Abstract
Introduction: Recent advances in tumor visualization have improved the extent of resection (EOR) of primary and secondary tumors of the central nervous system, while limiting the morbidity and mortality of the surgery. One area of recent interest has been the use of intraoperative fluorophores for tumor visualization such as 5-aminolevulinic acid (5-ala) and sodium fluorescein. We performed a systematic review and meta-analysis on the utility of fluorophore administration and EOR with each fluorophore to update the current literature. Methods: We conducted a systematic review and meta-analysis on the use of intraoperative 5-ala or fluorescein between 2021 and 2023 using the PubMed, SCOPUS, and WOS databases. The initial search yielded 8688 results. After inclusion and exclusion criteria were met, 44 studies remained for review. A meta-analysis was performed to compare the EOR between studies for each fluorophore and to compare the presence of intraoperative fluorescence by tumor type. Odds ratios (OR) were calculated for gross total resection (GTR), and two-way ANOVA tests were performed to compare rates of intraoperative fluorescence by fluorophore and tumor type. Results: In all groups except low-grade glioma, fluorescence was present after 5-ala administration; fluorescence was present for all groups after fluorescein administration. Two-way ANOVA analysis for both fluorophores demonstrated no statistically significant difference in presence of fluorescence between type of tumor resected. Meta-analysis of EOR did show a higher, but not significant, rate of GTR in the 5-ala group compared to controls (OR = 1.29, 95% CI = 0.49; 3.37). In the fluorescein group, there were statistically significant higher odds of GTR compared to the control group (OR = 2.10, 95% CI = 1.43; 3.10, I2 = 0%). Conclusions: Both 5-ala and sodium fluorescein demonstrated intraoperative fluorescence among various tumor types in both cranial and spinal tumors, as well as efficacy in improving EOR. Both fluorophores merit further investigation for use in surgery of CNS tumors.
1. Introduction/Background
Primary and secondary central nervous system tumors are a frequent source of morbidity and mortality in the population, with an estimated 6.2 new cases per 100,000 people annually in the U.S and a mortality rate of 4.4 per 100,000 people annually [1]. Perhaps more importantly, primary and metastatic central nervous system (CNS) tumors represent a significant source of morbidity, with neurologic symptoms such as weakness, aphasia, and cranial nerve dysfunction resulting from tumor location, degree of parenchymal invasion, and perilesional edema. The standard of care for most CNS lesions, primary or metastatic, typically involves an interdisciplinary approach that consists of a combination of resection, chemotherapy, and radiation therapy, though this varies based on tumor type. In glioblastoma multiforme (GBM), the most common primary malignant intracranial tumor, standard of care is maximally safe resection and subsequent radiotherapy, which has been shown to have significantly improved survival compared to surgery or radiation alone [2]. Conversely, benign tumors, such as typical meningiomas, can often be either observed or definitively cured with resection alone. Ultimately, surgical resection plays a central role in the resection of nervous system tumors.
Recent advances in surgical technique and adjunctive measures have improved the extent of resection (EOR) in patients with primary or metastatic CNS tumors. Though most of these advances have been noted in the resection of high-grade gliomas (HGG) and GBM, more recent advances are being made across the spectrum of tumor types. The utilization of fluorophores intraoperatively, sodium fluorescein and 5-aminolevulinic acid (5-ALA) in particular, has shown promise in improving EOR and is an area of significant focus for researchers.
1.1. 5-Aminolevulinic Acid
5-ALA (Gleolan®, Medexus Pharmaceuticals, Inc., Bolton, ON, Canada) is currently the only fluorophore with regulatory approval for use in high-grade glioma surgery in the United States [3,4]. First studied in 1980, it has been used in over 40 clinical trials and obtained regulatory approval in 2017 for standard of care in high-grade gliomas, offering a survival advantage of 6.2 months with some studies citing a specificity and sensitivity of 100% and 85%, respectively [3]. After being administered orally in the preoperative period, the drug demonstrates intracellular uptake and is metabolized secondary to blood–brain barrier disruption in diseased tissue. The oral bioavailability of 5-ALA is estimated to be 60% with a half-life of 45 min, and once absorbed, it is metabolized in the heme biosynthesis pathway in the mitochondria. Ferrochelatase converts 5-ALA to protoporphyrin, which fluoresces in the tumor’s intracellular space. It emits a violet-red fluorescence (640 to 710 nm) once activated with 375–440 nm light. The molecule is then effluxed by the ABCG2 pump into the blood, where it is excreted in the bile. The protoporphyrin accumulation is dependent on its efflux, biosynthesis, and protoporphyrin conversion to heme [5,6,7,8].
In general, Gleolan is given 2 to 4 h prior to anesthesia, since peak fluorescence occurs at six hours, aligning with the operative timing of dural incision [5,6,7,8]. Prolonged exposure to light can confer a photobleaching effect, minimizing the effectiveness of 5-ala. Fluorescence, although weak, can persist up to eight to nine hours after injection and include other areas of the CNS with diminished BBB, such as ependyma, choroid plexus, arcuate nucleus, median eminence, and areas with BBB breakdown secondary to malignancy [5,6,7,8]. The adverse events reported with Gleolan include skin sensitivity and clinically insignificant elevations in liver enzymes, with major adverse effects rarely cited in the literature [3].
1.2. Sodium Fluorescein
Fluorescein-sodium (C20H12O5-Na) is a water-soluble, orange-red powder that weighs 376 Da and has been widely utilized in the scientific and medical community. Its various applications include fluorescein isothiocyanate 1 (FITC) and the Alexa 488 fluorophore in cellular imaging, among others. The use of fluorescein for CNS tumors was first described by Moore and colleagues in 1947 [9]. The fluorescein biomarker is excited by a wavelength of 460 to 500 nm, and emits fluorescence in the 540 to 690 nm range [4,10]. Though fluorescein administration in for resection of CNS tumors is not currently FDA-approved, it has a wide breadth of ophthalmologic applications, and ongoing clinical studies are evaluating its use in CNS tumor resection.
Fluorescein’s intracranial mechanism of action relies on the disruption of the normal blood–brain barrier. The typical BBB is characterized by tight junctions between endothelial cells and astrocytic end feet separated by a basal laminar layer, responsible for maintaining the immunoprivileged nature of the CNS. It has been widely shown that tumors disrupt the BBB, allowing for leakage of fluids into the extracellular space. In this manner, fluorescein accumulates in the extracellular space and has been shown to correlate with gadolinium enhancement of glioma boundaries on MRI [11]. Importantly, this implies that fluorescein is not specifically targeting tumor cells, but rather targets areas with disrupted BBB [5,6,10]. Still, histopathologic analysis has demonstrated fluorescein administration to be highly specific in delineating tumor boundaries, with one study citing specificity at 90.9% and sensitivity at 82.2% [11]; these findings have since been corroborated by other studies [12,13,14,15,16].
