Human Health Risk Assessment of Chlorinated Hydrocarbons in Groundwater Based on Multi-Pathway Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Analysis
2.3. Health and Environmental Risk Assessment Methods for Groundwater
3. Results and Discussion
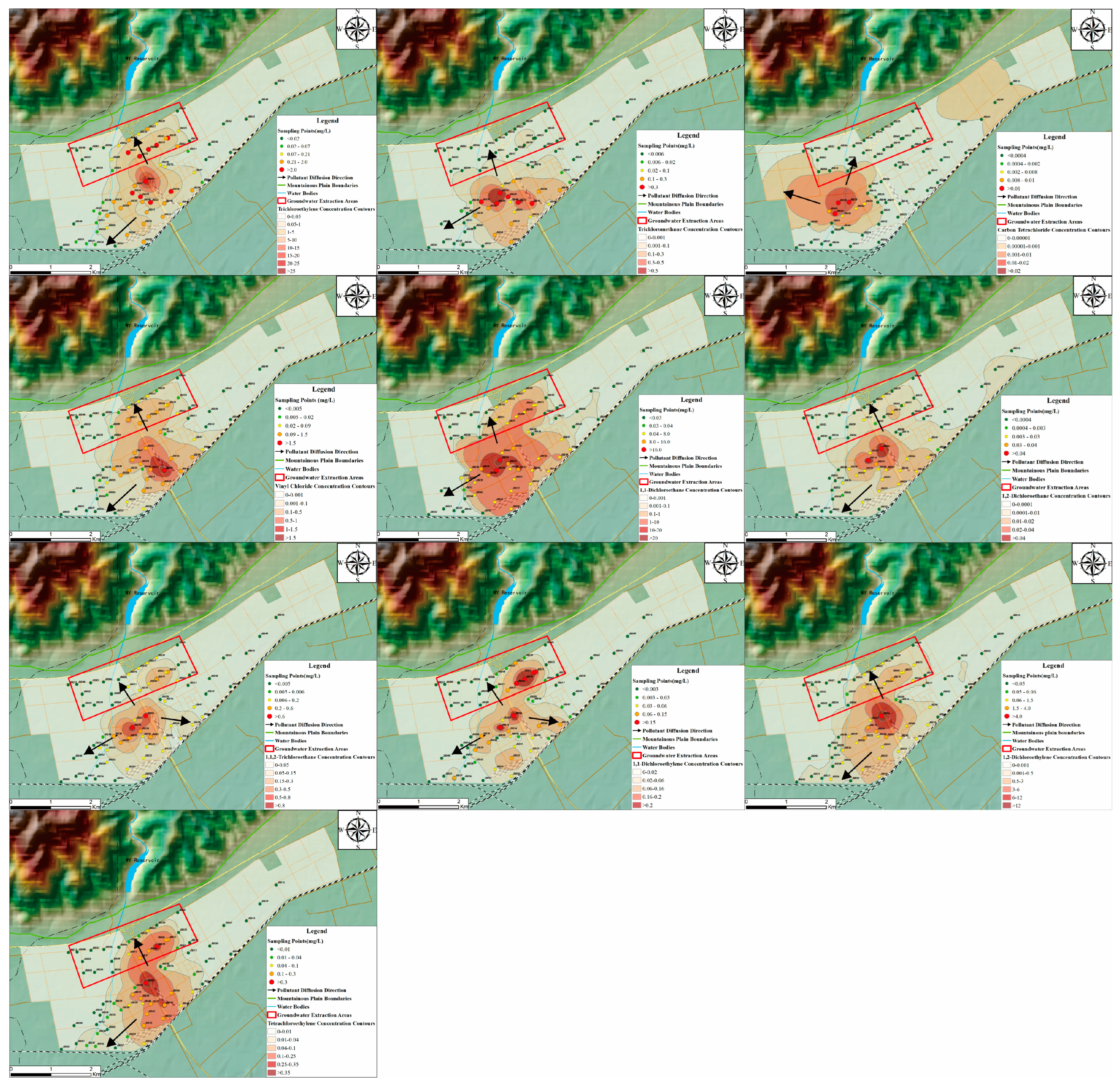
3.1. Chlorinated Hydrocarbons in Groundwater
3.2. Multi-Pathway Assessment of Chlorinated Hydrocarbons
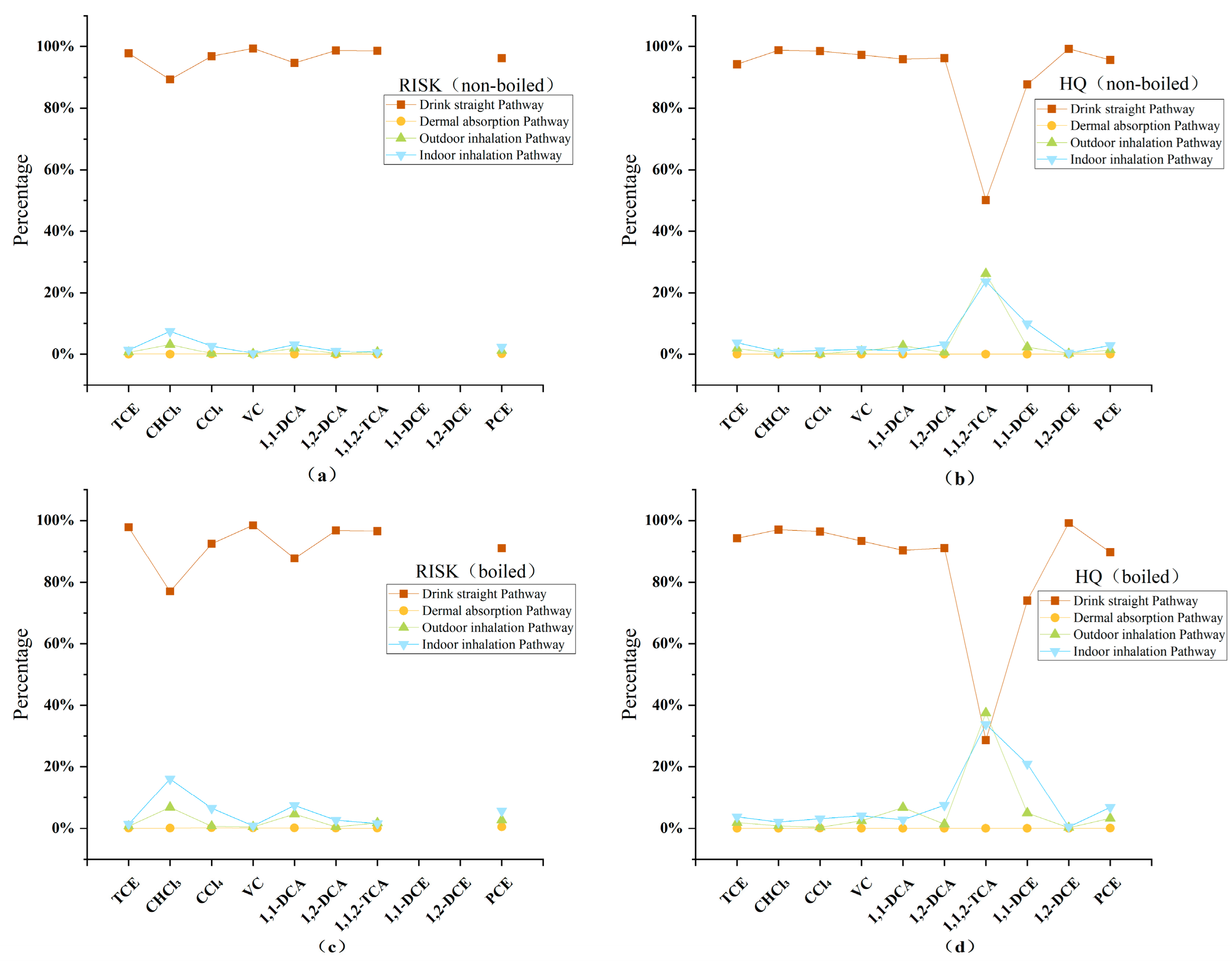
