Effect of Silver Nanoparticle Size on Antibacterial Activity
Abstract
1. Introduction
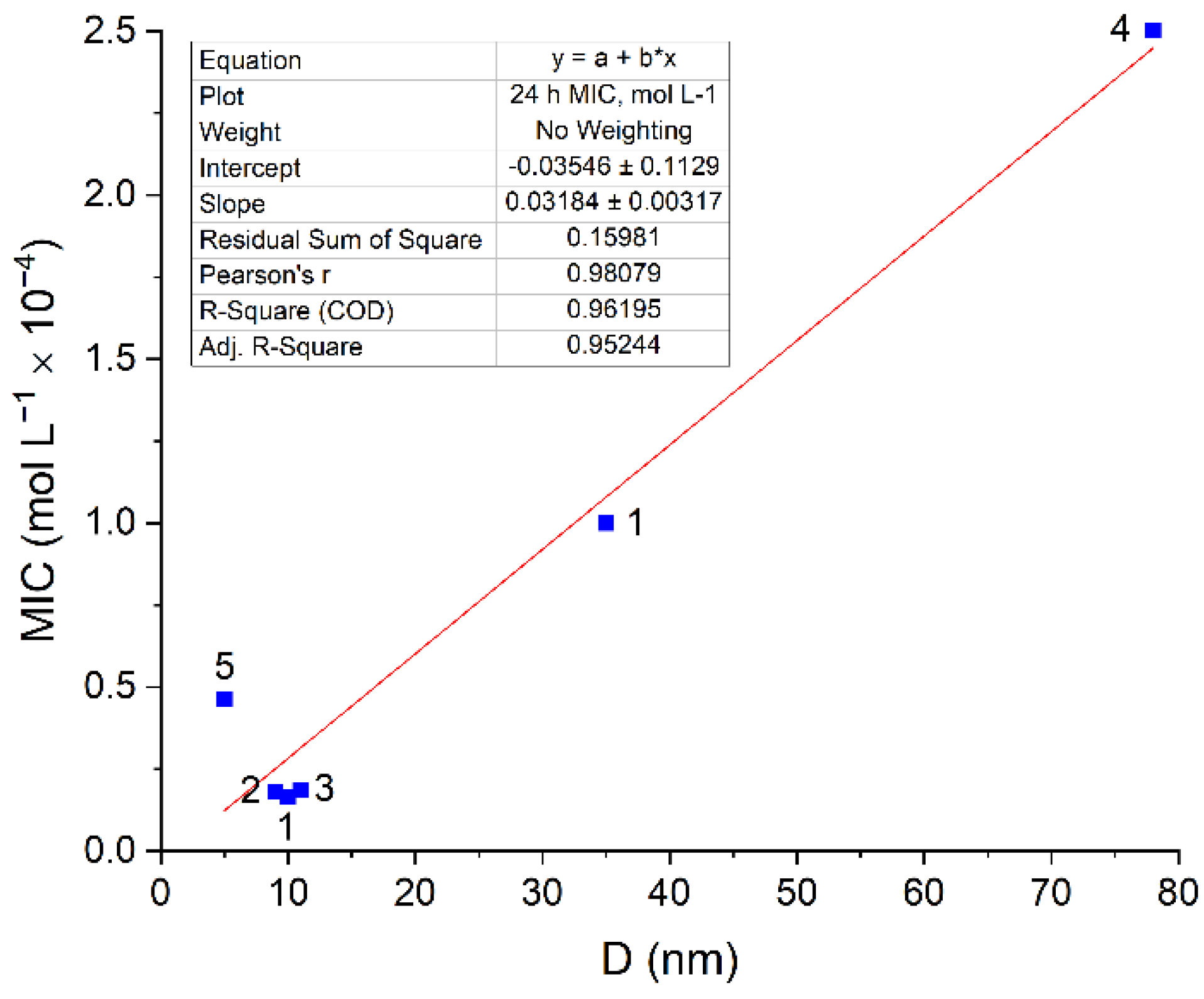
2. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the NanoFate Model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Li, H.; Xu, H. Mechanisms of Bacterial Resistance to Environmental Silver and Antimicrobial Strategies for Silver: A Review. Environ. Res. 2024, 248, 118313. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sandoval, M.J.; Caravaca, M.; López-García, I.; Hernández-Córdoba, M.; Vicente-Martínez, Y. Complete and Simultaneous Removal of Ionic Silver and Silver Nanoparticles by Using an Ionic Liquid Supported on a Magnetic Nanoparticle Core. Environ. Res. 2022, 214, 113943. [Google Scholar] [CrossRef]
- Angel, B.M.; Batley, G.E.; Jarolimek, C.V.; Rogers, N.J. The Impact of Size on the Fate and Toxicity of Nanoparticulate Silver in Aquatic Systems. Chemosphere 2013, 93, 359–365. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 Nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano. Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Ershov, V.; Tarasova, N.; Abkhalimov, E.; Safonov, A.; Sorokin, V.; Ershov, B. Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects. Int. J. Mol. Sci. 2022, 23, 949. [Google Scholar] [CrossRef]
- Ershov, B.; Ershov, V. Electrochemical Mechanism of Oxidative Dissolution of Silver Nanoparticles in Water: Effect of Size on Electrode Potential and Solubility. Nanomaterials 2023, 13, 1907. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C.; Rodríguez-González, V.; Ortega-Sigala, J.J.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosynthesis of Silver Nanoparticles by Fusarium scirpi and Its Potential as Antimicrobial Agent against Uropathogenic Escherichia coli Biofilms. PLoS ONE 2020, 15, e0230275. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Pfeiffer, P.A.; Soto Urzúa, L.; Sánchez-Mora, E.; González, A.L.; Romo-Herrera, J.M.; Gervacio Arciniega, J.J.; Martínez Morales, L.J. Damage on Escherichia coli and Staphylococcus aureus Using White Light Photoactivation of Au and Ag Nanoparticles. J. Appl. Phys. 2019, 125, 213102. [Google Scholar] [CrossRef]
- Haque, M.A.; Imamura, R.; Brown, G.A.; Krishnamurthi, V.R.; Niyonshuti, I.I.; Marcelle, T.; Mathurin, L.E.; Chen, J.; Wang, Y. An Experiment-Based Model Quantifying Antimicrobial Activity of Silver Nanoparticles on Escherichia coli. RSC Adv. 2017, 7, 56173–56182. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The Toxic Effect of Silver Ions and Silver Nanoparticles towards Bacteria and Human Cells Occurs in the Same Concentration Range. RSC Adv. 2012, 2, 6981. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- Long, Y.-M.; Hu, L.-G.; Yan, X.-T.; Zhao, X.-C.; Zhou, Q.-F.; Cai, Y.; Jiang, G.