Administration of fluorescein is typically performed ten to twenty minutes prior to incision in the dura. The majority of unbound fluorescein diffuses into the tumor while the remaining 30% of fluorescein remains in circulation, binds to albumin, and contributes to peak fluorescence. It fluoresces at 460 to 500 nm of light, and its characteristic yellow-green peaks at thirty minutes and last approximately four hours in the extracellular space. In addition, dura, the choroid plexus, and other circumventricular organs can also fluoresce. It ultimately washes out of the CNS circulation via renal excretion. The optimal dosing and timing of fluorescein administration to maximize tumor tagging and minimize fluorescence of normal brain tissue remains controversial [5,6], but reports of intravenous dosing for tumor resection vary from 3 mg/kg to 20 mg/kg. The more recent, lower dosing utilizes special operative microscopes with excitation and observation filters, while higher dosing is visible to the naked eye [17,18,19,20]. The administration of fluorescein has been shown to be typically well tolerated with minimal side effects. However, there have been two reported cases of anaphylaxis when used for resection of gliomas, both of which were treated in the ICU and resolved with supportive management [18,21,22].
2. Objectives
We performed a systematic review and meta-analysis on more recent literature surrounding 5-ala and sodium fluorescein to better understand the role of these fluorophores in resection of CNS tumors. The outcomes of interest were the presence or absence of fluorescence intraoperatively, and the overall EOR. A secondary outcome measured was the presence or absence of adverse effects.
3. Methods
3.1. Literature Search
A systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. PubMed, SCOPUS, and Web of Science were searched for English-language studies from January 2021 to March 2023, according to the following search terms for any field of the text: [(Fluorescein) AND (Glioma OR Brain Tumor OR Spinal OR Metastasis OR Tumor)] OR [{(5-Aminolevulinic Acid OR 5-ALA OR Gleolan) AND (Glioma OR Brain Tumor OR Spinal OR Metastasis OR Tumor)}] PubMed, SCOPUS, and Web of Science citations were imported into Rayyan.ai to remove duplicates and facilitate study selection. January 2021 to March 2023 was chosen as the study period to update the existing literature as several systematic reviews examine fluorophore use prior to January 2021, but very few have included data since 2021.
3.2. Study Selection
A priori inclusion and exclusion criteria were set using the PICOS framework. Randomized controlled trials (RCTs), retrospective cohort studies, and case series that included more than three patients were included. Only studies that included the use of 5-ala or sodium fluorescein were included for analysis. Studies were screened for the primary outcomes, which were inclusion about data regarding the presence or absence of intraoperative fluorescence after administration of the selected fluorophore, or extent of resection after fluorophore administration. Two investigators (S.S. and N.I.) independently reviewed and screened each abstract for inclusion in full-text review. Discrepancies between reviewers were resolved by consensus; if no consensus could be reached, the most senior author (N.A.) decided whether to include the study (however, no studies required this step). Upon full-text review, studies were excluded if they did not use the correct fluorophore, did not provide information about intraoperative fluorescence, included the wrong population, or included the wrong outcome. Studies were evaluated for bias using the JBI critical appraisal tool by S.S. [24].
3.3. Data Extraction
One reviewer (S.S.) extracted data from each article, which was then confirmed independently by one additional reviewer (N.I.). Missing data were not reported by the authors. Data included: author, study design, sample size, journal, fluorophore administered, presence of intraoperative fluorescence, extent of resection, safety events, and survival data. Presence of intraoperative fluorescence was sorted by type of tumor included in each respective study. Extent of resection was sorted by gross total resection (GTR) or subtotal resection (STR). Several studies included a category for near total resection, but the definition of near total resection was inconsistent, so all near total resections were included as subtotal resection.
3.4. Statistical Analysis
Meta-analysis was performed using R version 4.1.0 (The R Foundation for Statistical Computing, open source software). A two-tailed p-value < 0.05 was used to determine significance. When comparing extent of resection between studies, rates of gross total resection (GTR) as determined by each study were used for comparison. Dichotomous outcomes of presence of GTR and type of fluorophore administered were pooled using the Mantel–Haenszel method, and the Paule–Mandel estimator was used for t2. A random-effects meta-analysis model was then used to give a pooled estimate of the outcome as an odds ratio (OR) for GTR. Two-way ANOVA tests were run when comparing the type of tumor resected and presence of intraoperative fluorescence. The random-effects model was chosen over a fixed-effects model for all study variables due to differences in study design, patient selection, and measurement of outcomes, which may result in significant variation between studies not due to chance.
4. Results
4.1. Review Characteristics
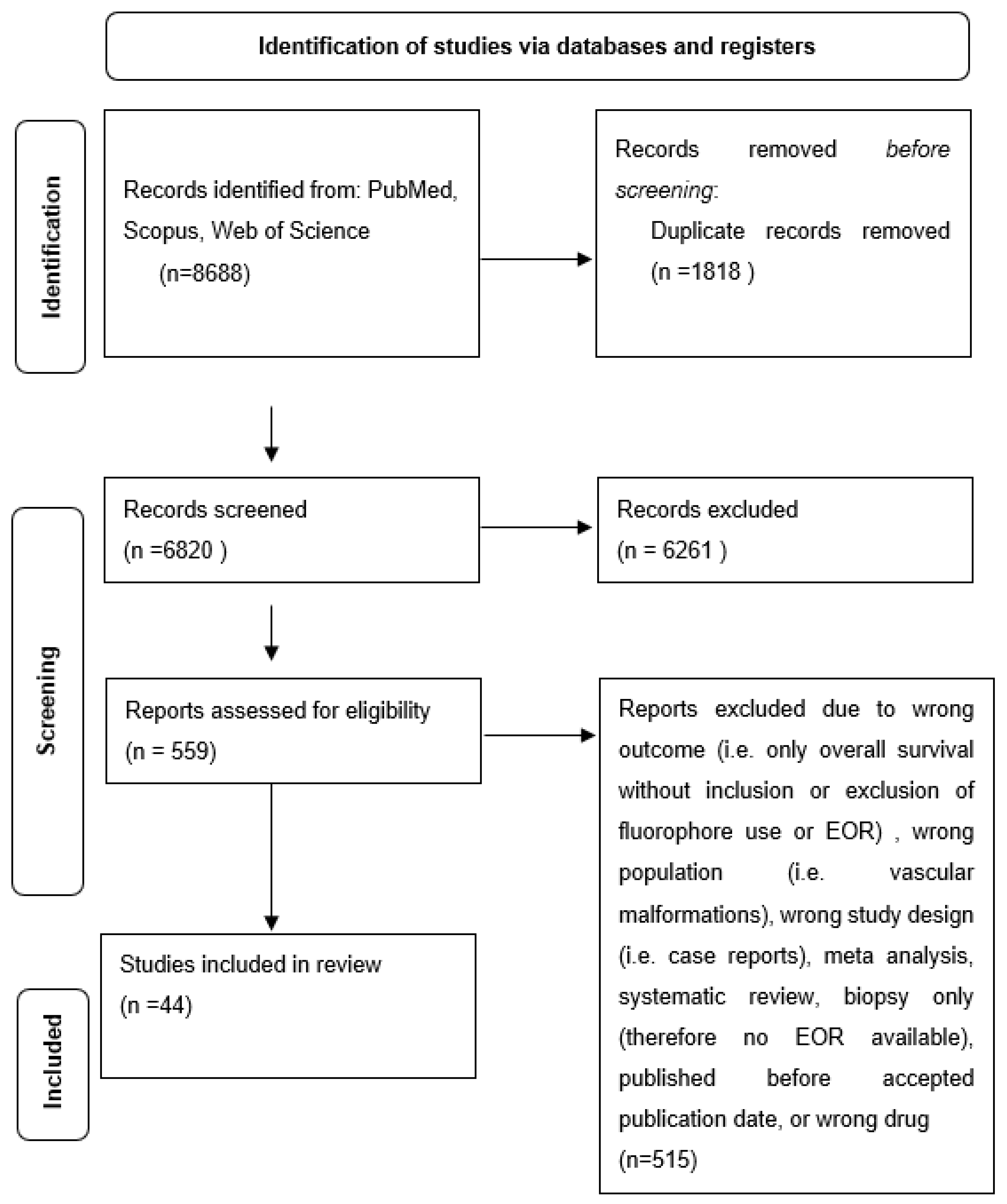
After all records had been screened for eligibility, the remaining studies were analyzed for study type, population type, outcome type, and appropriate drug administration (Figure 1). Ultimately, 44 studies were included in the study. The studies ultimately included for analysis are outlined in Table 1.