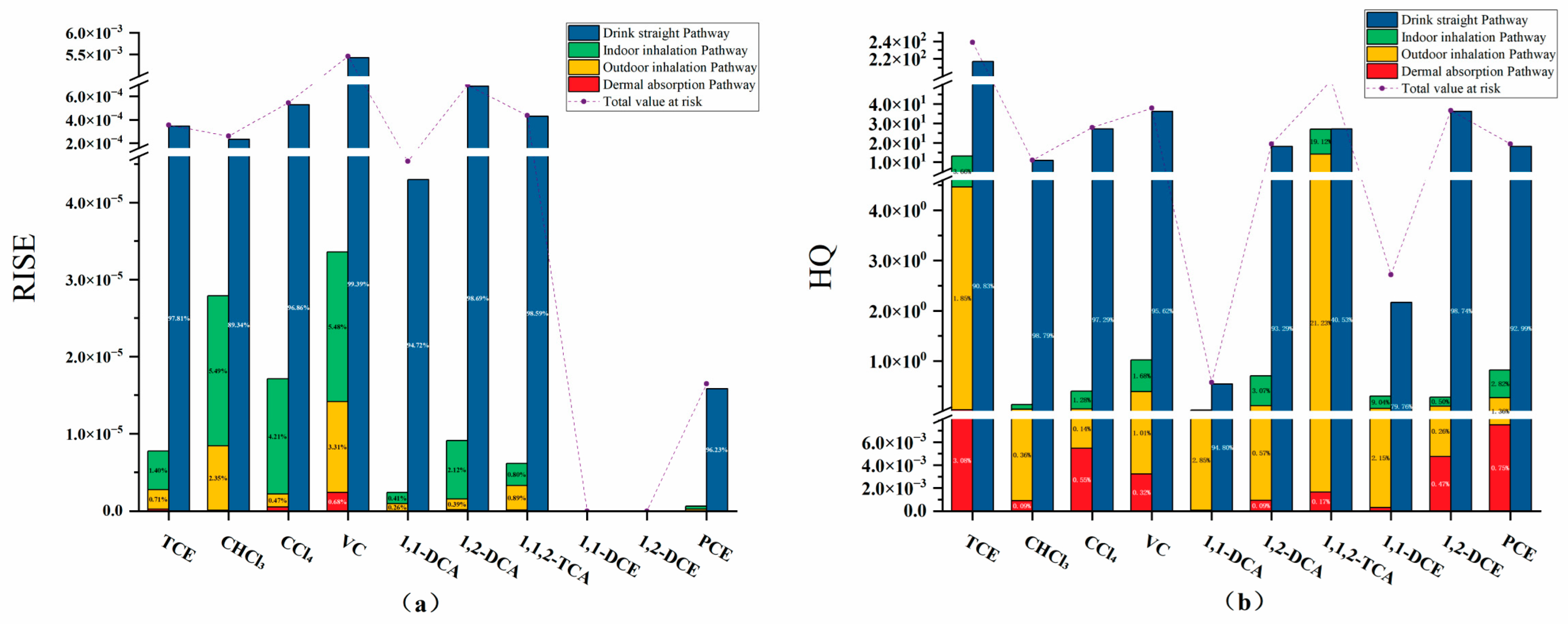
3.2.1. Carcinogenic and Non-Carcinogenic Risk Values
3.2.2. Contribution Rate and Standardization Analysis
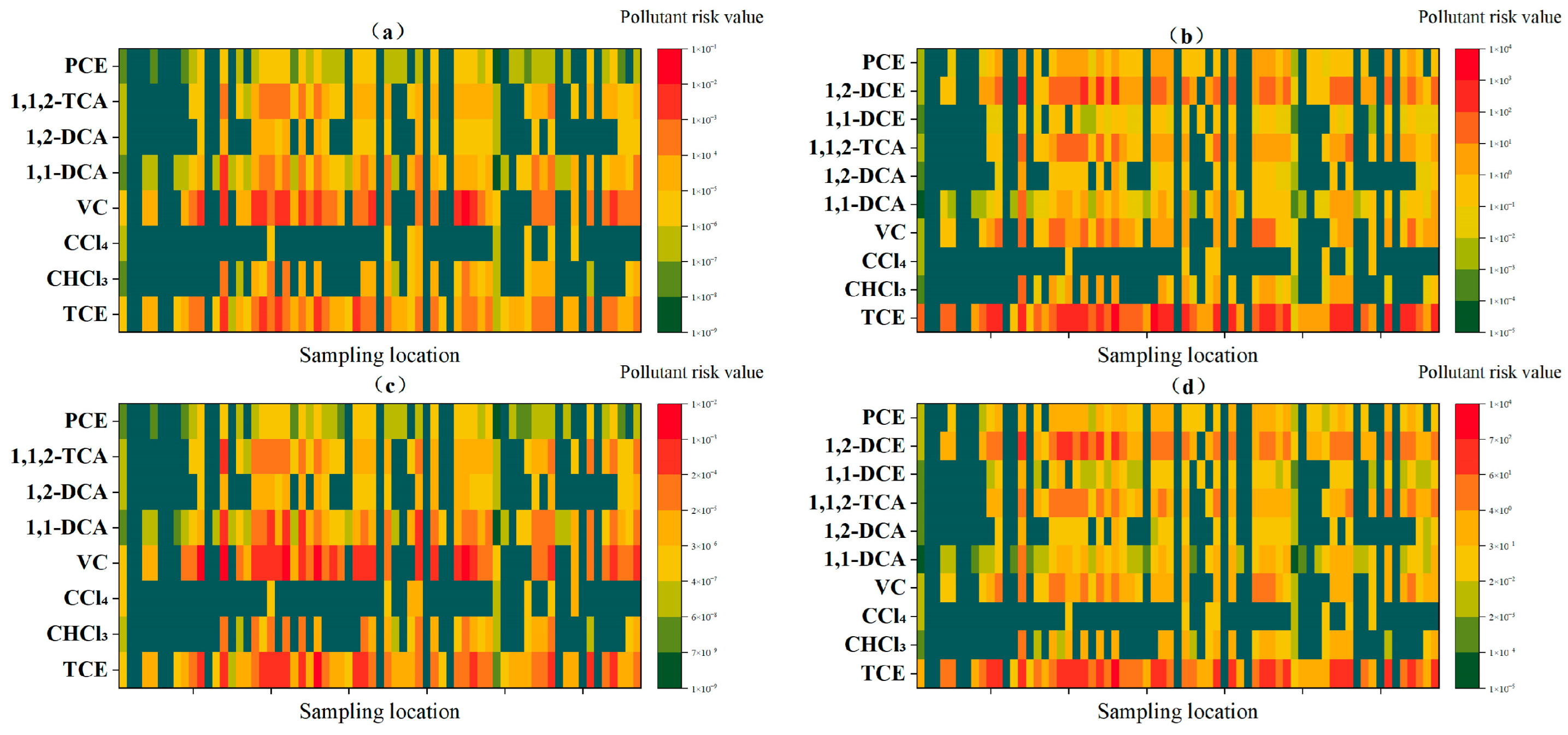
3.3. Analysis of Key Pollutants in Risk Assessment
3.4. Sensitivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.; He, R.; Cui, G.; Wei, J.; Li, X.; Xie, Y.; Shi, P. Quantitative tracing the sources and human risk assessment of complex soil pollution in an industrial park. Environ. Res. 2024, 257, 119185. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, C.; Xie, Y.C.; Wang, M.; Zhang, Z.; Yan, Z.G.; Guo, G.L. Key influencing factors of soil risk screening values in heavy metal contaminated sites: A case study of arsenic. Chin. J. Ecotoxicol. 2018, 13, 11. [Google Scholar]
- Wei, J.; Chen, M.; Song, J.; Luo, F.; Han, L.; Li, C.; Dong, M. Assessment of human health risk for an area impacted by a large-scale metallurgical refinery complex in Hunan, China. Hum. Ecol. Risk Assess. 2015, 21, 863–881. [Google Scholar] [CrossRef]
- CRAG. Technical Guidelines for Risk Assessment of Contaminated Sites; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2014. Available online: https://english.mee.gov.cn/Resources/standards/Soil/Method_Standard4/201605/t20160506_337324.shtml (accessed on 20 May 2024).
