Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Reagents and Chemicals
2.3. Instrumental Analysis
2.4. Recommended Procedures
2.4.1. Wet Digestion
2.4.2. Analysis of Labile (Water-Soluble) Trace Elements in B. elegans Tissues
2.5. Statistical Method
3. Results and Discussion
3.1. Trace Metals in Plant
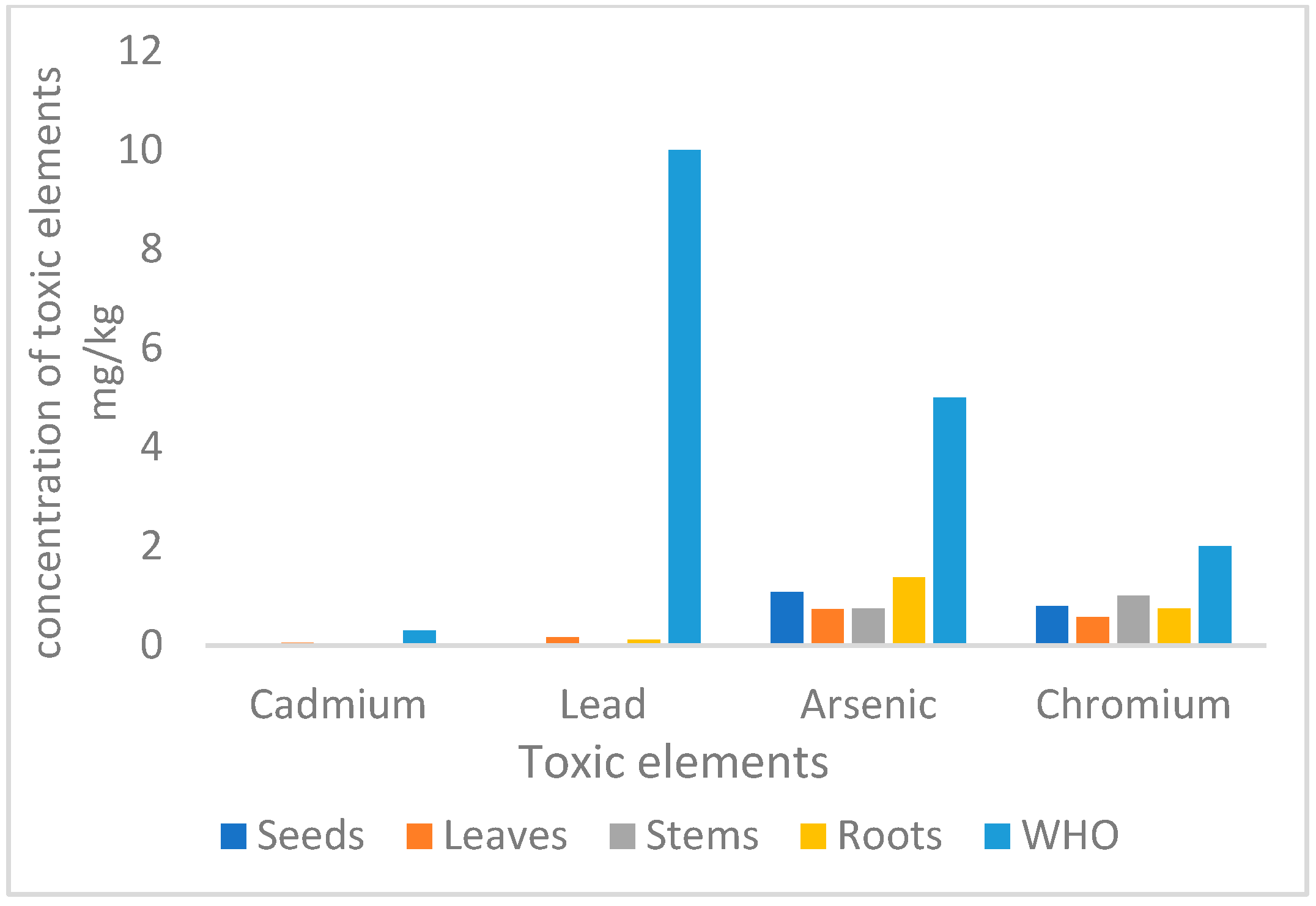
3.2. Toxic Elements
3.3. Macronutrient Elements
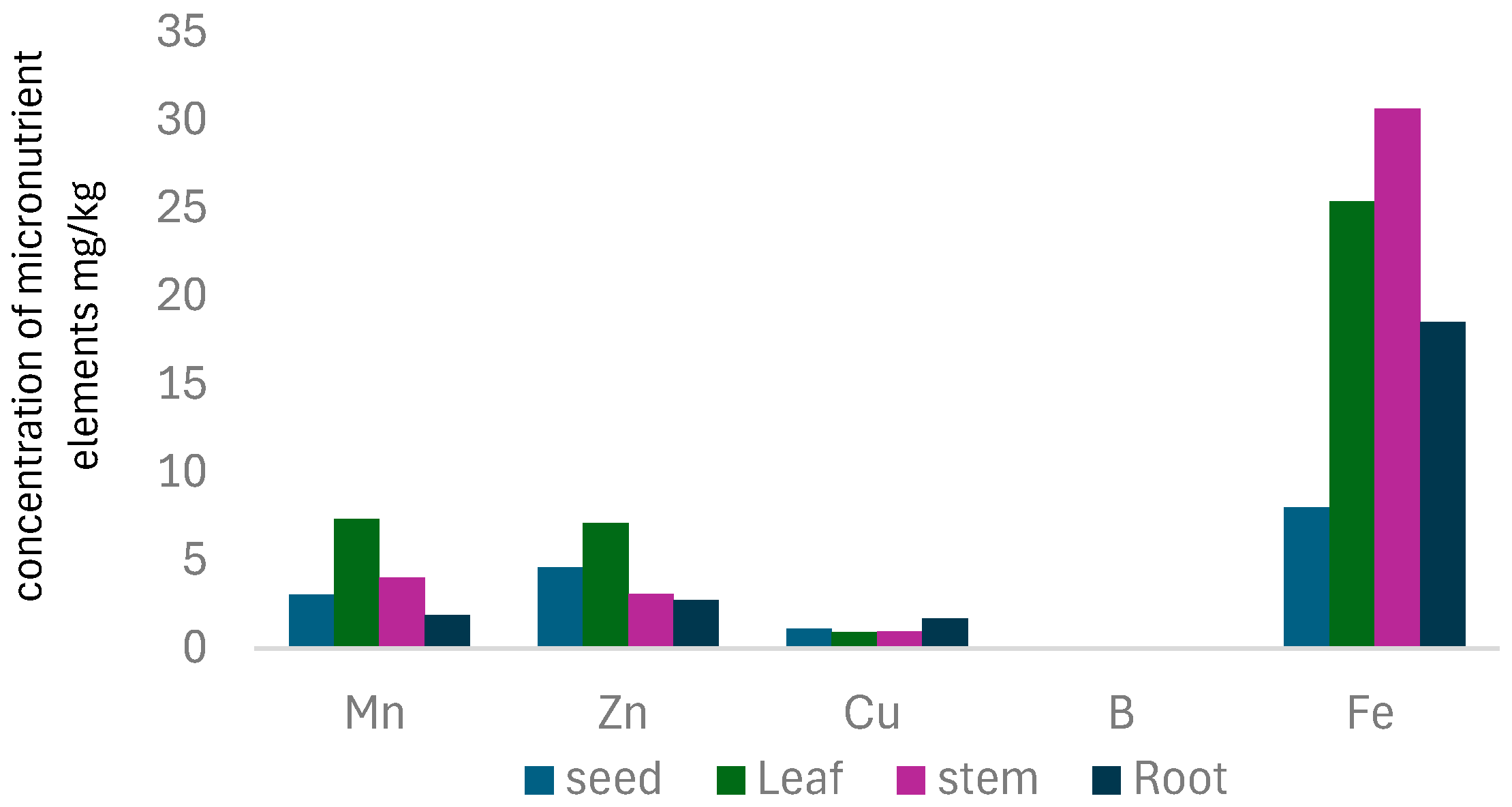
3.4. Micronutrient Elements
3.5. Beneficial Elements
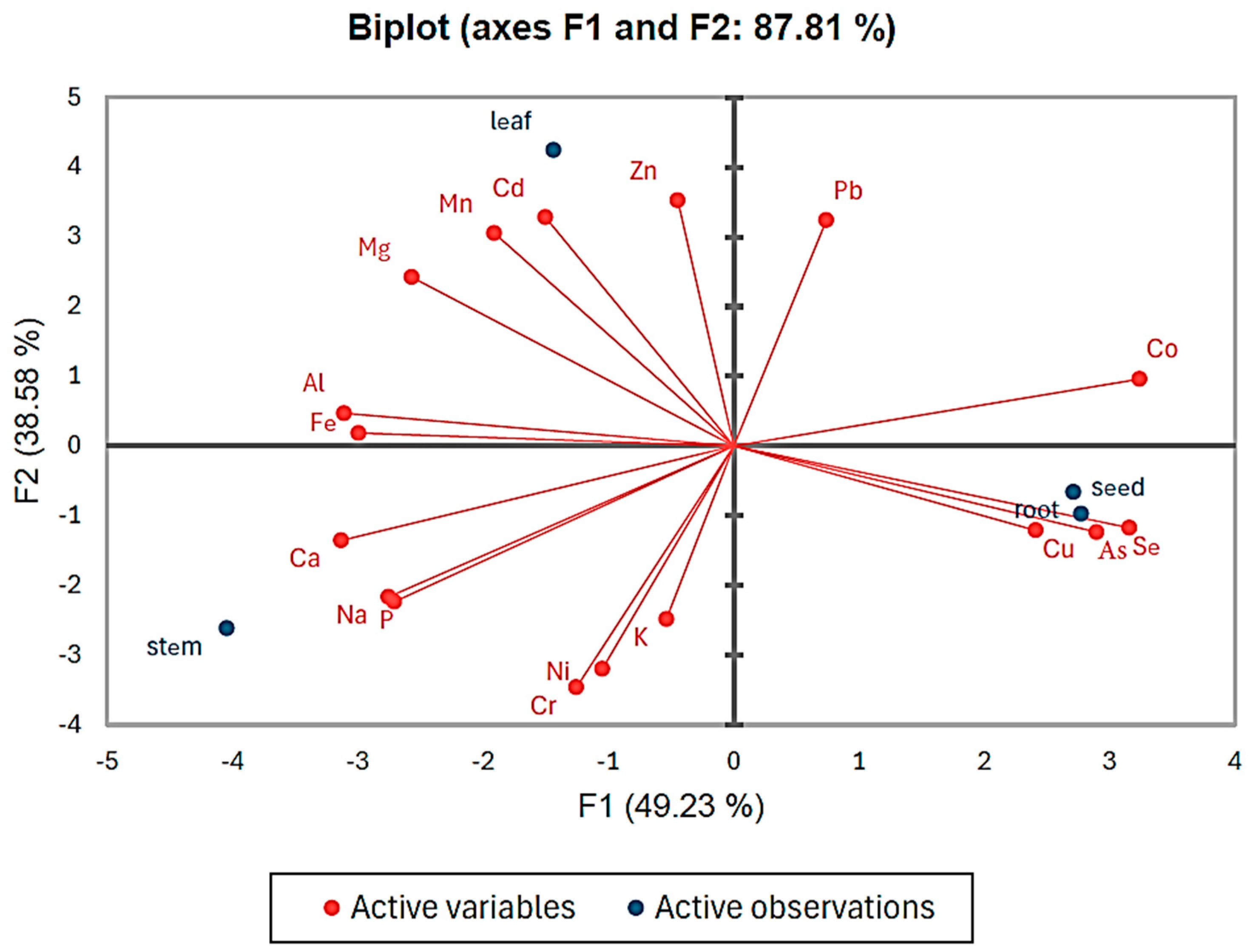
3.6. Principal Component Analysis (PCA)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, F.U.; Faridullah, F.; Irshad, M.; Bacha, A.U.R.; Ullah, Z.; Fawad, M.; Hafeez, F.; Iqbal, A.; Nazir, R.; Alrefaei, A.F.; et al. Distribution and Speciation of Trace Elements in Soils of Four Land-Use Systems. Land 2023, 12, 1894. [Google Scholar] [CrossRef]
- Ertaş, A.; Boğa, M.; Haşimi, N.; Yılmaz, M.A. Fatty acid and essential oil compositions of Trifolium angustifolium var. Angustifolium with antioxidant, anticholinesterase and antimicrobial activities. Iran. J. Pharm. Res. 2015, 14, 233–241. [Google Scholar]
- Yener, I. Trace Element Analysis in Some Plants Species by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). J. Inst. Sci. Technol. 2019, 9, 1492–1502. [Google Scholar] [CrossRef]
- Başgel, S.; Erdemoǧlu, S.B. Determination of mineral and trace elements in some medicinal herbs and their infusions consumed in Turkey. Sci. Total Environ. 2006, 359, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Brantner, A.; Grein, E. Antibacterial activity of plant extracts used externally in traditional medicine. J. Ethnopharmacol. 1994, 44, 35–40. [Google Scholar] [CrossRef]