-B. Surface Ligand Controls Silver Ion Release of Nanosilver and Its Antibacterial Activity against Escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The Inhibitory Effects of Silver Nanoparticles, Silver Ions, and Silver Chloride Colloids on Microbial Growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Núñez, N.V.; Lara Villegas, H.H.; del Carmen Ixtepan Turrent, L.; Rodríguez Padilla, C. Silver Nanoparticles Toxicity and Bactericidal Effect Against Methicillin-Resistant Staphylococcus aureus: Nanoscale Does Matter. NanoBiotechnology 2009, 5, 2–9. [Google Scholar] [CrossRef]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution on Staphylococcus aureus and Candida spp. J. Nanomater. 2014, 2014, 545279. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [PubMed]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol 2016, 7, 760. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial Effect of Silver Nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Sánchez Reyna, P.A.; Olea Mejía, O.F.; González-Pedroza, M.G.; Montiel-Bastida, N.M.; Rebollo-Plata, B.; Morales-Luckie, R.A. Inhibition of the Growth of Escherichia coli and Staphylococcus aureus Microorganisms in Aesthetic Orthodontic Brackets through the In Situ Synthesis of Ag, TiO2 and Ag/TiO2 Nanoparticles. Microorganisms 2024, 12, 1583. [Google Scholar] [CrossRef]
- Mayouf, F.; Hamidouche, M.; Maouche, N.; Cherif-Silini, H.; Balla, A. Fast Pulsed Electrodeposition of Silver Nanoparticles on Polypyrrole Thin Films for Antibacterial and Biomedical Applications. J. Alloys Compd. 2023, 968, 172086. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Oćwieja, M.; Mrowiec, H.; Walas, S.; Lupa, D. Oxidative Dissolution of Silver Nanoparticles: A New Theoretical Approach. J. Colloid Interface Sci. 2016, 469, 355–364. [Google Scholar] [CrossRef]
- Ho, C.-M.; Yau, S.K.-W.; Lok, C.-N.; So, M.-H.; Che, C.-M. Oxidative Dissolution of Silver Nanoparticles by Biologically Relevant Oxidants: A Kinetic and Mechanistic Study. Chem. Asian J. 2010, 5, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Pattadar, D.K.; Nambiar, H.N.; Allen, S.L.; Jasinski, J.B.; Zamborini, F.P. Effect of Metal Nanoparticle Aggregate Structure on the Thermodynamics of Oxidative Dissolution. Langmuir 2021, 37, 7320–7327. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. https://doi.org/10.3390/toxics12110801
Ershov VA, Ershov BG. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics. 2024; 12(11):801. https://doi.org/10.3390/toxics12110801
Chicago/Turabian StyleErshov, Vadim A., and Boris G. Ershov. 2024. "Effect of Silver Nanoparticle Size on Antibacterial Activity" Toxics 12, no. 11: 801. https://doi.org/10.3390/toxics12110801
APA StyleErshov, V. A., & Ershov, B. G. (2024). Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics, 12(11), 801. https://doi.org/10.3390/toxics12110801