- Li, X.; Tian, T.; Shang, X.; Zhang, R.; Xie, H.; Wang, X.; Wang, H.; Xie, Q.; Chen, J.; Kadokami, K. Occurrence and health risks of organic micro-pollutants and metals in groundwater of Chinese rural areas. Environ. Health Perspect. 2020, 128, 107010. [Google Scholar] [CrossRef]
- Yang, X.; Du, J.; Jia, C.; Yang, T.; Shao, S. Groundwater pollution risk, health effects and sustainable management of halocarbons in typical industrial parks. Environ. Res. 2024, 250, 118422. [Google Scholar] [CrossRef]
- Namocatcat, J.A.; Fang, J.; Barcelona, M.J.; Quibuyen, A.T.O.; Abrajano, T.A., Jr. Trimethylbenzoic acids as metabolite signatures in the biogeochemical evolution of an aquifer contaminated with jet fuel hydrocarbons. J. Contam. Hydrol. 2003, 67, 177–194. [Google Scholar] [CrossRef]
- Rail, C.D. Groundwater Contamination: Sources, Control, and Preventive Measures; Technomic: Lancaster, PA, USA, 1989. [Google Scholar]
- Gao, C.R.; Wang, J.T. Detection and characteristics of volatile halogenated hydrocarbon contamination in groundwater from 69 cities in China. J. Earth Sci. Environ. 2012, 34, 66–71. [Google Scholar]
- Shi, J.S.; Wang, Z.; Zhang, Z.J.; Fei, Y.H.; Zhang, F.E.; Li, Y.S.; Chen, J.S.; Qian, Y. Preliminary analysis of organic contamination characteristics in groundwater of the North China Plain. J. Ecol. Environ. 2011, 20, 1695. [Google Scholar]
- Zhu, L.; Guan, X.; Peng, Y.; Li, J.; Zhang, X.; Gong, A.; Li, M.; Xie, H.; Chen, S.; Li, J.; et al. Characterization of VOCs emissions and associated health risks inherent to the packaging and printing industries in Shandong Province, China. Sci. Total Environ. 2024, 946, 174108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhao, X.; Tang, Z.; Zhao, T.; Teng, M.; Liang, W.; Wang, J.; Niu, L. Using deterministic and probabilistic approaches to assess the human health risk of 7 polycyclic aromatic hydrocarbons. J. Clean. Prod. 2021, 331, 129811. [Google Scholar] [CrossRef]
- Xiong, Y.; Bari, A.; Xing, Z.; Du, K. Ambient volatile organic compounds (VOCs) in two coastal cities in western Canada: Spatiotemporal variation, source apportionment, and health risk assessment. Sci. Total Environ. 2019, 706, 135970. [Google Scholar] [CrossRef]
- Han, L.; Qian, L.; Yan, J.; Liu, R.; Du, Y.; Chen, M. A comparison of risk modeling tools and a case study for human health risk assessment of volatile organic compounds in contaminated groundwater. Environ. Sci. Pollut. Res. 2015, 23, 1234–1245. [Google Scholar] [CrossRef]
- Kawabe, Y.; Komai, T. A case study of natural attenuation of chlorinated solvents under unstable groundwater conditions in Takahata, Japan. Bull. Environ. Contam. Toxicol. 2019, 102, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, L.; Liu, X.; Chen, J.; Liu, R.; Niu, H. Comparison of the health risks associated with different exposure pathways of multiple volatile chlorinated hydrocarbons in contaminated drinking groundwater. Environ. Pollut. 2019, 255, 113339. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Gupta, S.K.; Mishra, B.K. Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies—A case study of Eastern region of India. Ecotoxicol. Environ. Saf. 2015, 113, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Mishaqa, E.S.I.; Radwan, E.K.; Ibrahim, M.B.M.; Hegazy, T.A.; Ibrahim, M.S. Multi-exposure human health risks assessment of trihalomethanes in drinking water of Egypt. Environ. Res. 2022, 207, 112643. [Google Scholar] [CrossRef]
- Yuan, B.; Cao, H.; Du, P.; Ren, J.; Chen, J.; Zhang, H.; Zhang, Y.; Luo, H. Source-oriented probabilistic health risk assessment of soil potentially toxic elements in a typical mining city. J. Hazard. Mater. 2023, 443, 130222. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K.; Singh, R.P.; Anshumali; Kumari, D.; Jha, P.K.; Mehta, P. Characterization of heavy metal pollution in an anthropogenically and geologically influenced semi-arid region of east India and assessment of ecological and human health risks. Sci. Total Environ. 2020, 705, 135801. [Google Scholar] [CrossRef] [PubMed]
- Technical Specifications for Environmental Monitoring of Groundwater. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2004. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/method_standard2/200807/t20080707_125109.shtml (accessed on 26 May 2024).