- Merusomayajula, K.V.; Tirukkovalluri, S.R.; Kommula, R.S.; Chakkirala, S.V.; Vundavilli, J.K.; Kottapalli, P.K.S.R. Development and validation of a simple and rapid ICP-OES method for quantification of elemental impurities in voriconazole drug substance. Future J. Pharm. Sci. 2021, 7, 45. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; Taylor & Francis Group: Abingdon, UK, 2016; pp. 1–773. Available online: https://www.kufunda.net/publicdocs/Barker-2007-Handbook_of_Plant_Nutrition.pdf (accessed on 13 December 2024).
- Abugassa, I.O.; Bashir, A.T.; Doubali, K.; Etwir, R.H.; Abu-Enawel, M.; Abugassa, S.O. Characterization of trace elements in medicinal herbs by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 2008, 278, 559–563. [Google Scholar] [CrossRef]
- Maiga, A.; Diallo, D.; Bye, R.; Paulsen, B.S. Determination of some toxic and essential metal ions in medicinal and edible plants from Mali. J. Agric. Food Chem. 2005, 53, 2316–2321. [Google Scholar] [CrossRef]
- Xie, G.; Ye, M.; Wang, Y.; Ni, Y.; Su, M.; Huang, H.; Qiu, M.; Zhao, A.; Zheng, X.; Chen, T.; et al. Characterization of pu-erh tea using chemical and metabolic profiling approaches. J. Agric. Food Chem. 2009, 57, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- AL-Oud, S.S. Heavy Metal Contents in Tea and Herb Leaves. Pak. J. Biol. Sci. 2003, 6, 208–212. [Google Scholar] [CrossRef]
- Altıntıg, E.; Altundağ, H.; Tuzen, M. Determination of Multi Element Levels in Leaves and Herbal. Bull. Chem. Soc. Ethiop. 2014, 28, 9–16. [Google Scholar] [CrossRef]
- Izol, E.; Çiçek, İ.; Behçet, L.; Kaya, E.; Tarhan, A. Trace Element Analysis of Some Medicinal and Aromatic Plant Species by ICP-MS. Türk Doğa Fen Dergisi 2023, 12, 21–29. [Google Scholar] [CrossRef]
- Bin, C.; Xiaoru, W.; Lee, F.S.C. Pyrolysis coupled with atomic absorption spectrometry for the determination of mercury in Chinese medicinal materials. Anal. Chim. Acta 2001, 447, 161–169. [Google Scholar] [CrossRef]
- Leśniewicz, A.; Jaworska, K.; Zyrnicki, W. Macro- and micro-nutrients and their bioavailability in polish herbal medicaments. Food Chem. 2006, 99, 670–679. [Google Scholar] [CrossRef]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Brima, E.I. Toxic elements in different medicinal plants and the impact on human health. Int. J. Environ. Res. Public Health 2017, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Tokalioǧlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef]
