacol. 2016, 34, 146–154. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The Comparative Toxicities of BPA, BPB, BPS, BPF, and BPAF on the Reproductive Neuroendocrine System of Zebrafish Embryos and Its Mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, X.; Zhang, Y.; Li, Y.; Qiu, J.; Mu, X.; Jiang, J.; Qian, Y. Advances in potential impacts of bisphenol A and its alternatives on gut-brain regulation and involved mechanisms. Asian J. Ecotoxicol. 2023, 18, 159–174. [Google Scholar]
- Xiong, S.; Wang, X.; Luo, W.; Ma, Y.; Lin, Y.; Wang, M.; Zheng, J. Spatia Distribution, Ecological Risk and Industry-Dependence of Endocrine Disrupting Chemicals in the Beijiang River, South China. Environ. Chem. 2021, 40, 3803–3814. [Google Scholar]
- Wang, Q.; Feng, Q.; Zhu, X. Determination of bisphenols in sediment by accelerated solvent extraction and solid-phase extraction purification coupled with ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2023, 41, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Zhu, A.; Liu, G.; Liu, P. Distribution and ecological risk assessment of bisphenols in Fuhe River and Baiyang Lake. J. Environ. Health 2025, 1–5. Available online: http://kns.cnki.net/kcms/detail/12.1095.r.20240410.1710.004.html (accessed on 9 January 2025).
- Qin, Y.; Jian, B. Impact of Bisphenol A Pollution on Nervous Immune System of Children with Autism Spectrum Disease. Environ. Sci. Manag. 2016, 41, 28–31. [Google Scholar]
- Chen, W.; Lau, S.-W.; Fan, Y.; Wu, R.S.S.; Ge, W. Juvenile Exposure to Bisphenol A Promotes Ovarian Differentiation but Suppresses Its Growth—Potential Involvement of Pituitary Follicle-Stimulating Hormone. Aquat. Toxicol. 2017, 193, 111–121. [Google Scholar] [CrossRef]
- Usman, A.; Ahmad, M. From BPA to Its Analogues: Is It a Safe Journey? Chemosphere 2016, 158, 131–142. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, J.; Gao, C.; Qiu, R.; Li, Y.; Li, X.; Huang, M.; Kannan, K. Urinary Concentrations of Bisphenols and Their Association with Biomarkers of Oxidative Stress in People Living Near E-Waste Recycling Facilities in China. Environ. Sci. Technol. 2016, 50, 4045–4053. [Google Scholar] [CrossRef]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Jiménez Cisneros, B. Accumulation and Leaching Potential of Some Pharmaceuticals and Potential Endocrine Disruptors in Soils Irrigated with Wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, J.; Sakkiah, S.; Guo, W.; Hong, H. BPA Replacement Compounds: Current Status and Perspectives. ACS Sustain. Chem. Eng. 2021, 9, 2433–2446. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Bisphenol A Alternatives in Thermal Paper. 2014. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/bpa_final.pdf (accessed on 9 January 2025).
- Li, J.; Wang, G. Airborne Particulate Endocrine Disrupting Compounds in China: Compositions, Size Distributions and Seasonal Variations of Phthalate Esters and Bisphenol A. Atmos. Res. 2015, 154, 138–145. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Qin, H.; Gao, J.; Qu, G.; Jiang, G. The effect of bisphenols on the secretion of exosomes. Environ. Chem. 2024, 43, 1–7. [Google Scholar]
- Pastor-Belda, M.; Bastida, D.; Campillo, N.; Pérez-Cárceles, M.D.; Motas, M.; Viñas, P. A Study of the Influence on Diabetes of Free and Conjugated Bisphenol A Concentrations in Urine: Development of a Simple Microextraction Procedure Using Gas Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 458–465. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, L.; Qiu, Z.; Lee, D.Y.; Settle, S.J.; Que Hee, S.; Telesca, D.; Yang, X.; Allard, P. Exposure to the BPA-Substitute Bisphenol S Causes Unique Alterations of Germline Function. PLoS Genet 2016, 12, e1006223. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA), EPI SuiteTM-Estimation Program Interface. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 9 January 2025).
