Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Maintenance and Embryo Collection
2.3. Embryos Treatment and Morphological Observation
2.4. Motor Behavior Evaluation
2.5. RNA Extraction and Real-Time Quantitative PCR
2.6. Oxidative Stress Analysis
2.7. Transcriptome Analysis
2.8. Statistical Analysis
3. Results
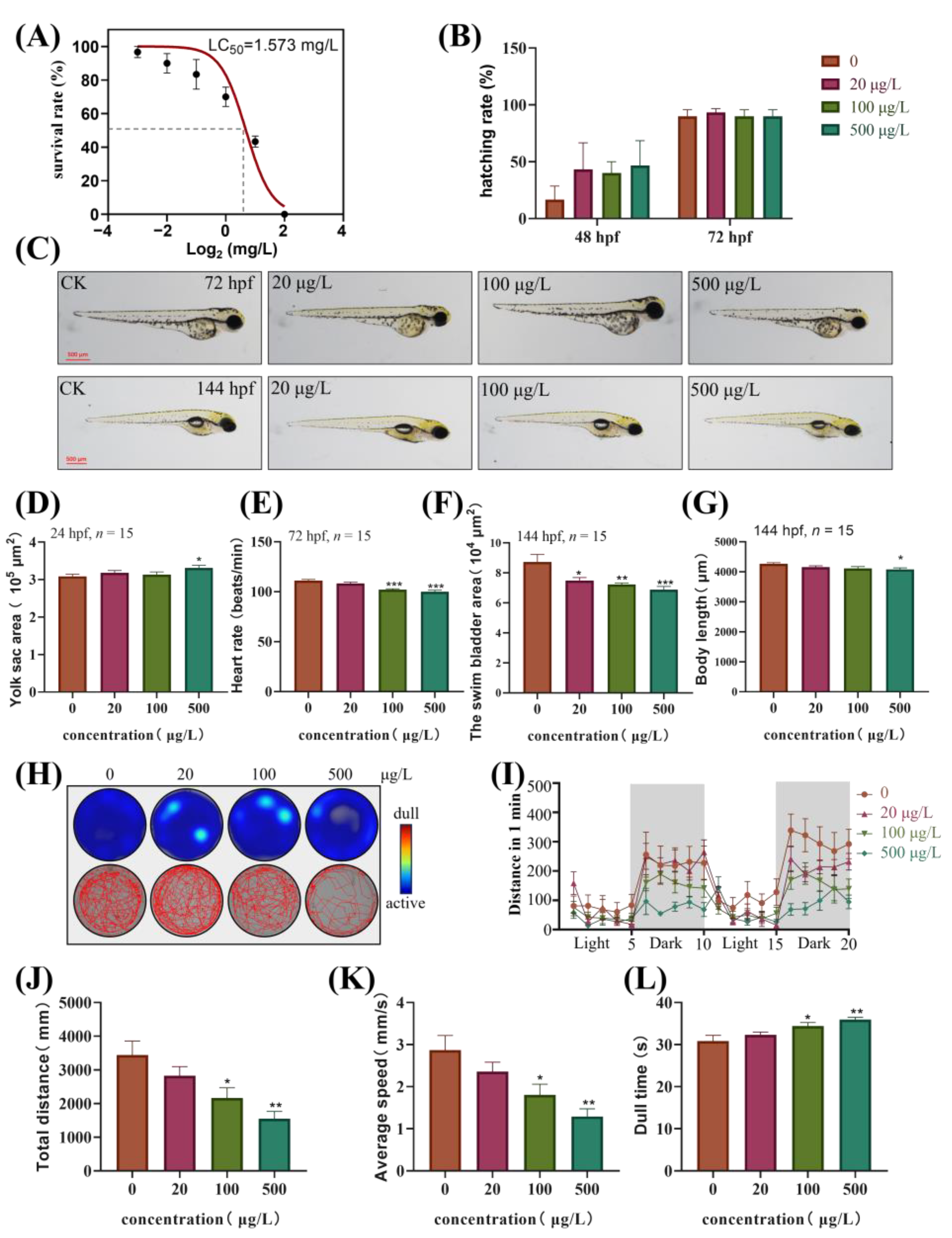
3.1. Developmental Toxicity of TBBPA-DHEE in Zebrafish Embryos and Larvae
3.2. The Motor Ability of Zebrafish Larvae is Inhibited After Exposure to TBBPA-DHEE
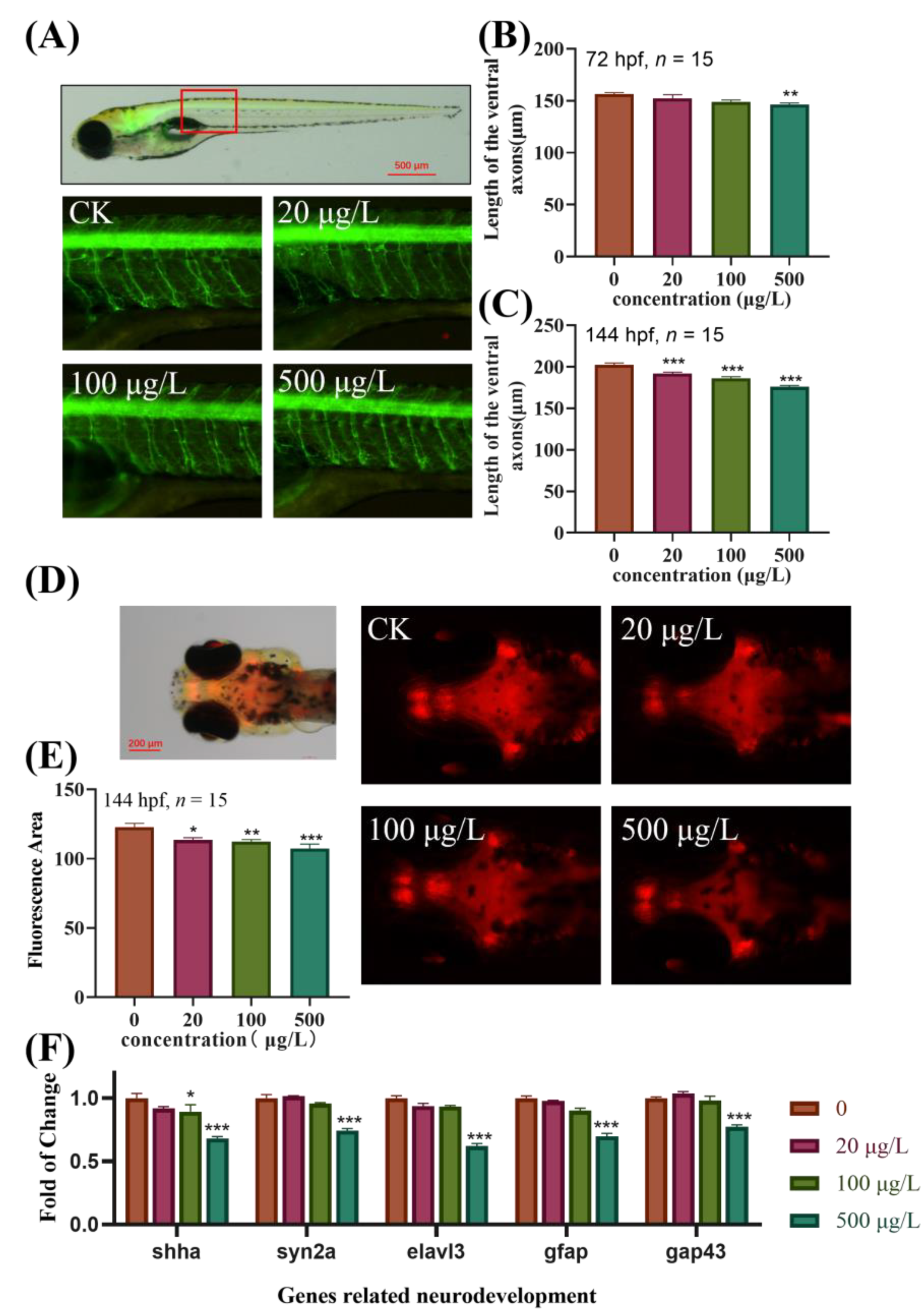
3.3. Effects of TBBPA-DHEE on the Development of Specific Target Organs in Transgenic Zebrafish
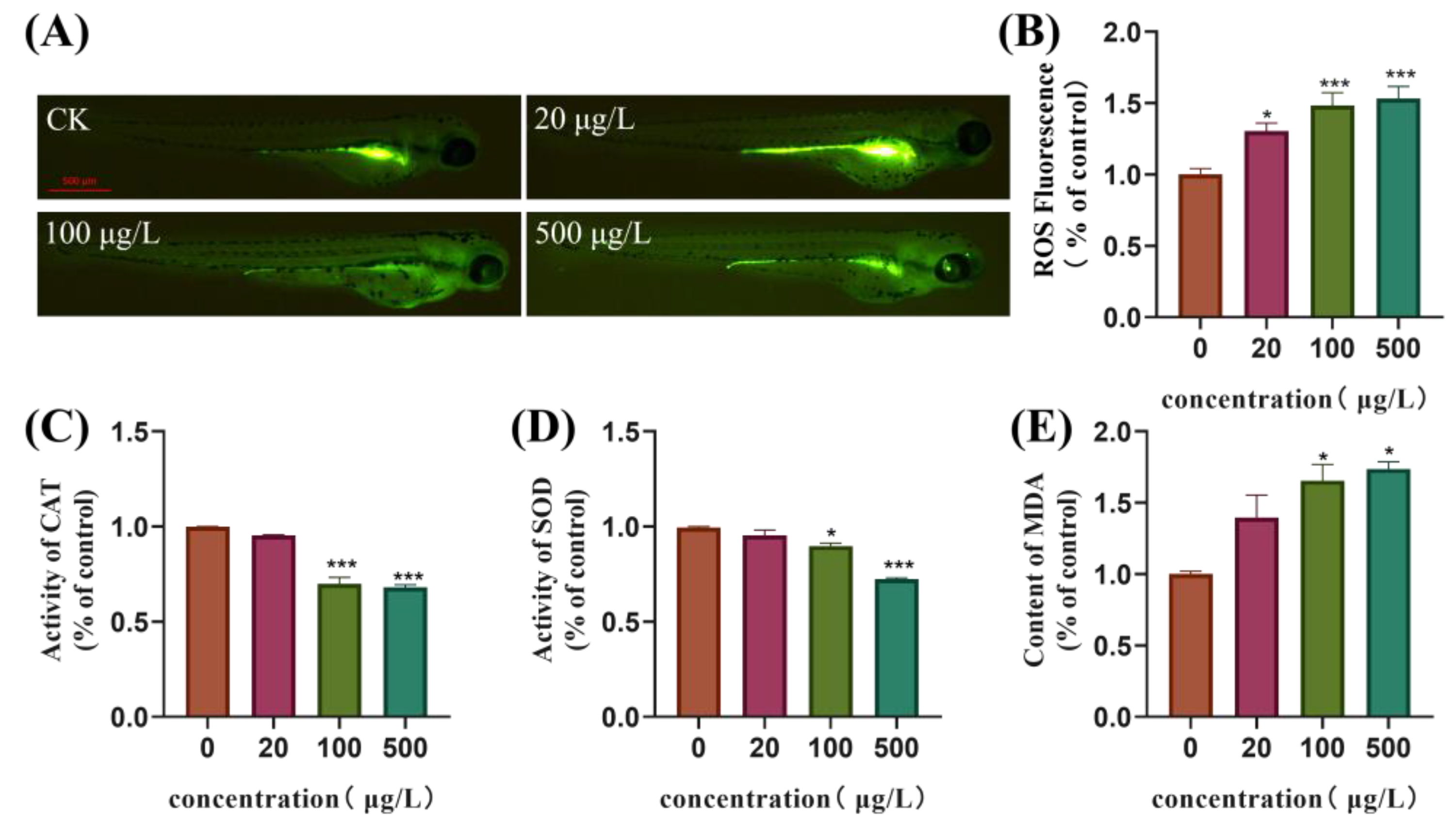
3.4. Oxidative Stress Is Induced in Zebrafish Embryos Following Exposure to TBBPA-DHEE
3.5. TBBPA-DHEE Affects the Brain Transcriptome of Juvenile Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakajima, A.; Saigusa, D.; Tetsu, N.; Yamakuni, T.; Tomioka, Y.; Hishinuma, T. Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol. Lett. 2009, 189, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Segev, O.; Kushmaro, A.; Brenner, A. Environmental impact of flame retardants (persistence and biodegradability). Int. J. Environ. Res. Public Health 2009, 6, 478–491. [Google Scholar] [CrossRef]
- Zhu, B.; Lei, L.; Fu, K.; Zhao, S.; Hua, J.; Yang, L.; Han, J.; Li, R.; Zhou, B. Neurotoxicity of tetrabromobisphenol A and SiO2 nanoparticle co-exposure in zebrafish and barrier function of the embryonic chorion. Sci. Total Environ. 2022, 845, 157364. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Xiang, Y.; Li, L.; Qie, H.; Ren, M.; Lin, A.; Qi, F. Effects of tetrabromobisphenol A (TBBPA) on the reproductive health of male rodents: A systematic review and meta-analysis. Sci. Total Environ. 2021, 781, 146745. [Google Scholar] [CrossRef]
- Yin, N.; Liang, S.; Liang, S.; Yang, R.; Hu, B.; Qin, Z.; Liu, A.; Faiola, F. TBBPA and Its Alternatives Disturb the Early Stages of Neural Development by Interfering with the NOTCH and WNT Pathways. Environ. Sci. Technol. 2018, 52, 5459–5468. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yan, R.; Lin, H.; Liu, Z.; Shen, L.; Yang, H.; Wu, H.; Shan, X.; Zhang, H. TBBPA and its alternative TCBPA induced ROS-dependent mitochondria-mediated apoptosis in the liver of Rana nigromaculata. Environ. Pollut. 2022, 297, 118791. [Google Scholar] [CrossRef]