- Marguí, E.; Dalipi, R.; Sangiorgi, E.; Štefan, M.B.; Sladonja, K.; Rogga, V.; Jablan, J. Determination of essential elements (Mn, Fe, Cu and Zn) in herbal teas by TXRF, FAAS and ICP-OES. X-Ray Spectrom. 2022, 51, 204–213. [Google Scholar] [CrossRef]
- Satish, S.; Girish, H. V Antibacterial Activity of Important Medicinal Plants on Human Pathogenic Bacteria-a Comparative Analysis. World Appl. Sci. J. 2008, 5, 267–271. [Google Scholar]
- Sadeghi, Z.; Valizadeh, J.; Azyzian Shermeh, O.; Akaberi, M. Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J. Phytomed. 2015, 5, 1–9. [Google Scholar]
- Mehrnia, M.A.; Bashti, A. Evaluation of toxic element contents in infant foods commercially available in Iran. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 249–253. [Google Scholar]
- El Aal, S.A.; Bayoumi, M.A.; A., E.I. Heavy metals residues and trace elements in milk powder marketed in Dakahlia Governorate. Int. Food Res. J. 2013, 20, 1807–1812. [Google Scholar]
- Viñas, P.; Pardo-Martı, M.; Hernández-Córdoba, M. Rapid determination of selenium, lead and cadmium in baby food samples using electrothermal atomic absorption spectrometry and slurry atomization. Anal. Chim. Acta 2000, 412, 121–130. [Google Scholar] [CrossRef]
- Kiani, A.; Arabameri, M.; Moazzen, M.; Shariatifar, N.; Aeenehvand, S.; Khaniki, G.J.; Abdel-Wahhab, M.; Shahsavari, S. Probabilistic Health Risk Assessment of Trace Elements in Baby Food and Milk Powder Using ICP-OES Method. Biol. Trace Elem. Res. 2022, 200, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Ikem, A.; Nwankwoala, A.; Odueyungbo, S.; Nyavor, K.; Egiebor, N. Levels of 26 elements in infant formula from USA, UK, and Nigeria by microwave digestion and ICP-OES. Food Chem. 2002, 77, 439–447. [Google Scholar] [CrossRef]