- Liu, J.; Jin, Y.; Wei, Q.; Jin, Z.; Wei, D.; Jin, Y. Research progress on reproductive toxicity and reproductive system tumors induced by environmental endocrine disrupting chemicals. J. Environ. Occup. Med. 2022, 39, 833–839. [Google Scholar]
- Devi, T.; Saleh, N.M.; Kamarudin, N.H.N.; Roslan, N.J.; Jalil, R.; Hamid, H.A. Efficient Adsorption of Organic Pollutants Phthalates and Bisphenol A (BPA) Utilizing Magnetite Functionalized Covalent Organic Frameworks (MCOFs): A Promising Future Material for Industrial Applications. Ecotoxicol. Environ. Saf. 2023, 268, 115706. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, L.; Liu, Y.; Tang, X.; Miao, Y.; Zhang, J.; Chen, J. Genotoxicity of Bisphenol AF in Rats: Detrimental to Male Reproductive System and Probable Stronger Micronucleus Induction Potency than BPA. J. Appl. Toxicol. 2024, 44, 428–444. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K. Ubiquity of Bisphenol A in the Atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef]
- Jiang, X.; Rotily, L.; Villermaux, E.; Wang, X. Submicron Drops from Flapping Bursting Bubbles. Proc. Natl. Acad. Sci. USA 2022, 119, e2112924119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rao, Z.; Guo, F.; Liu, C.; Zhan, N.; Wang, Y.; Peng, J.; Yang, H. Occurrence Characteristics and Health Risk Assessment of Endocrine Disrupting Chemicals in Groundwater in Wuxi-Changzhou. Environ. Sci. 2021, 42, 166–174. [Google Scholar] [CrossRef]
- Wang, P. Study on Migration and Transformation of Representative Endocrine Disrupt Chemicals during Reclaimed Water Infltrating into Groundwater. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Ju, X.; Gao, Z.; Zheng, W.; Zhang, Q. Identification and Derivation of Emerging Contaminants in the Roof Rainwater Confluence. Environ. Sci. 2024, 45, 4032–4043. [Google Scholar] [CrossRef]
- Zhao, B.; Tan, X.; Xue, M.; Lu, J.; Xu, D.; Yang, R.; Zhang, L.; Gou, W. Pollution Status and Distribution Characteristics of Bisphenols in Rivers of Guangyuan City. Environ. Monit. Forewarning 2023, 15, 17–23. [Google Scholar]
- Dueñas-Moreno, J.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J. Groundwater Contamination Pathways of Phthalates and Bisphenol A: Origin, Characteristics, Transport, and Fate – A Review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef]
- Kumawat, M.; Sharma, P.; Pal, N.; James, M.M.; Verma, V.; Tiwari, R.R.; Shubham, S.; Sarma, D.K.; Kumar, M. Occurrence and Seasonal Disparity of Emerging Endocrine Disrupting Chemicals in a Drinking Water Supply System and Associated Health Risk. Sci Rep 2022, 12, 9252. [Google Scholar] [CrossRef]
- Chen, M.; Guo, M.; Liu, D.; Li, J.; Zhang, S.; Shi, L. Occurrence and distribution of typical endocrine disruptors in surface water and sediments from Taihu Lake and its tributaries. Chin. Environ. Sci. 2017, 37, 4323–4332. [Google Scholar]
- Ma, B.; Wang, L.; Tao, W.; Liu, M.; Zhang, P.; Zhang, S.; Li, X.; Lu, X. Phthalate Esters in Atmospheric PM2.5 and PM10 in the Semi-Arid City of Xi’an, Northwest China: Pollution Characteristics, Sources, Health Risks, and Relationships with Meteorological Factors. Chemosphere 2020, 242, 125226. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, Q.; Mou, J.; Luo, W. Bisphenol A secondary pollution in barreled drinking water and its exposure assessment. Pract. Prev. Med. 2017, 24, 43–45. [Google Scholar]