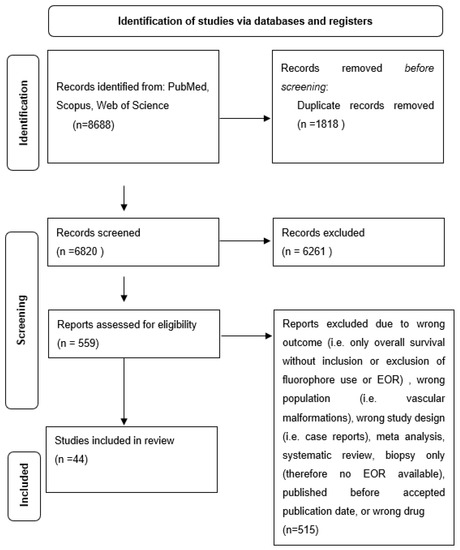
Figure 1.
Flow diagram of included studies in review.
4.2. Intraoperative Fluorescence
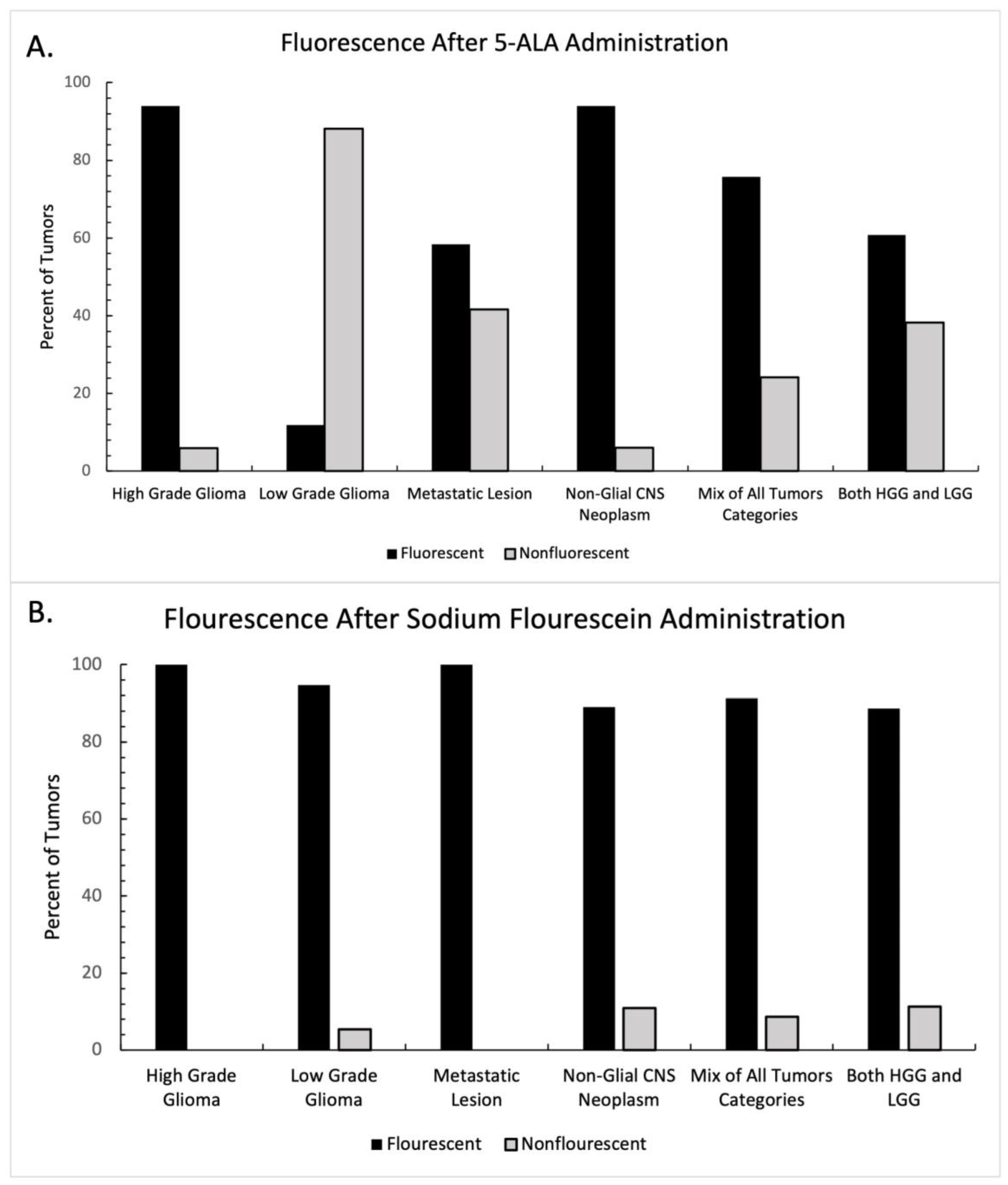
The presence or absence of fluorescence was reported in thirty-five of the studies included in this systematic review, 20 in the 5-ala group and 15 in the fluorescein group. Each study was categorized according to the class of tumor being resected (Table 1). Tumors in the “mixed tumor” category and the “all glioma” category were excluded from statistical analysis, as the presence or absence of fluorescence was not regularly reported in the context of tumor type. The graphical breakdown of intraoperative fluorescence by tumor type is shown in Figure 2A. In the 5-ala group, administration of the fluorophore demonstrated intraoperative fluorescence more frequently than not in all groups except for low-grade gliomas, where intraoperative fluorescence was seen infrequently. Intraoperative fluorescence also demonstrated mixed results in the setting of metastatic disease with 5-ala. A two-way ANOVA was performed between tumor type and the presence of fluorescence, which did not show statistical significance (F = 2.67, p = 0.316).