- Fathabad, A.E.; Shariatifar, N.; Moazzen, M.; Nazmara, S.; Fakhri, Y.; Alimohammadi, M.; Azari, A.; Khaneghah, A.M. Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: A risk assessment study. Food Chem. Toxicol. 2018, 115, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Al Khalifa, A.S.; Ahmad, D. Determination of key elements by ICP-OES in commercially available infant formulae and baby foods in Saudi Arabia. Afr. J. Food Sci. 2010, 4, 464–468. [Google Scholar]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO: Geneva, Switzerland, 2007.
- Tang, Z.; Wang, H.Q.; Chen, J.; Chang, J.D.; Zhao, F.J. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2023, 65, 570–593. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100064. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. Enhancing Cleanup Environ. Pollut. 2017, 2, 97–140. [Google Scholar] [CrossRef]
- Neidhardt, H.; Kramar, U.; Tang, X.; Guo, H.; Norra, S. Arsenic accumulation in the roots of Helianthus annuus and Zea mays by irrigation with arsenic-rich groundwater: Insights from synchrotron X-ray fluorescence imaging. Geochemistry 2015, 75, 261–270. [Google Scholar] [CrossRef]
- Khan Niazi, N.; Bibi, I.; Fatimah, A.; Shahid, M.; Tariq Javed, M.; Wang, H.; Sik Ok, Y.; Bashir, S.; Murtaza, B.; Ahmad Saqib, Z.; et al. Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: Morphological and physiological response. Int. J. Phytoremediat. 2017, 19, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Rathinasabapathi, B.; Ma, L.Q. Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria. Chemosphere 2015, 134, 1–6. [Google Scholar] [CrossRef]
- Quantin, C.; Ettler, V.; Garnier, J.; Šebek, O. Sources and extractibility of chromium and nickel in soil profiles developed on Czech serpentinites. C. R.-Geosci. 2008, 340, 872–882. [Google Scholar] [CrossRef]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium stress in plants: Toxicity, tolerance and phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-R.; Cai, M.