- Xu, Y.; Hu, A.; Li, Y.; He, Y.; Xu, J.; Lu, Z. Determination and Occurrence of Bisphenol A and Thirteen Structural Analogs in Soil. Chemosphere 2021, 277, 130232. [Google Scholar] [CrossRef]
- Ye, Z. Studies on the Pollution Distribution of Typical Bisphenol Compounds in Soil and Earthworms and Toxicity Effects. Master’s Thesis, Shenzhen University, Shenzhen, China, 2021. [Google Scholar]
- Zhang, Y. Research on Pollution Characteristics and Health Risks of Bisphenols in Soil at National Scale. Master’s Thesis, Harbin Institute of Technology, Shenzhen, China, 2022. [Google Scholar]
- Teil, M.-J.; Moreau-Guigon, E.; Blanchard, M.; Alliot, F.; Gasperi, J.; Cladière, M.; Mandin, C.; Moukhtar, S.; Chevreuil, M. Endocrine Disrupting Compounds in Gaseous and Particulate Outdoor Air Phases According to Environmental Factors. Chemosphere 2016, 146, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.; Yu, J.; Xu, J. Investigation and Environmental Risk Assessment of Octylphenol, Nonylphenol and Bisphenol A in the Sediment of Xiangjiang River in Zunyi City; Chinese Society for Environmental Sciences: Beijing, China, 2019; pp. 1274–1278.
- Loganathan, S.N.; Kannan, K. Occurrence of Bisphenol A in Indoor Dust from Two Locations in the Eastern United States and Implications for Human Exposures. Arch. Env. Contam. Toxicol. 2011, 61, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Liu, Y.; Sun, Y.; Wu, M.; Ma, J. Comparative assessment of human exposure to phthalate esters and bisphenol A from different indoor dust. J. Shanghai Univ. Nat. Sci. 2019, 25, 282–292. [Google Scholar]
- Wu, W.; Guan, C.; Wang, G.; Wang, Z.; Chen, Z.; Li, L.; Feng, D. A Study on Determination of 8 Kinds of Bisphenols in Packaged Drinking Water by High Per-Formance Liguid Chromatography-Tandem Mass Spectrometry. China Meas. Test 2022, 48, 78–82. [Google Scholar]
- Liu, Y.; Jiang, W.; Hu, R.; Wu, X. Study of the Method for the Determination of Bisphenol a in Vegetable Oil by High Performance Liquid Chromatography. Sci. Technol. Cereals Oils Foods 2022, 30, 153–158. [Google Scholar] [CrossRef]
- Ling, Y.; Liu, Z. Determination of 14 Environmental Hormones in Workplace Air by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. PTCA Part B Chem. Anal. 2022, 58, 1449–1456. [Google Scholar]
- Zhang, J.; Cui, Y.; He, L.; Zhang, Y.; Meng, X.; Zhang, S. Modification of P(NVP-DVB) and its application in the detection of BPA in aqueous solution. Fine Chem. 2023, 40, 75–86. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, X.; Cui, X.; Zhu, Y.; Fan, J.; Liu, T.; Zheng, S. Electrochemical Detection of Bisphenol A Based on Co Nanoparticles/N-Doped Carbon Nano-Tubes Composites. Chin. J. Anal. Lab. 2023, 42, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bai, B.; Guo, C.; Zhang, J.; Yang, Y. A self- powered molecular imprinting photoelectrochemical sensor based on CdSe ZnS QDs/HOFs heterojunction for the detection of bisphenol A. In Proceedings of the Chinese Society of Food Science and Technology 19th Annual Meeting Paper Abstracts Col-Lection; Chinese Society of Food Science and Technology: Beijing, China, 2022; pp. 28–29. [Google Scholar]
- Zhang, Y.; Li, Y.; Yu, H.; Wang, H. Preparation of magnesium phytate-based modified electrode and its detection for bisphenol A. Chin. J. Anal. Lab. 2023, 42, 324–330. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Liu, J.; Tan, F. Gold nanoparticles-aptamer electrochemical sensor for detection of bisphenol A in environmental waters. Environ. Chem. 2023, 42, 379–387. [Google Scholar]