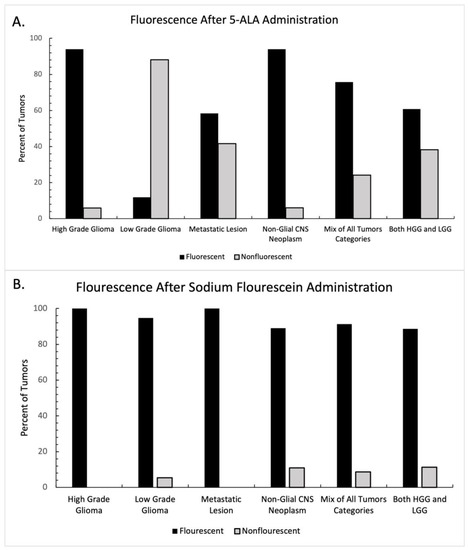
Figure 2.
Intraoperative fluorescence after fluorophore administration, distributed by tumor type. Percentage of tumors with intraoperative fluorescence after (A) 5-ala administration and (B) sodium fluorescein administration. 5-ala demonstrated heterogenous enhancement depending on tumor type, while sodium fluorescein demonstrated consistent intraoperative fluorescence across tumor type.
A similar analysis was performed for studies reporting sodium fluorescein use. The graphical breakdown of intraoperative fluorescence by tumor type is shown in Figure 2B. In all groups, fluorescence was present far more frequently in the sodium fluorescein group than not. A two-way ANOVA was again performed between tumor type and the presence of fluorescence, which did not show statistical significance (F = 0.266, p = 0.848).
4.3. Extent of Resection
Extent of resection was reported in 32 studies, 15 in which 5-ala was used, and 17 where fluorescein was used. Table 1 shows the extent of resection by study. Studies in which a control group was not present were excluded from analysis. In studies with a control group, odds ratios were calculated for the presence of gross total resection (GTR) by use of fluorophore. Only one study in which 5-ala was used during the time frame for this meta-analysis presented data on EOR for both an experimental and control group; results are demonstrated in Figure 3A and were not found to be statistically significant (OR = 1.29, 95% CI = 0.49; 3.37). In contrast, six studies using fluorescein included data on EOR in both groups with and without fluorophore use (Figure 3B). The use of sodium fluorescein was found to have statistically significantly higher odds of achieving GTR than in cases without fluorescein (OR = 2.10, 95% CI = 1.43; 3.10, I2 = 0%).
4.4. Fluorophore Safety
In total, 27 studies commented on the safety of fluorophore use, 14 in the 5-ala group and 13 with fluorescein use. Figure 4 demonstrates the frequency of both major adverse events and minor side effects with use of each fluorophore. As noted, side effects were rare in both groups, with only four reported major adverse events with 5-ala use, and only sixteen cases of minor side effects. There were no major or minor adverse effects reported with the use of sodium fluorescein.
5. Discussion
The use of intraoperative fluorophores in tumor resection has become increasingly frequent, and with improving technology, has led to improvements in extent of tumor resection. In this study, we aimed to assess the utility of fluorophore administration, its impact on extent of resection, and the safety of each fluorophore in studies conducted during the past three years. Our approach was unique as it included all CNS tumors, regardless of location (cranial or spine) or tumor type. Largely, our findings support the use of either fluorophore during resection of CNS tumors, though in the case of 5-ala, utility in low-grade gliomas and metastatic disease requires further evaluation.
In our study, the use of either 5-ala or sodium fluorescein largely demonstrated intraoperative fluorescence of the tumor and tumor boundaries. The use of 5-ala in high-grade gliomas was particularly useful, but as reported in the literature, use of 5-ala in low-grade gliomas was of limited utility and demonstrated mixed results in metastatic disease. In 2015, Jaber and colleagues noted that out of 82 low-grade gliomas, only 13 demonstrated intraoperative fluorescence with 5-ala [25]. Similarly, Hossman et al. found that only 7 out of 59 low-grade gliomas demonstrated intraoperative fluorescence with 5-ala; further analysis by this group noted that fluorescence was focal and limited to more aggressive areas of the tumor [26]. Other groups have reported similar rates of low intraoperative fluorescence of LGG with 5-ala [27,28,29]. Our study found that detection of metastatic lesions with 5-ala was heterogenous, again in line with the published literature. Mercea and colleagues reported a 69% rate of fluorescence in one cohort, while Marhold reported only 28% fluorescence in another recent study [30,31]. Previously published cohort studies report similar findings, with fluorescence noted to be heterogenous with regard to tumor boundaries [32,33,34]. Bettag and colleagues noted that although there was only 57% fluorescence under a standard white light microscope, fluorescence in the same group of tumors approached 84% with the use of an endoscope [35]. This underscores the value that advances in adjunct technologies might have on improving the role of intraoperative fluorophore use in surgery. Our study corroborates previously extensively reported evidence that 5-ala fluorescence is particularly useful in high-grade glioma surgery [36,37,38]. Notably, our study also found similar utility of 5-ala in identifying other CNS neoplasms (pleiomorphic xanthastrocytomas, oligodendrogliomas, etc.) [39,40]. Though our analysis did not reach statistical significance when comparing the rate of fluorescence by tumor type, we did observe that LGG demonstrated remarkably different fluorescence rates and patterns to other CNS tumors.
The use of sodium fluorescein in our study, by contrast, showed far more homogenous results. Regardless of tumor type, sodium fluorescein demonstrated no significant difference in rate of fluorescence. Out of all studies included in our analysis, all demonstrated fluorescence rates above 77%, and 11 out of 15 demonstrated fluorescence rates > 90% [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Previous prospective cohort studies by Acerbi have shown a high specificity and sensitivity of fluorescein in glioma detection, citing numbers as high as 79.1% and 80.8%, respectively [12]. Diaz et al. reported even higher rates of sensitivity and specificity at 82.2% and 90.9%, respectively [11]. One study showed 88% homogenous and the remaining 12% heterogenous fluorescence of meningiomas [56]. Part of the reason that intraoperative fluorescence was higher across tumor groups in the fluorescein cohort may be that, unlike 5-ala, fluorescein is not dependent on an intracellular metabolic process for activation, but rather accumulates in the extracellular space at areas of BBB breakdown.
Extent of resection was also evaluated in our meta-analysis. In the 5-ala group, only one study that reported EOR also reported the results of a control group [57]. Though the odds of GTR did not reach statistical significance in this study, it is worth noting that multiple studies have published their EOR using 5-ala, with consistently improved odds of GTR or improved EOR when using 5-ala [36,38,58]. As noted in Table 1, rates of GTR in 5-ala cohorts without a control group were variable. This suggests that further investigation into the rate of GTR using 5-ala is warranted, especially when considering resection of CNS tumors that do not fall into the HGG category. By contrast, six studies in the fluorescein group reported statistics for both EOR in the fluorescein group and the control group. The odds of GTR with fluorescein were shown to be significantly higher than those in the control groups; moreover, the studies showed little heterogeneity in outcome and no single study unduly influenced the outcome. Notably, unlike the 5-ala group, these studies were variable in the type of tumor being resected; these results provide convincing evidence that the use of intraoperative fluorescein does improve the odds of GTR. As early as 2008, Koc et al. showed a GTR rate of 83% with fluorescein compared to 55% in the control group [14]. In the prospective cohort FLUGLIO Phase II study, Acerbi found that fluorescein was effective with a GTR rate of 82.6% [12]. While Table 1 shows a trend toward generally favorable rates of GTR with fluorescein in all tumor types, more stringent studies will be required to better characterize this effect. Ultimately, both 5-ala and fluorescein demonstrate promising results with regard to the extent of tumor resection.