- Abou-Elwafa Abdallah, M. Environmental occurrence, analysis and human exposure to the flame retardant tetrabromobisphenol-A (TBBP-A)—A review. Environ. Int. 2016, 94, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; He, M.J.; Yang, Z.H.; Wei, S.Q. Occurrence of tetrabromobisphenol a (TBBPA) and hexabromocyclododecane (HBCD) in soil and road dust in Chongqing, western China, with emphasis on diastereoisomer profiles, particle size distribution, and human exposure. Environ. Pollut. 2018, 242, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, J.; Ouyang, Y.Z.; Qu, G.B.; Liu, A.F.; Wang, X.M.; Liu, C.X.; Shi, J.B.; Chen, H.W.; Jiang, G.B. Reactive extractive electrospray ionization tandem mass spectrometry for sensitive detection of tetrabromobisphenol A derivatives. Anal. Chim. Acta 2014, 814, 49–54. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, Z.; Liu, L.; Shao, J.; Gu, L.; Liu, H.; Qu, G.; Shi, J.; Jiang, G.B. A typical derivative and byproduct of tetrabromobisphenol A: Development of novel high-throughput immunoassays and systematic investigation of their distributions in Taizhou, an e-waste recycling area in eastern China. Environ. Pollut. 2020, 263, 114382. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Zheng, M.; Lin, Y.; Liu, A.; Wang, Y.; Li, Y. Identification of lower brominated bisphenol A analogs as the photooxidation products of tetrabromobisphenol A bis(2,3-dibromopropyl) ether (TBBPA-BDBPE). Sci. Total Environ. 2023, 890, 164227. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yuan, K.; Ai, F.; Li, M.; Zhu, N.; Wang, Y.; Zeng, K.; Yin, D.; Bu, Y.; Zhang, Z. Occurrence, spatial distributions, and temporal trends of bisphenol analogues in an E-waste dismantling area: Implications for risk assessment. Sci. Total Environ. 2023, 867, 161498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ren, X.; Long, Y.; Hu, L.; Qu, G.; Zhou, Q.; Jiang, G. The potential neurotoxicity of emerging tetrabromobisphenol A derivatives based on rat pheochromocytoma cells. Chemosphere 2016, 154, 194–203. [Google Scholar] [CrossRef]