- Wu, W.; Guan, C.; Wang, G.; Wang, Z.; Chen, Z.; Li, L.; Chen, Q.; Cao, J.; Feng, D. Determination of 8 Kinds of Bisphenols in Urineby Solid Phase Extraction Combined with High Performance Liquid Chromatography-Tandem Mass Spectrometry. China Meas. Test 2023, 49, 75–80. [Google Scholar]
- Jiang, K.; Zhang, H.; Cao, H.; Wang, J.; Zhou, X.; Li, X. Salting out Assisted Liquid Liquid Extraction Coupled with HPLC-MS/MS for Determination of 13 Kinds of Bisphenols and Alkyl Phenol Compounds in Liquid Dairy Products. Food Mach. 2022, 225, 32–36. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Q.; Li, J.; Li, J.; Tong, W. Simultaneous determination of 9 alkyl phenols and bisphenol A in water by SPE combined with LC-MS/MS. Water Wastewater Eng. 2023, 59, 529–534. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Wang, R.; Su, X. Advances on pollution status and endocrine disrupting effects of bisphenols. Asian J. Ecotoxicol. 2022, 17, 60–81. [Google Scholar]
- Li, Y.; Jiao, Z.; Zhang, X.; Fu, W.; Wang, L.; Gong, H.; Jiang, G. Progress in the treatment technologies toward endocrine disrupter bisphenol A. Environ. Chem. 2023, 42, 4019–4031. [Google Scholar]
- Hong, S.; Hong, Y.; Kim, J.; Park, E.; Shin, M.; Kim, B.; Yoo, H.; Cho, I.; Bhang, S.; Cho, S. Bisphenol A in Relation to Behavior and Learning of School-age Children. Child Psychol. Psychiatry 2013, 54, 890–899. [Google Scholar] [CrossRef]
- Xie, N.; Wang, H.; Wang, S. Research progress on the effects of bisphenol A exposure on children’s health. Matern. Child Health Care China 2022, 37, 1539–1542. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.; Kim, Y. The Association between Bisphenol A Exposure and Obesity in Children—A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2521. [Google Scholar] [CrossRef]
- Tian, H.; Shan, L.; Cui, K.; Ru, S. Pollution status and adverse effects of bisphenols on early life. Asian J. Ecotoxicol. 2022, 17, 96–111. [Google Scholar]
- Wu, Y.; Lu, X.; Ren, Y.; Sun, S. The effect of environmental endocrine disruptoe bisphenol A on female reproductive system. J. Zunyi Med. Univ. 2022, 45, 399–406. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, Y.; Tao, F. Childhood and adolescent obesity and maternal phthalate exposure during pregnancy. Chin. J. Sch. Health 2019, 40, 1434–1437. [Google Scholar] [CrossRef]
- Tian, J.; Ding, Y.; She, R.; Ma, L.; Du, F.; Xia, K.; Chen, L. Histologic Study of Testis Injury after Bisphenol A Exposure in Mice: Direct Evidence for Impairment of the Genital System by Endocrine Disruptors. Toxicol. Ind. Health 2017, 33, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ling, Y.; Zhao, C.; Chen, J.; Zhang, J.; Liu, Y.; Xie, M. Mechanism of meiotic arrest of spermatogenic cells in testis of mice exposed to bisphenol A during lactation. J. Environ. Occup. Med. 2021, 38, 769–774. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Q.; Dang, X.; He, Y.; Li, X.; Sun, Y. Local Effect of Bisphenol A on the Estradiol Synthesis of Ovarian Granulosa Cells from PCOS. Gynecol. Endocrinol. 2017, 33, 21–25. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.; Zhai, L.; Bai, Y.; Wei, W.; Sun, Q.; Jia, L. Urinary Concentrations of Bisphenol A and Its Alternatives: Potential Predictors of and Associations with Antral Follicle Count among Women from an Infertility Clinic in Northern China. Environ. Res. 2024, 249, 118433. [Google Scholar] [CrossRef]