- Water Quality-Determination of Volatile Organic Compounds Purge and Trap/Gas Chromatography-Mass Spectrometer. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2012. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201212/t20121207_243474.shtml (accessed on 26 May 2024).
- Amjad, H.; Hashmi, I.; Rehman, M.S.U.; Awan, M.A.; Ghaffar, S.; Khan, Z. Cancer and non-cancer risk assessment of trihalomethanes in urban drinking water supplies of Pakistan. Ecotoxicol. Environ. Saf. 2013, 91, 25–31. [Google Scholar] [CrossRef]
- Lee, S.C.; Guo, H.; Lam, S.M.J.; Lau, S.L.A. Multipathway risk assessment on disinfection by-products of drinking water in Hong Kong. Environ. Res. 2004, 94, 47–56. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Removal of aromatic and hydrophobic fractions of natural organic matter (NOM) using surfactant modified magnetic nanoadsorbents (MNPs). Environ. Sci. Pollut. Res. 2018, 25, 25565–25579. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rodriguez, M.J.; Serodes, J. Model development for predicting changes in DBP exposure concentrations during indoor handling of tap water. Sci. Total Environ. 2010, 408, 4733–4743. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Wagner, E.D.; Osiol, J.; Plewa, M.J. Boiling of simulated tap water: Effect on polar brominated disinfection byproducts, halogen speciation, and cytotoxicity. Environ. Sci. Technol. 2014, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y. Distribution characteristics of chlorinated hydrocarbons in contaminated plots of typical organic chemical plants and risk assessment based on Monte Carlo simulation. J. Environ. Eng. Technol. 2024, 14, 98–111. [Google Scholar]
- Ministry of Health of the People’s Republic of China. China Standards for Drinking Water Quality; Ministry of Health of the People’s Republic of China: Beijing, China, 2022. [Google Scholar]
- Pollicino, L.C.; Masetti, M.; Stevenazzi, S.; Colombo, L.; Alberti, L. Spatial statistical assessment of groundwater PCE (tetrachloroethylene) diffuse contamination in urban areas. Water 2019, 11, 1211. [Google Scholar] [CrossRef]
- Cheng, Z. Migration, Distribution, and Mass Desorption of Tetrachloroethylene in Saturated Porous Media. Doctoral Dissertation, Nanjing University, Nanjing, China, 2016. [Google Scholar]
- U.S. EPA. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A, Interim Final); 1989 EPA/540/189/002; U.S. EPA: Washington, DC, USA, 1989. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (accessed on 22 June 2024).