- Liu, Q.S.; Liu, N.; Sun, Z.; Zhou, Q.; Jiang, G. Intranasal administration of tetrabromobisphenol A bis(2-hydroxyethyl ether) induces neurobehavioral changes in neonatal Sprague Dawley rats. J. Environ. Sci. 2018, 63, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Feng, W.; Song, C.; Mao, G.; Chen, Y.; Xu, H.; Qian, X.; Luo, M.; Wu, X.; Yang, L. Transcriptomic profiling reveals the neuroendocrine-disrupting effect and toxicity mechanism of TBBPA-DHEE exposure in zebrafish (Danio rerio) during sexual development. Sci. Total Environ. 2023, 858, 160089. [Google Scholar] [CrossRef]
- Okeke, E.S.; Feng, W.; Mao, G.; Chen, Y.; Qian, X.; Luo, M.; Xu, H.; Qiu, X.; Wu, X.; Yang, L. A transcriptomic-based analysis predicts the neuroendocrine disrupting effect on adult male and female zebrafish (Danio rerio) following long-term exposure to tetrabromobisphenol A bis(2-hydroxyethyl) ether. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 264, 109527. [Google Scholar] [CrossRef]
- Gu, L.; Zou, Y.; Li, Y.; Zeng, K.; Zhu, N.; Zhu, F.; Gyimah, E.; Yakubu, S.; Meng, H.; Zhang, Z. High-throughput chemiluminescence immunoassay based on Co(2+)/hemin synergistic catalysis for sensitive detection tetrabromobisphenol A bis(2-hydroxyethyl) ether in the environments. Sci. Total Environ. 2020, 714, 136880. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef]
- Beattie, C.E. Control of motor axon guidance in the zebrafish embryo. Brain Res. Bull. 2000, 53, 489–500. [Google Scholar] [CrossRef]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef]
- Vanwalleghem, G.C.; Ahrens, M.B.; Scott, E.K. Integrative whole-brain neuroscience in larval zebrafish. Curr. Opin. Neurobiol. 2018, 50, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, H.; Zhou, L.; Fan, D.; Shi, L.; Ji, G.; Gu, A. Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci. Total Environ. 2020, 731, 139190. [Google Scholar] [CrossRef]