Safety data for both fluorescein and 5-ala were assessed during the study. In our review, we found no adverse reactions associated with fluorescein use, and a limited number of severe adverse events and side effects associated with 5-ala use. With regard to fluorescein, the dye has been widely applied in the field of ophthalmology, with side effects being fairly infrequent [59,60]. At least two case reports of anaphylaxis after fluorescein administration for CNS tumor surgery have been reported, but in both the dosing was 20 mg/kg, the highest dose reported for this administration [18,22]. Restelli and colleagues reported a patient who erroneously received 30 mg/kg of fluorescein without any adverse effects [21]. The safety of 5-ala has been well documented, including in large randomized controlled trials (RCTs) with no significant difference between the rate of adverse events in groups treated with 5-ala compared to control groups [37]. Both 5-ala and fluorescein appear safe for clinical use with regard to improving intraoperative visualization.
Of note, one unique characteristic of our study is the breadth of tumor cohorts we included. Unlike many studies, we included data from both pediatric and adult patients, as well as cranial and spine tumors, and all CNS tumor types, both primary and secondary. The inclusiveness of this study was deliberate, as our goal was to understand how the broad spectrum of CNS tumors behaved with regard to 5-ala and fluorescein administration, and whether EOR was improved. The results presented above do suggest that 5-ala differentially fluoresces for HGG compared to LGG and metastatic lesions, but the same does not hold true for fluorescein. Similar conclusions can be drawn for EOR, as in the lone 5-ala study the sole tumor type was HGG, while in the six fluorescein studies in which EOR was analyzed, tumor type varied from HGG to metastatic lesion to other non-glial CNS primary malignancy.
One major limitation of our study is that we did not report overall survival (OS) or progression-free survival (PFS) as an outcome. This was in part due to the heterogenous nature in which survival has been reported throughout the literature, obscuring the ability of statistical analyses to be performed. A 2021 retrospective cohort study by Baig Mirza et al. showed improved OS, EOR, and performance status in GBM with 5-ala-assisted surgery when compared to the control group; subgroup analysis showed an even greater OS benefit and performance status improvement in patients undergoing GTR [58]. Though Stummer and Eljamel both showed a significant improvement in PFS with 5-ala use during early RCTs on 5-ala use [36,37], a 2017 systematic review showed varying results for OS between studies, fluctuating between significance and non-significance [61]. Multiple cohort studies have shown promising data on OS or PFS for GBM, but have been heterogenous in their approach and without a control group to provide Level 1 evidence [38,39,61,62,63,64]. In another recent study regarding Grade III tumor resection, OS was not significantly improved in 5-ala-assisted surgery compared to the control groups; however, a subgroup analysis showed that patients with 5-ala-guided resection who underwent GTR showed a highly significant improvement in OS compared to the control group [57]. Hosmann and colleagues noted that, in patients with LGG, fluorescence of 5-ala was associated with shorter PFS (2.3 ± 0.7 vs. 5.0 ± 0.4 years; p = 0.01), suggesting that fluorescence may portend a more aggressive tumor and thus worse prognosis [26]. When evaluating non-glial CNS tumors or metastatic lesions, survival data for 5-ala guided resection was sparse.
One additional limitation of our study was that individual studies did not include homogenous criteria for what constituted gross total resection. Indeed, resection of ring enhancing lesion in GBM might be easier to identify radiologically than resection of low-grade gliomas, which often are poorly defined radiologically. As such, one weakness of our study is the underlying assumption that GTR was generally assessed in a similar fashion by radiologists and neurosurgeons across the included studies. Similarly, each individual study had a heterogenous set of inclusion and exclusion criteria, leaving the systematic review and meta-analysis vulnerable to selection bias based on the published studies included. In addition, publication bias would favor positive results with the use of both fluorescein and 5-ALA, again potentially biasing the results of this study. Similarly, confounding factors such as demographic factors of patients, comorbidities, and surgeon experience were unable to be controlled for given the nature of our review, again exposing the study to confounding variables.
It is also possible that other drugs routinely administered in the treatment of CNS tumors, such as antiepileptics and steroids, may impact the efficacy of fluorophores. One recent study by Goryaynov and colleagues noted a negative correlation between antiepileptic administration and intraoperative fluorescence, with 73% of patients treated with antiepileptic medications prior to surgery having no visible 5-ALA fluorescence, in contrast to 83% of antiepileptic naïve patients demonstrating visible fluorescence [65]. A multivariate analysis of intraoperative 5-ALA fluorescence in 175 IDH-1 wildtype GBM patients revealed levetiracetam use as the only significant risk factor for reduced fluorescence [66]. These findings are corroborated by in vitro studies testing protoporphyrin accumulation in glioma cell lines when treated with either dexamethasone or commonly used antiepileptics [67,68]. However, a recent large retrospective cohort series by Wadiura and colleagues yielded no significant impact of corticosteroids or antiepileptics on intraoperative fluorescence [69]. Ultimately, the effect of antiepileptic drugs and corticosteroids on intraoperative fluorescence requires further study.
In fluorescein-guided resection, there are no current RCTs comparing resection with a control group. However, Schebesch et al. found significantly improved OS with the use of SF during multivariate analysis during a large retrospective cohort review, and Falco and colleagues showed similarly promising OS (16 months) with the use of fluorescein in HGG [43,70]. These findings align with early data provided by Acerbi in the large FLUGLIO Phase II trial, as well as other studies [12,13]. Data behind survival benefit in metastatic lesions is more controversial, as Cheng and Kofoed both showed statistically significant increases in OS in the fluorescein-assisted surgery group [44,71], while Chen and Kerschbaumer showed no significant difference with the use of fluorophore [45,72]. Again, survival data on other tumor types is sparse. In the largest study utilizing both fluorescein and 5-ala, there did appear to be a trend towards improved survival with concomitant use of both fluorophores [73]. Future studies will likely focus even more on survival, making it feasible to quantify the survival benefits of 5-ala and fluorescein use further.
Another source of bias in our study was limiting the study period to 2021–2023; as many of the 5-ala studies were performed before this period, comparative data on 5-ala EOR was limited, as noted in Figure 2A. We chose this period as most systematic reviews or meta-analyses on this topic include studies from before 2021, and as such exclude the more recent advances made using fluoresecein. However, a broader study period would likely yield a statistically significant OR with regard to 5-ala-assisted resection, as several RCTs have already shown improved EOR with 5-ala [36,38,58].