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Human health risk assessment for exposure to BTEXN in an urban aquifer using deterministic and probabilistic methods: A case study of Chennai city, India. Environ. Pollut. 2020, 265, 114814. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; López, F.; Becerra, S.; Jamhoury, H.; Le Menach, K.; Dévier, M.-H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking water quality in areas impacted by oil activities in Ecuador: Associated health risks and social perception of human exposure. Sci. Total Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef]
- Pardakhti, A.R.; Bidhendi, G.R.N.; Torabian, A.; Karbassi, A.; Yunesian, M. Comparative cancer risk assessment of THMs in drinking water from well water sources and surface water sources. Environ. Monit. Assess. 2011, 179, 499–507. [Google Scholar] [CrossRef]
- Singha, W.J.; Deka, H. Ecological and human health risk associated with heavy metals (HMs) contaminant sourced from petroleum refinery oily sludge. J. Hazard. Mater. 2024, 476, 135077. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, D.; Ding, D.; Deng, S.; Kong, L.; Wei, J.; Xia, F.; Li, M.; Long, T. Spatiotemporal characteristics and dynamic risk assessment of a multi-solvents abandoned pesticide-contaminated site with a long history, in China. J. Environ. Manag. 2023, 336, 117633. [Google Scholar] [CrossRef]
- Su, A.Q.; Han, L.; Yan, J.C.; Qian, L.B.; Ouyang, D.; Chen, M.F. Groundwater risk assessment of chlorinated hydrocarbon-contaminated sites based on health and water environment protection. Environ. Eng. 2018, 36, 138–143. [Google Scholar]
- Swartjes, F.A. Human health risk assessment related to contaminated land: State of the art. Environ. Geochem. Health 2015, 37, 651–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.-H.; Chan, C.-C.; Chung, C.-W.; Ma, Y.-C.; Wang, G.-S.; Wang, J.-D. Health risk assessment on residents exposed to chlorinated hydrocarbons contaminated in groundwater of a hazardous waste site. J. Toxicol. Environ. Health Part A 2002, 65, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Kujlu, R.; Mahdavianpour, M.; Ghanbari, F. Multi-route human health risk assessment from trihalomethanes in drinking and non-drinking water in Abadan, Iran. Environ. Sci. Pollut. Res. 2020, 27, 42621–42630. [Google Scholar] [CrossRef]
- Tong, R.P.; Yang, X.Y. Environmental health risk assessment of contaminated soil based on Monte Carlo method: A case of PAHs. Huanjing Kexue 2017, 38, 2522–2529. [Google Scholar] [PubMed]
- Zhang, L. Comprehensive Assessment of Shallow Groundwater Quality and Human Health Risks in Liaoning Province. Doctoral Dissertation, Jilin University, Changchun, China, 2023. [Google Scholar]



| TCE | CHCl₃ | CCl₄ | VC | 1,1-DCA | 1,2-DCA | 1,1,2-TCA | 1,1-DCE | 1,2-DCE | PCE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overstandard rate | 71.6% | 26.9% | 10.4% | 61.2% | 55.2% | 4.5% | 53.7% | 34.3% | 52.2% | 31.3% |
| Detection rate | 82.1% | 41.8% | 11.9% | 62.7% | 80.6% | 40.3% | 61.2% | 56.7% | 71.6% | 65.7% |
| Maximum (mg/L) | 28 | 0.95 | 0.021 | 1.92 | 26.5 | 0.06 | 1.08 | 0.33 | 17.6 | 0.45 |
| Average value (mg/L) | 1.07 | 0.08 | 0.00 | 0.15 | 2.01 | 0.01 | 0.09 | 0.04 | 0.72 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, G.; Li, X.; Yang, Y.; Ding, K.; Xing, S.; Zhang, Y.; Zhang, L. Human Health Risk Assessment of Chlorinated Hydrocarbons in Groundwater Based on Multi-Pathway Analysis. Toxics 2024, 12, 894. https://doi.org/10.3390/toxics12120894
Wang Y, Li G, Li X, Yang Y, Ding K, Xing S, Zhang Y, Zhang L. Human Health Risk Assessment of Chlorinated Hydrocarbons in Groundwater Based on Multi-Pathway Analysis. Toxics. 2024; 12(12):894. https://doi.org/10.3390/toxics12120894
Chicago/Turabian StyleWang, Yidi, Guilan Li, Xiaohan Li, Ye Yang, Kaifang Ding, Shilu Xing, Yilong Zhang, and Luxing Zhang. 2024. "Human Health Risk Assessment of Chlorinated Hydrocarbons in Groundwater Based on Multi-Pathway Analysis" Toxics 12, no. 12: 894. https://doi.org/10.3390/toxics12120894
APA StyleWang, Y., Li, G., Li, X., Yang, Y., Ding, K., Xing, S., Zhang, Y., & Zhang, L. (2024). Human Health Risk Assessment of Chlorinated Hydrocarbons in Groundwater Based on Multi-Pathway Analysis. Toxics, 12(12), 894. https://doi.org/10.3390/toxics12120894