- Sunday, O.E.; Bin, H.; Guanghua, M.; Yao, C.; Zhengjia, Z.; Xian, Q.; Xiangyang, W.; Weiwei, F. Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ. Res. 2022, 206, 112594. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, S.; Ge, D.; Zhu, N.; Wang, K.; Zhu, G.; Xu, W.; Xu, H. An ultrasensitive competitive immunosensor using silica nanoparticles as an enzyme carrier for simultaneous impedimetric detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether. Biosens. Bioelectron. 2018, 105, 77–80. [Google Scholar] [CrossRef]
- Luo, M.; Song, C.; Zuo, J.; Feng, W.; Wu, C.; Geng, X.; Okeke, E.S.; Mao, G.; Chen, Y.; Zhao, T.; et al. Neurodevelopmental toxicity and molecular mechanism of environmental concentration of tetrabromobisphenol A bis (2- hydroxyethyl) ether exposure to sexually developing male SD rats. Chemosphere 2024, 353, 141378. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, J.; Chen, Y.; Wang, H.; Guo, M.; Wang, L.; Wang, Z.; Wu, S.; Shi, L.; Gu, A.; et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere 2019, 217, 629–635. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2016, 31, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Schroeder, H.; Olivier, J.L.; Turner, J.D. Epigenetic and Neurological Impairments Associated with Early Life Exposure to Persistent Organic Pollutants. Int. J. Genom. 2019, 2019, 2085496. [Google Scholar] [CrossRef]
- Sano, K.; Isobe, T.; Yang, J.; Win-Shwe, T.T.; Yoshikane, M.; Nakayama, S.F.; Kawashima, T.; Suzuki, G.; Hashimoto, S.; Nohara, K.; et al. In utero and Lactational Exposure to Acetamiprid Induces Abnormalities in Socio-Sexual and Anxiety-Related Behaviors of Male Mice. Front. Neurosci. 2016, 10, 228. [Google Scholar] [CrossRef]
- Okeke, E.S.; Feng, W.; Luo, M.; Mao, G.; Chen, Y.; Zhao, T.; Wu, X.; Yang, L. RNA-Seq analysis offers insight into the TBBPA-DHEE-induced endocrine-disrupting effect and neurotoxicity in juvenile zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2024, 350, 114469. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, S.E.; Layden, M.J.; Von Ohlen, T.; Doe, C.Q.; Eisen, J.S. Zebrafish and fly Nkx6 proteins have similar CNS expression patterns and regulate motoneuron formation. Development 2004, 131, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Guo, M.; Yin, X.; Huang, C.; Qian, L.; Zhou, L.; Wang, Z.; Wang, L.; Shi, L.; Ji, G. A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci. Total Environ. 