- Zhou, Y.; Lou, R. The effect of bisphenol A on the reproductive system and its mechanism. Matern. Child Health Care China 2019, 34, 468–474. [Google Scholar]
- Li, Y.; Zhang, H.; Kuang, H.; Fan, R.; Cha, C.; Li, G.; Luo, Z.; Pang, Q. Relationship between Bisphenol A Exposure and Attention-Deficit/ Hyperactivity Disorder: A Case-Control Study for Primary School Children in Guangzhou, China. Environ. Pollut. 2018, 235, 141–149. [Google Scholar] [CrossRef]
- Wei, J.; He, Z.; Wang, C.; Hu, Y.; Wang, C.; Cao, H.; Cao, M.; Liang, Y. Progress in Reproductive Toxicity and Human Reproductive Health Risk of Bisphenol A Analogues. Asian J. Ecotoxicol. 2022, 17, 85–107. [Google Scholar]
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.-H.; Svoboda, D.; Auerbach, S.S.; et al. A Scoping Review of the Health and Toxicological Activity of Bisphenol A (BPA) Structural Analogues and Functional Alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Z.; Zhou, Y.; Guo, W.; Tian, L.; Zhang, L.; Li, X.; Chen, J. Mode of Action Exploration of Reproductive Toxicity Induced by Bisphenol S Using Human Normal Ovarian Epithelial Cells through ERβ-MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2024, 272, 116037. [Google Scholar] [CrossRef]
- Liao, K.; Zhao, Y.; Qu, J.; Yu, W.; Hu, S.; Fang, S.; Zhao, M.; Jin, H. Association of Serum Bisphenols, Parabens, and Triclosan Concentrations with Sjögren Syndrome in the Hangzhou, China Population. Sci. Total Environ. 2024, 915, 170031. [Google Scholar] [CrossRef] [PubMed]
- Gély, C.A.; Lacroix, M.Z.; Morin, M.; Vayssière, C.; Gayrard, V.; Picard-Hagen, N. Comparison of the Materno-Fetal Transfer of Fifteen Structurally Related Bisphenol Analogues Using an Ex Vivo Human Placental Perfusion Model. Chemosphere 2021, 276, 130213. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, B.; Nikaeen, M.; Amin, M.M.; Mohammadi, F. Health Risk Assessment of Exposure to Bisphenol A in Polymeric Baby Bottles. Env. Health Insights 2023, 17, 117863022311515. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Xu, X.; Liu, Z.; Xian, J.; Yang, S.; Zhang, C. Pollution Characteristics and Health Risk Assessment of Phthalate Esters in Household Dust in Chengdu, China. Hum. Ecol. Risk Assess. Int. J. 2022, 28, 958–971. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, L.; Zhang, P.; Zhang, D.; Li, X. Pollution Characteristics and Health Risk Assessment of Phthalate Esters (PAEs) in Soils of Typical Agricultural Areas in Qingdao. Environ. Chem. 2024, 43, 1857–1870. [Google Scholar]
- Karrer, C.; Andreassen, M.; Von Goetz, N.; Sonnet, F.; Sakhi, A.K.; Hungerbühler, K.; Dirven, H.; Husøy, T. The EuroMix Human Biomonitoring Study: Source-to-Dose Modeling of Cumulative and Aggregate Exposure for the Bisphenols BPA, BPS, and BPF and Comparison with Measured Urinary Levels. Environ. Int. 2020, 136, 105397. [Google Scholar] [CrossRef]
- Hong, H.; Harvey, B.; Palmese, G.; Stanzione, J.; Ng, H.; Sakkiah, S.; Tong, W.; Sadler, J. Experimental Data Extraction and in Silico Prediction of the Estrogenic Activity of Renewable Replacements for Bisphenol A. Int. J. Environ. Res. Public Health 2016, 13, 705. [Google Scholar] [CrossRef]
- Several States in the United States Have Issued New Regulations to Control Bisphenol A. Available online: https://bz.hgcm.cn/zggmsb/2019-03-25/details.html?edition=2&details=12 (accessed on 9 January 2025).