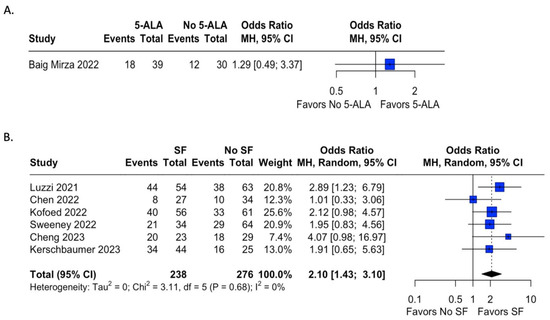
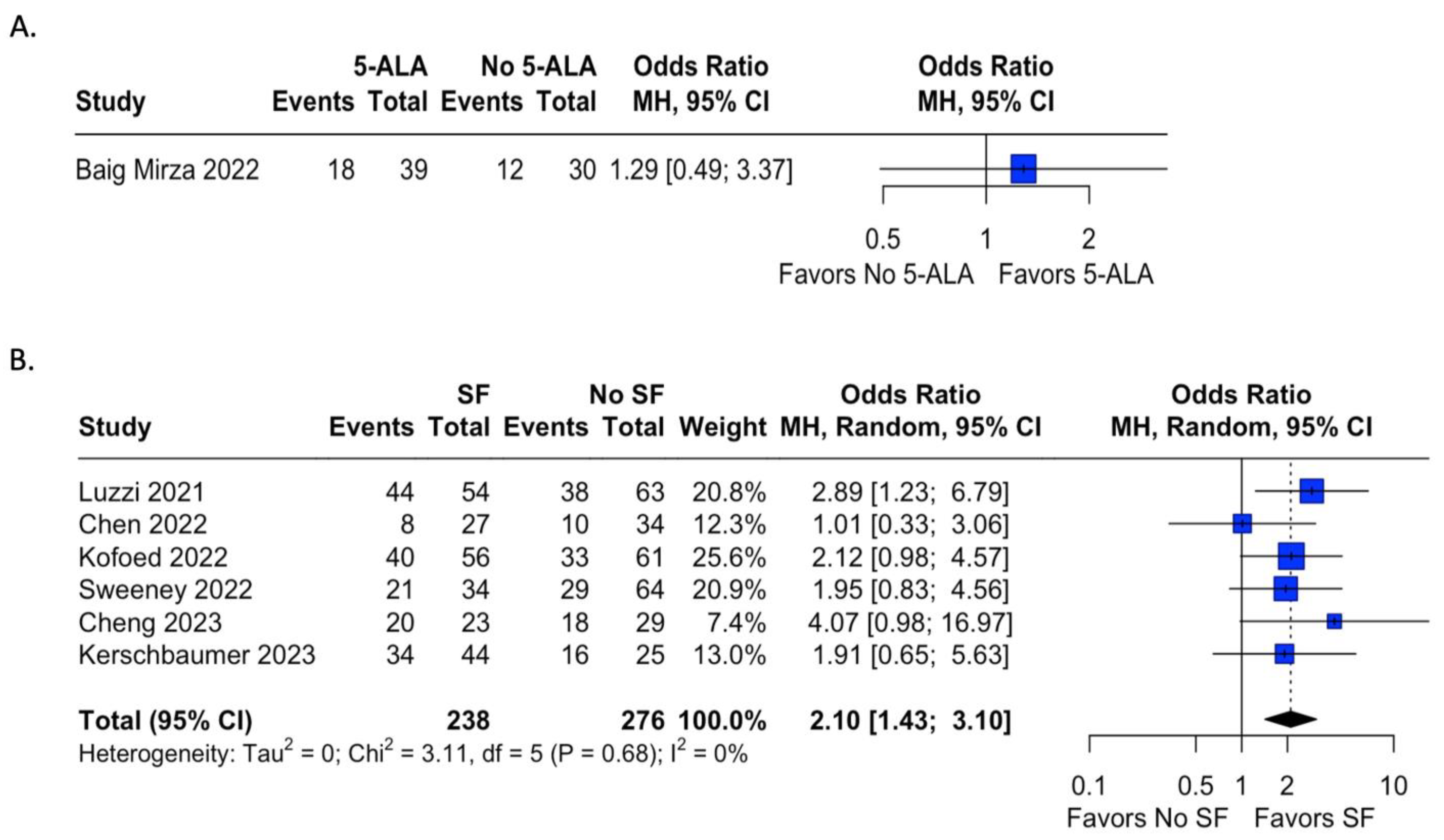



Table 1.
List of papers included in systematic review and meta-analysis. In many studies, data on visualization, EOR, side effect profile, and survival benefit of fluorophore was incomplete. * Study was found to evaluate both 5-ALA and fluorescein; resections in which both were administered were excluded from our analysis but mentioned for survival benefit.
Table 1.
List of papers included in systematic review and meta-analysis. In many studies, data on visualization, EOR, side effect profile, and survival benefit of fluorophore was incomplete. * Study was found to evaluate both 5-ALA and fluorescein; resections in which both were administered were excluded from our analysis but mentioned for survival benefit.
| Author | Year | Type of Study | Fluorophore Used | Tumors Visualized (# of Visualization/Total Number Resected) | Type of Tumor | EOR with Fluorophore (#GTR/Total Number Resected) | EOR in Control Group (#GTR/Total Number Resected) | Adverse Events | Survival Benefit of Fluorophore |
|---|---|---|---|---|---|---|---|---|---|
| Alcazar et al. [46] | 2023 | Retrospective Case Series | Fluorescein | 3/3 | Other Non-Glial CNS Neoplasm (Various) | 3/3 | -- | None | -- |
| Baig Mirza et al. [57] | 2022 | Retrospective Cohort Study | 5-ALA | 28/39 | HGG (Grade III Anaplastic Astrocytoma) | 18/39 | 12/30 | 3 Major Adverse Events | No difference |
| Baig Mirza et al. [58] | 2021 | Retrospective Cohort Study | 5-ALA | -- | HGG (GBM) | 120/253 | -- | -- | Improved compared to non-5-ALA guided surgery |
| Batalov et al. [74] | 2021 | Retrospective Case Series | 5-ALA | 62/75 | HGG & LGG | -- | -- | -- | -- |
| Bettag et al. [35] | 2022 | Retrospective Case Series | 5-ALA | 22/26 | Metastatic Lesions | -- | -- | -- | -- |
| Cardali et al. [53] | 2022 | Retrospective Case Series | Fluorescein | 13/14 | Mixed Tumors (Various) | 11/14 | -- | None | -- |
| Certo et al. [39] | 2021 | Prospective Case Series | 5-ALA | 68/68 | HGG (GBM/Gliosarcoma) | 64/68 | -- | -- | -- |
| Chen et al. [50] | 2022 | Retrospective Cohort Study | Fluorescein | 27/27 | Other Non-Glial CNS Neoplasm (Medulloblastoma) | 8/27 | 10/34 | None | No difference |
| Cheng et al. [49] | 2023 | Retrospective Cohort Study | Fluorescein | 23/23 | Metastatic Lesions | 20/23 | 18/29 | None | Improved with Fluorescein-guided surgery |
| da Silva et al. [75] | 2022 | Retrospective Case Series | 5-ALA | 195/255 | Mixed Tumors (Various) | -- | -- | 1 Major Adverse Event | -- |
| de Laurentis et al. [54] | 2022 | Retrospective Case Series | Fluorescein | 35/45 | Mixed Tumors (Various) | -- | -- | -- | -- |
| Falco et al. [48] | 2023 | Retrospective Case Series | Fluorescein | 93/93 | HGG (GBM) | 77/93 | -- | None | -- |
| Falco et al. [41] | 2022 | Retrospective Case Series | Fluorescein | 41/44 | LGG (Pilocytic Astrocytoma) | 24/39 | -- | None | -- |
| Falco et al. [42] | 2022 | Retrospective Case Series | Fluorescein | 12/12 | Other Non-Glial CNS Neoplasm (Pleiomorphic Xanthoastrocytoma) | 8/12 | -- | None | -- |
| Goryaynov et al. [76] | 2022 | Retrospective Case Series | 5-ALA | 20/34 | HGG & LGG | -- | -- | None | -- |
| Hohne et al. [55] | 2021 | Retrospective Case Series | Fluorescein | 12/12 | Mixed Tumors (Various) | -- | -- | None | -- |
| Hosmann et al. [26] | 2021 | Retrospective Case Series | 5-ALA | 7/52 | LGG | 29/59 | -- | None | -- |
| Ibrahim et al. [62] | 2021 | Retrospective Case Series | 5-ALA | -- | HGG & LGG | 24/40 | -- | One Minor Side Effect | -- |
| Kerschbaumer et al. [72] | 2022 | Retrospective Cohort Study | Fluorescein | -- | Metastatic Lesions | 34/44 | 16/25 | -- | No Difference |
| Kiesel et al. [64] | 2021 | Retrospective Case Series | 5-ALA | 161/163 | HGG (GBM) | 84/94 | -- | None | -- |
| Kofoed et al. [71] | 2022 | Retrospective Case Series | Fluorescein | -- | Metastatic Lesions | 40/56 | 33/61 | -- | Associated With Increased Overall Survival |
| Kutlay et al. [43] | 2021 | Retrospective Case Series | Fluorescein | 18/18 | Mixed Tumors (Various) | 16/18 | -- | None | -- |
| Lavrador et al. [77] | 2023 | Retrospective Case Series | 5-ALA | 6/6 | HGG | -- | -- | -- | -- |
| Luzzi et al. [78] | 2021 | Retrospective Cohort Study | Fluorescein | -- | HGG (GBM + Anaplastic Astrocytoma) | 44/54 | 38/63 | -- | Improved Progression Free Survival, Similar Overall Survival |
| Maragkos et al. [79] | 2021 | Retrospective Case Series | 5-ALA | 16/16 | HGG | -- | -- | None | -- |
| Marhold et al. [31] | 2022 | Retrospective Case Series | 5-ALA | 8/29 | Metastatic Lesions | -- | -- | -- | -- |