2022, 805, 150210. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tanguay, R.L.; Xiao, Y.; Haggard, D.E.; Ge, X.; Jia, Y.; Zheng, Y.; Dong, Q.; Huang, C.; Lin, K. TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ. Pollut. 2016, 216, 53–63. [Google Scholar] [CrossRef]
- Song, Y.; Tao, B.; Chen, J.; Jia, S.; Zhu, Z.; Trudeau, V.L.; Hu, W. GABAergic Neurons and Their Modulatory Effects on GnRH3 in Zebrafish. Endocrinology 2017, 158, 874–886. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, G.; Yang, L.; Zhou, B. Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere 2018, 197, 353–361. [Google Scholar] [CrossRef]
- Garbarino, G.; Costa, S.; Pestarino, M.; Candiani, S. Differential expression of synapsin genes during early zebrafish development. Neuroscience 2014, 280, 351–367. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Shi, X.; Luo, C.; Huang, W.; Lin, H.; Peng, J.; Tan, W.; Wu, K. Neurodevelopmental toxicity of organophosphate flame retardant triphenyl phosphate (TPhP) on zebrafish (Danio rerio) at different life stages. Environ. Int. 2023, 172, 107745. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Yano, M.; Okano, H. Acute reduction of neuronal RNA binding Elavl2 protein and Gap43 mRNA in mouse hippocampus after kainic acid treatment. Biochem. Biophys. Res. Commun. 2015, 466, 46–51. [Google Scholar] [CrossRef]
- Gyimah, E.; Xu, H.; Dong, X.; Qiu, X.; Zhang, Z.; Bu, Y.; Akoto, O. Developmental neurotoxicity of low concentrations of bisphenol A and S exposure in zebrafish. Chemosphere 2021, 262, 128045. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Jørgensen, A.L. Structural and functional characterization of the zebrafish gene for glial fibrillary acidic protein, GFAP. Gene 2003, 310, 123–132. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.; Wang, J.; Xie, H.; Wang, J.; Han, Y.; Yang, J. Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ. Sci. Pollut. Res. Int. 2013, 20, 201–208. [Google Scholar] [CrossRef]