-L.; Chen, S.-H.; Huang, X.-Y.; Zhao, F.-J.; Wang, P. High-affinity sulfate transporter Sultr1; 2 is a major transporter for Cr (VI) uptake in plants. Environ. Sci. Technol. 2021, 55, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, E.A.; Mengel, K. The role of magnesium in plant nutrition. Z. Pflanzenernährung Bodenkd. 1976, 139, 209–222. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015; pp. 15–16. [Google Scholar] [CrossRef]
- Gardner, R.C. Genes for magnesium transport. Curr. Opin. Plant Biol. 2003, 6, 263–267. [Google Scholar] [CrossRef]
- González-Fontes, A.; Navarro-Gochicoa, M.T.; Ceacero, C.J.; Herrera-Rodríguez, M.B.; Camacho-Cristóbal, J.J.; Rexach, J. Understanding calcium transport and signaling, and its use efficiency in vascular plants. In Plant Macronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 165–180. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Njira, K.; Nabwami, J. A review of effects of nutrient elements on crop quality. Afr. J. Food Agric. Nutr. Dev. 2015, 15, 9777–9793. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; Mckenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; Glover, C.J.; et al. In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef]
- Ryan, B.M.; Kirby, J.K.; Degryse, F.; Harris, H.; Mclaughlin, M.J.; Scheiderich, K. Copper speciation and isotopic fractionation in plants: Uptake and translocation mechanisms. New Phytol. 2013, 199, 367–378. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.; Xiao, J.; Wang, S. Molecular and functional analyses of COPT/Ctr-type copper transporter-like gene family in rice. BMC Plant Biol. 2011, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responsesand prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Minz, A.; Sinha, A.K.; Kumar, R.; Kumar, B.; Deep, K.P.; Kumar, S.B. A review on importance of cobalt in crop growth and production. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2978–2984. [Google Scholar]