| Mercea et al. [30] | 2021 | Retrospective Case Series | 5-ALA | 36/58 | Metastatic Lesions | 17/25 | -- | None | -- |
| Millesi et al. [40] | 2021 | Retrospective Case Series | 5-ALA | 25/31 | Other Non-Glial CNS Neoplasm (Ependymoma/Subependymoma) | 25/31 | -- | None | -- |
| Milos et al. [80] | 2023 | Prospective Case Series | 5-ALA | 5/14 | Mixed Tumors (Various) | 10/14 | -- | None | -- |
| Muscas et al. [81] | 2022 | Retrospective Case Series | 5-ALA | -- | HGG (GBM) | 41/65 | -- | -- | -- |
| Muther et al. [82] | 2022 | Retrospective Case Series | 5-ALA | 81/173 | HGG & LGG | -- | -- | -- | -- |
| Olguner et al. [44] | 2021 | Retrospective Case Series | Fluorescein | 47/49 | Mixed Tumors (Various) | 46/49 | -- | -- | -- |
| Ott et al. [51] | 2022 | Retrospective Case Series | Fluorescein | -- | Mixed Tumors (Various) | 11/14 | -- | None | -- |
| Schebesch et al. [70] | 2022 | Retrospective Case Series | Fluorescein | -- | HGG | -- | -- | None | -- |
| Schupper et al. [63] | 2022 | Prospective Cohort Study | 5-ALA | 69/69 | HGG | -- | -- | 15 minor adverse events | -- |
| Strickland et al. [83] | 2022 | Retrospective Case Series | 5-ALA | 24/30 | HGG (GBM + Anaplastic Astrocytoma + Anaplastic Oligodendroglioma) | 9/21 | -- | None | -- |
| Sun et al. [52] | 2022 | Retrospective Case Series | Fluorescein | 47/59 | Other Non-Glial CNS Neoplasm (Ependymoma) | 56/56 | -- | -- | -- |
| Sweeney et al. [84] | 2022 | Retrospective Cohort Study | Fluorescein | -- | GBM | 21/34 | 29/64 | -- | -- |
| Takeda et al. [85] | 2022 | Retrospective Case Series | 5-ALA | 4/7 | Mixed Tumors (Various) | 6/7 | -- | None | -- |
| Ung et al. [45] | 2022 | Retrospective Case Series | Fluorescein | 12/12 | Mixed Tumors (Various) | -- | -- | None | -- |
| Watts et al. [86] | 2023 | Prospective Case Study | 5-ALA | 85/99 | HGG (GBM) | 75/99 | -- | -- | -- |
| Xue et al. [47] | 2021 | Retrospective Case Series | Fluorescein | 44/50 | HGG + LGG | 41/50 | -- | -- | -- |
| Zeppa et al. * [73] | 2022 | Retrospective Case Series | 5-ALA, Fluorescein | -- | HGG | 18/40 (5-ALA) 21/44 (Fluorescein) | -- | -- | Advantage after concomitant use |
| Zhang et al. [87] | 2022 | Retrospective Case Series | 5-ALA | 10/11 | HGG | -- | -- | None | -- |

Figure 3.
Meta-analysis of extent of resection of tumor after fluorophore administration. Odds ratios were calculated for rates of GTR for studies reporting either 5-ala or fluorescein use and a control cohort. Analysis of 5-ala favored the use of 5-ala but did not reach statistical significance, while analysis of sodium fluorescein demonstrated statistically significant odds of achieving GTR with fluorescein administration compared to the control group with relatively low heterogeneity [15,49,57,71,72,79,85].
Figure 3.
Meta-analysis of extent of resection of tumor after fluorophore administration. Odds ratios were calculated for rates of GTR for studies reporting either 5-ala or fluorescein use and a control cohort. Analysis of 5-ala favored the use of 5-ala but did not reach statistical significance, while analysis of sodium fluorescein demonstrated statistically significant odds of achieving GTR with fluorescein administration compared to the control group with relatively low heterogeneity [15,49,57,71,72,79,85].


Figure 4.
Safety profile of fluorophores. Both fluorophores were generally well tolerated with minimal to no adverse events or side effects.
Figure 4.
Safety profile of fluorophores. Both fluorophores were generally well tolerated with minimal to no adverse events or side effects.

6. Conclusions
Both 5-ala and fluorescein appear to have utility in primary and secondary CNS tumor resection. In our review and analysis of the recent literature, both fluorophores appear to fluoresce in various tumor types. 5-ala may have limited utility in low-grade glioma, while fluorescein appears to more homogenously enhance intraoperatively, likely due to their respective mechanisms of action. While 5-ala shows a trend toward improving EOR, though limited due to our study period, fluorescein showed compelling evidence of improving rates of GTR regardless of tumor type and location. Both fluorophores were found to be generally safe, but careful selection of the fluorophore based on preoperative imaging, expected tumor type, and the patient’s current medications may better inform the surgeon which fluorophore to utilize. Further research on the use of both fluorophores will better elucidate their utility on the various types of CNS neoplasms.