- Mu, X.; Shen, G.; Huang, Y.; Luo, J.; Zhu, L.; Qi, S.; Li, Y.; Wang, C.; Li, X. The enantioselective toxicity and oxidative stress of beta-cypermethrin on zebrafish. Environ. Pollut. 2017, 229, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, F.; Liu, X.; Xu, J.; Wu, X.; Liu, W.; Zheng, Y. Crosstalk of oxidative damage, apoptosis, and autophagy under endoplasmic reticulum (ER) stress involved in thifluzamide-induced liver damage in zebrafish (Danio rerio). Environ. Pollut. 2018, 243, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Xia, Y.; Tang, K.; Xu, J.; Wang, A.; Hu, S.; Wen, L.; Wang, B.; Yao, W.; et al. Toxic effects of isofenphos-methyl on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2023, 254, 114723. [Google Scholar] [CrossRef] [PubMed]
- Haridevamuthu, B.; Murugan, R.; Seenivasan, B.; Meenatchi, R.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; M, K.K.; Arockiaraj, J. Synthetic azo-dye, Tartrazine induces neurodevelopmental toxicity via mitochondria-mediated apoptosis in zebrafish embryos. J. Hazard. Mater. 2024, 461, 132524. [Google Scholar] [CrossRef]
- Givaudan, N.; Binet, F.; Le Bot, B.; Wiegand, C. Earthworm tolerance to residual agricultural pesticide contamination: Field and experimental assessment of detoxification capabilities. Environ. Pollut. 2014, 192, 9–18. [Google Scholar] [CrossRef]
- Gil-Martins, E.; Barbosa, D.J.; Silva, V.; Remião, F.; Silva, R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol. Ther. 2020, 213, 107554. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Jha, N.K.; Kar, R.; Niranjan, R. ABC Transporters in Neurological Disorders: An Important Gateway for Botanical Compounds Mediated Neuro-Therapeutics. Curr. Top. Med. Chem. 2019, 19, 795–811. [Google Scholar] [CrossRef]
- Cannon, R.E.; Trexler, A.W.; Knudsen, G.A.; Evans, R.A.; Birnbaum, L.S. Tetrabromobisphenol A (TBBPA) Alters ABC Transport at the Blood-Brain Barrier. Toxicol. Sci. 2019, 169, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.E.; Richards, A.C.; Trexler, A.W.; Juberg, C.T.; Sinha, B.; Knudsen, G.A.; Birnbaum, L.S. Effect of GenX on P-Glycoprotein, Breast Cancer Resistance Protein, and Multidrug Resistance-Associated Protein 2 at the Blood-Brain Barrier. Environ. Health Perspect. 2020, 128, 37002. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Chen, J.; He, X.R.; Yang, S.L.; Ma, B.J.; Yu, J.; Qiu, J.; Qian, Y.Z.; Xu, Y.Y. Perfluorooctane sulfonate (PFOS) and benzo[a]pyrene (BaP) synergistically induce neurotoxicity in C6 rat glioma cells via the activation of neurotransmitter and Cyp1a1-mediated steroid hormone synthesis pathways. Food Chem. Toxicol. 2024, 193, 115058. [Google Scholar] [CrossRef]
- Dredge, B.K.; Polydorides, A.D.; Darnell, R.B. The splice of life: Alternative splicing and neurological disease. Nat. Rev. Neurosci. 2001, 2, 43–50. [Google Scholar] [CrossRef]
- Nik, S.; Bowman, T.V. Splicing and neurodegeneration: Insights and mechanisms. Wiley Interdiscip. Rev. RNA 2019, 10, e1532. [Google Scholar] [CrossRef]
- Kim, K.K.; Nam, J.; Mukouyama, Y.S.; Kawamoto, S. Rbfox3-regulated alternative splicing of Numb promotes neuronal differentiation during development. J. Cell Biol. 2013, 200, 443–458. [Google Scholar] [CrossRef]
- Ohnishi, T.; Shirane, M.; Nakayama, K.I. SRRM4-dependent neuron-specific alternative splicing of protrudin transcripts regulates neurite outgrowth. Sci. Rep. 2017, 7, 41130. [Google Scholar] [CrossRef]
- Zheng, S.; Black, D.L. Alternative pre-mRNA splicing in neurons: Growing up and extending its reach. Trends Genet. 2013, 29, 442–448. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Guo, L.; Luo, Y.; Xu, X.; Han, Y.; Chen, H.; Sun, H.; Xue, Y.; Ji, G. Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure. Toxics 2025, 13, 76. https://doi.org/10.3390/toxics13020076
Zhang X, Guo L, Luo Y, Xu X, Han Y, Chen H, Sun H, Xue Y, Ji G. Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure. Toxics. 2025; 13(2):76. https://doi.org/10.3390/toxics13020076
Chicago/Turabian StyleZhang, Xinyu, Liguo Guo, Yiwen Luo, Xia Xu, Ying Han, Hui Chen, Haohao Sun, Yingang Xue, and Guixiang Ji. 2025. "Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure" Toxics 13, no. 2: 76. https://doi.org/10.3390/toxics13020076
APA StyleZhang, X., Guo, L., Luo, Y., Xu, X., Han, Y., Chen, H., Sun, H., Xue, Y., & Ji, G. (2025). Neurotoxicity and Mechanism in Zebrafish Embryo Induced by Tetrabromobisphenol A bis (2-Hydroxyethyl) Ether (TBBPA-DHEE) Exposure. Toxics, 13(2), 76. https://doi.org/10.3390/toxics13020076