- Dadlani, M.; Yadava, D.K. Seed Science and Technology: Biology, Production, Quality; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 1–430. [Google Scholar] [CrossRef]


| Tissue | Seeds | Leaves | Stems | Roots | ||||
|---|---|---|---|---|---|---|---|---|
| Elements | HNO3 | H2O | HNO3 | H2O | HNO3 | H2O | HNO3 | H2O |
| Cd | 0.009 ± 0.0003 | 0 ± 0.0012 | 0.054 ± 0.0019 | 0 ± 0.0007 | 0.021 ± 0.0049 | 0 ± 0.0014 | 0.018 ± 0.0002 | 0.061 ± 0.0025 |
| Co | 0.124 ± 0.0009 | 0.051 ± 0.0011 | 0.097 ± 0.0004 | 0.08 ± 0.0001 | 0.056 ± 0.0009 | 0.061 ± 0.0009 | 0.118 ± 0.0004 | 0.059 ± 0.0008 |
| Cr | 0.793 ± 0.0025 | 0 ± 0.0003 | 0.565 ± 0.003 | 0 ± 0.0006 | 1.004 ± 0.001 | 0 ± 0.0001 | 0.738 ± 0.0014 | 0 ± 0.0003 |
| Cu | 1.13 ± 0.0052 | 4.958 ± 0.0354 | 0.91 ± 0.0066 | 0.207 ± 0.0019 | 0.954 ± 0.0189 | 18.21 ± 0.053 | 1.692 ± 0.0126 | 0.292 ± 0.0008 |
| Fe | 8.021 ± 0.0653 | 0.081 ± 0.0015 | 25.37 ± 0.15 | 0 ± 0.0004 | 30.66 ± 0.083 | 0.655 ± 0.0045 | 18.54 ± 0.13 | 0 ± 0.0004 |
| Mg | 367.8 ± 0.75 | 14.5 ± 0.121 | 914.7 ± 3.89 | 259.9 ± 1.32 | 683.4 ± 2.85 | 102 ± 0.33 | 392.2 ± 2.91 | 59.84 ± 0.351 |
| Mn | 3.034 ± 0.0091 | 0.194 ± 0.0003 | 7.343 ± 0.0314 | 1.264 ± 0.0062 | 4.033 ± 0.0449 | 1.004 ± 0.0141 | 1.887 ± 0.0119 | 0.157 ± 0.0007 |
| Ni | 0.5 ± 0.0019 | 0.323 ± 0.002 | 0.377 ± 0.0026 | 0 ± 0.0012 | 0.551 ± 0.0023 | 0.752 ± 0.0037 | 0.423 ± 0.0027 | 0 ± 0.0007 |
| Pb | 0.033 ± 0.0061 | 0.282 ± 0.0009 | 0.165 ± 0.0053 | 0 ± 0.0078 | 0 ± 0.0162 | 1.333 ± 0.0061 | 0.113 ± 0.0078 | 0.04 ± 0.0048 |
| Al | 24.49 ± 2.154 | 0 ± 0.0018 | 59.71 ± 1.255 | 0 ± 0.002 | 67.53 ± 1.062 | 0 ± 0.0059 | 40.67 ± 0.426 | 0 ± 0.0079 |
| As | 1.068 ± 0.1653 | 0.109 ± 0.0264 | 0.728 ± 0.0407 | 0.177 ± 0.0252 | 0.742 ± 0.0164 | 0.099 ± 0.0138 | 1.374 ± 0.0185 | 0.221 ± 0.0306 |
| Se | 1.386 ± 0.1493 | 0.126 ± 0.0134 | 0.501 ± 0.0063 | 0.147 ± 0.0149 | 0.499 ± 0.0072 | 0.119 ± 0.0128 | 1.253 ± 0.0375 | 0.258 ± 0.0191 |