- Ministry of Ecology and Environment of the People’s Republic of China. Comprehensive List of Environmental Protection (2021 Edition). Available online: https://www.mee.gov.cn/ywdt/hjywnews/202111/t20211103_959023.shtml (accessed on 9 January 2025).
- Chau, K. Request for Relevant Information on the Carcinogenicity of Bisphenol A (BPA). Available online: https://oehha.ca.gov/proposition-65/crnr/request-relevant-information-carcinogenicity-bisphenol-bpa (accessed on 9 January 2025).
- Program, H.F. Bisphenol A (BPA). Food and Drug Administration (FDA) 2024. Available online: https://www.fda.gov/food/food-packaging-other-substances-come-contact-food-information-consumers/bisphenol-bpa (accessed on 9 January 2025).
- Food and Drug Administration, US. Department of Health and Human Services Food and Drug Administration. Available online: https://www.federalregister.gov/documents/2022/07/11/2022-14682/environmental-defense-fund-maricel-maffini-breast-cancer-prevention-partners-clean-water-actionclean (accessed on 9 January 2025).
| Parameter | Age | Reference Data |
|---|---|---|
| Rfd (µg/g/d) | - | 0.05 |
| IRWater (L/d) | Adult | 1.85 |
| Child | 0.86 | |
| IRSoil (mg/d) | Adult | 100 |
| Child | 200 | |
| IRAtmosphere (m3/d) | Adult | 12.8 |
| Child | 7.8 | |
| BW (kg) | Adult | 58.6 |
| Child | 15 |
| Region/Organization | Bill | Stipulation | Reference |
|---|---|---|---|
| US. Hawaii | HB139(HD1) Toxin-Free Keiki Bill | BPA is banned from reusable food or drink containers for children as young as three years old | [87] |
| US. Illinois | HB2076 Bill | BPA is prohibited from being used in commercial or bank record paper | [87] |
| US. New York | S1076 Bill | No BPA in toys or in cans and other containers containing liquids or beverages intended for children aged three years or younger | [87] |
| US. New York | S3056 Bill | BPA is prohibited in childcare products for children aged three years or younger | [87] |
| EU | 2011/8/EU | The chemical BPA is prohibited from being used in the production of baby bottles, requiring that all plastic materials that come into contact with food have no more than 0.6 mg/kg of BPA allowed to migrate | [90] |
| Food and Drug Administration (FDA) | 2022-14682 | The authorized use of BPA as a food additive is revoked and limited to establish a maximum limit of 0.5 ng/kg in food | [91] |
| CN, Ministry of Ecology and Environment of the People’s Republic of China | Comprehensive List of Environmental Protection (2021 version) | BPA is listed as a “high-pollution, high-environmental-risk” product | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, F.; Ren, Y.; Bi, F.; Wu, Z.; Zhang, H.; Li, J.; Gao, R.; Liu, Z.; Li, H. Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives. Toxics 2025, 13, 109. https://doi.org/10.3390/toxics13020109
Long F, Ren Y, Bi F, Wu Z, Zhang H, Li J, Gao R, Liu Z, Li H. Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives. Toxics. 2025; 13(2):109. https://doi.org/10.3390/toxics13020109
Chicago/Turabian StyleLong, Fangyun, Yanqin Ren, Fang Bi, Zhenhai Wu, Haijie Zhang, Junling Li, Rui Gao, Zhengyang Liu, and Hong Li. 2025. "Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives" Toxics 13, no. 2: 109. https://doi.org/10.3390/toxics13020109
APA StyleLong, F., Ren, Y., Bi, F., Wu, Z., Zhang, H., Li, J., Gao, R., Liu, Z., & Li, H. (2025). Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives. Toxics, 13(2), 109. https://doi.org/10.3390/toxics13020109