| Zn | 4.619 ± 0.1817 | 34.52 ± 0.194 | 7.117 ± 0.0995 | 1.279 ± 0.0059 | 3.108 ± 0.0453 | 64.03 ± 0.37 | 2.754 ± 0.0035 | 0.643 ± 0.0039 |
| Na | 0 ± 0.0128 | 9.737 ± 0.2996 | 0.234 ± 0.1354 | 30.3 ± 5.058 | 9.728 ± 1.3931 | 50.68 ± 1.328 | 0 ± 0.0385 | 29.2 ± 0.61 |
| K | 2.649 ± 0.0068 | 103.2 ± 0.37 | 9.937 ± 0.6758 | 67.76 ± 0.064 | 152.9 ± 6.95 | 76.53 ± 3.66 | 173 ± 5.24 | 66.46 ± 0.357 |
| P | 0 ± 0.0031 | 9.151 ± 0.2234 | 0 ± 0.026 | 4.922 ± 0.3786 | 1.012 ± 0.8824 | 0.31 ± 0.0252 | 0 ± 0.0032 | 15.61 ± 0.279 |
| B | 0 ± 0.0012 | 0.714 ± 156 | 0 ± 0.0012 | 2.669 ± 2051 | 0 ± 0.0041 | 0.173 ± 0.0184 | 0 ± 0.0003 | 0.244 ± 0.004 |
| Ca | 1.061 ± 0.0501 | 20.99 ± 0.149 | 30.17 ± 0.0501 | 255.4 ± 3.61 | 102.8 ± 7.08 | 8.533 ± 0.0554 | 6.939 ± 0.8491 | 1.38 ± 0.0587 |
| Groups | Count | Sum | Average | Variance |
|---|---|---|---|---|
| seed | 18 | 2083.585 | 115.7547 | 185,790.4 |
| leaves | 18 | 5289.89 | 293.8828 | 1,146,789 |
| stems | 18 | 5294.99 | 294.1661 | 653,386 |
| roots | 18 | 3208.595 | 178.2553 | 239,611.8 |
| Tissue | Seed | Leaves | Stem | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction |
| cd | 0.045 | 0 | ND | 0.27 | 0 | ND | 0.105 | 0 | ND | 0.09 | 1.22 | ND |
| pb | 0.165 | 2.82 | ND | 0.825 | 0 | ND | 0 | 13.33 | ND | 0.565 | 0.8 | ND |
| As | 5.34 | 1.09 | 4.25 | 3.64 | 3.54 | 0.1 | 3.71 | 0.99 | 2.72 | 6.87 | 4.42 | 2.45 |
| Cr | 3.965 | 0 | ND | 2.825 | ND | ND | 5.02 | ND | 6.01 | 3.69 | 0 | ND |
| Tissue | Seed | Leaves | Stem | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction |
| Mg | 1839 | 145 | 1694 | 4573.5 | 5198 | ND | 3417 | 1020 | 2397 | 1961 | 1196.8 | 764.2 |
| Ca | 5.305 | 209.9 | ND | 150.85 | 5108 | ND | 514 | 85.33 | 428.67 | 34.695 | 27.6 | 7.095 |
| K | 13.245 | 1032 | ND | 49.685 | 1355.2 | ND | 764.5 | 765.3 | ND | 865 | 1329.2 | ND |
| P | 0 | 91.51 | ND | 0 | 98.44 | ND | 5.06 | 3.1 | 1.96 | 0 | 312.2 | ND |
| Tissue | Seed | Leaves | Stem | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction |
| Cu | 5.65 | 49.58 | ND | 4.55 | 4.14 | 0.41 | 4.77 | 182.1 | ND | 8.46 | 5.84 | 2.62 |
| Fe | 40.105 | 0.81 | 39.295 | 126.85 | 0 | 126.85 | 153.3 | 6.55 | 146.75 | 92.7 | 0 | 92.7 |
| Mn | 15.17 | 1.94 | 13.23 | 36.715 | 25.28 | 11.435 | 20.165 | 10.04 | 10.125 | 9.435 | 3.14 | 6.295 |
| Zn | 23.095 | 345.2 | ND | 35.585 | 25.58 | 10.005 | 15.54 | 640.3 | ND | 13.77 | 12.86 | 0.91 |
| Ni | 2.5 | 3.23 | ND | 1.885 | 0 | 1.885 | 2.755 | 7.52 | ND | 2.115 | 0 | 2.115 |
| B | 0 | 7.14 | ND | 0 | 53.38 | ND | 0 | 1.73 | ND | 0 | 4.88 | ND |
| Tissue | Seed | Leaves | Stem | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction | Total Concentration | Labile | Complex Fraction |
| Na | 0 | 97.37 | ND | 1.17 | 606 | ND | 48.64 | 506.8 | ND | 0 | 584 | ND |
| Co | 0.62 | 0.51 | 0.11 | 0.485 | 1.6 | ND | 0.28 | 0.61 | ND | 0.59 | 1.18 | ND |
| Se | 6.93 | 1.26 | 5.67 | 2.505 | 2.94 | ND | 2.495 | 1.19 | 1.305 | 6.265 | 5.16 | 1.105 |
| Al | 122.45 | 0 | 122.45 | 298.55 | 0 | 298.55 | 337.65 | 0 | 337.65 | 203.35 | 0 | 203.35 |
| Na | 0 | 97.37 | ND | 1.17 | 606 | ND | 48.64 | 506.8 | ND | 0 | 584 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Raddadi, T.M.; Al-Khateeb, L.A.; Sadaka, M.W.; Bahaffi, S.O. Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues. Toxics 2025, 13, 14. https://doi.org/10.3390/toxics13010014
Al-Raddadi TM, Al-Khateeb LA, Sadaka MW, Bahaffi SO. Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues. Toxics. 2025; 13(1):14. https://doi.org/10.3390/toxics13010014
Chicago/Turabian StyleAl-Raddadi, Tahreer M., Lateefa A. Al-Khateeb, Mohammad W. Sadaka, and Saleh O. Bahaffi. 2025. "Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues" Toxics 13, no. 1: 14. https://doi.org/10.3390/toxics13010014
APA StyleAl-Raddadi, T. M., Al-Khateeb, L. A., Sadaka, M. W., & Bahaffi, S. O. (2025). Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues. Toxics, 13(1), 14. https://doi.org/10.3390/toxics13010014