Author Contributions
All authors contributed to this work. S.S. and N.I. were responsible for study design, data collection, literature review, and study screening. S.S. and A.M. were responsible for statistical analysis. All authors were involved in data interpretation, manuscript drafting and editing, and submission process. S.S. is corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as this was a review of the literature using already published data.
Informed Consent Statement
There was no a priori registration for this review or the protocol used in the review.
Data Availability Statement
Data extracted from included studies, used for analysis, analytic code, and forms used for bias assessment are available for public access per request from corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Cancer Institute. Cancer Stat Facts: Brain and Other Nervous System Cancer. The Surveillance, Epidemiology, and End Results (SEER). Published 2018. Available online: https://seer.cancer.gov/statfacts/html/brain.html (accessed on 3 November 2020).
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Yong, R.L.; Hadjipanayis, C.G. The Neurosurgeon’s Armamentarium for Gliomas: An Update on Intraoperative Technologies to Improve Extent of Resection. J. Clin. Med. 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Martirosyan, N.L.; Yagmurlu, K.; Miller, E.J.; Eschbacher, J.M.; Izadyyazdanabadi, M.; Bardonova, L.A.; Byvaltsev, V.A.; Nakaji, P.; Preul, M.C. Intraoperative Fluorescence Imaging for Personalized Brain Tumor Resection: Current State and Future Directions. Front. Surg. 2016, 3, 55. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Di Perna, G.; Melcarne, A.; Altieri, R.; La Rocca, G.; Sabatino, G.; et al. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Chanbour, H.; Chotai, S. Review of Intraoperative Adjuncts for Maximal Safe Resection of Gliomas and Its Impact on Outcomes. Cancers 2022, 14, 5705. [Google Scholar] [CrossRef]
- Moore, G.E.; Peyton, W.T.; French, L.A.; Walker, W.W.; Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Raza, H.; Hassan, M.; Seo, S.-Y.; et al. The Clinical Use of Fluorescein in Neurosurgery. J. Neurosurg. 1948, 5, 392–398. [Google Scholar] [CrossRef]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, B.; Yao, X.; Yang, Y. Outcome comparisons of high-grade glioma resection with or without fluorescein sodium-guidance. Curr. Probl. Cancer 2019, 43, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, H.; Ge, P.; Zhao, J.; Li, W.; Gu, H.; Wang, G.; Luo, Y.; Chen, D. Gross Total Resection of Glioma with the Intraoperative Fluorescence-guidance of Fluorescein Sodium. Int. J. Med. Sci. 2012, 9, 708–714. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.S.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C.; Lv, A.; Ga, R.; et al. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, E11. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Broggi, M.; Cordella, R.; Anghileri, E.; Eoli, M.; Schiariti, M.; Broggi, G.; Ferroli, P. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg. Rev. 2014, 37, 547–557. [Google Scholar] [CrossRef]
- Dilek, O.; Ihsan, A.; Tulay, H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J. Clin. Neurosci. 2011, 18, 430–431. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Kajimoto, Y.; Ohta, T. Development of a Fluorescein Operative Microscope for use During Malignant Glioma Surgery: A Technical Note and Preliminary Report. Surg. Neurol. 1998, 50, 41–49. [Google Scholar] [CrossRef]
- Wallace, M.B.; Meining, A.; Canto, M.I.; Fockens, P.; Miehlke, S.; Roesch, T.; Lightdale, C.J.; Pohl, H.; Carr-Locke, D.; Löhr, M.; et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2010, 31, 548–552. [Google Scholar] [CrossRef]
- Restelli, F.; Bonomo, G.; Monti, E.; Broggi, G.; Acerbi, F.; Broggi, M. Safeness of sodium fluorescein administration in neurosurgery: Case-report of an erroneous very high-dose administration and review of the literature. Brain Spine 2022, 2, 101703. [Google Scholar] [CrossRef]
- Tanahashi, S.; Lida, H.; Dohi, S. An Anaphylactoid Reaction After Administration of Fluorescein Sodium During Neurosurgery. Obstet. Anesth. Dig. 2006, 103, 503. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18 F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecula. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Thomas, C. Comment on Hosmann et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)—Experience at Two Specialized Centers. Cancers 2021, 13, 2540. Cancers 2021, 13, 5634. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.-J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Clin. Neurosurg. 2019, 84, 1214–1224. [Google Scholar] [CrossRef]
- Mercea, P.A.; Mischkulnig, M.; Kiesel, B.; Wadiura, L.I.; Roetzer, T.; Prihoda, R.; Heicappell, P.; Kreminger, J.; Furtner, J.; Woehrer, A.; et al. Prognostic Value of 5-ALA Fluorescence, Tumor Cell Infiltration and Angiogenesis in the Peritumoral Brain Tissue of Brain Metastases. Cancers 2021, 13, 603. [Google Scholar] [CrossRef]
- Marhold, F.; Roetzer-Pejrimovsky, T.; Scheichel, F.; Mercea, P.A.; Mischkulnig, M.; Wadiura, L.I.; Kiesel, B.; Weber, M.; Popadic, B.; Prihoda, R.; et al. Does pigmentation, hemosiderin and blood effect visible 5-ALA fluorescence in cerebral melanoma metastasis? Photodiagnosis Photodyn. Ther. 2022, 39, 102864. [Google Scholar] [CrossRef]
- Kamp, M.A.; Grosser, P.; Felsberg, J.; Slotty, P.J.; Steiger, H.-J.; Reifenberger, G.; Sabel, M. 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir. 2012, 154, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Schatlo, B.; Stockhammer, F.; Barrantes-Freer, A.; Bleckmann, A.; Siam, L.; Pukrop, T.; Rohde, V. 5-Aminolevulinic Acid Fluorescence Indicates Perilesional Brain Infiltration in Brain Metastases. World Neurosurg. X 2020, 5, 100069. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.-R.; Coluccia, D.; Fandino, J. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg. Focus 2014, 36, E10. [Google Scholar] [CrossRef] [PubMed]
- Bettag, C.; Hussein, A.; Schatlo, B.; Barrantes-Freer, A.; Abboud, T.; Rohde, V.; Mielke, D. Endoscope-assisted visualization of 5-aminolevulinic acid fluorescence in surgery for brain metastases. J. Neurosurg. 2022, 137, 1650–1655. [Google Scholar] [CrossRef]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Valle, R.D.; Slof, J.; Galván, J.; Arza, C.; Romariz, C.; Vidal, C. Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurología 2014, 29, 131–138. [Google Scholar] [CrossRef]
- Certo, F.; Altieri, R.; Maione, M.; Schonauer, C.; Sortino, G.; Fiumanò, G.; Tirrò, E.; Massimino, M.; Broggi, G.; Vigneri, P.; et al. FLAIRectomy in Supramarginal Resection of Glioblastoma Correlates with Clinical Outcome and Survival Analysis: A Prospective, Single Institution, Case Series. Oper. Neurosurg. 2021, 20, 151–163. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Mazanec, V.; Wadiura, L.I.; Wöhrer, A.; Herta, J.; Wolfsberger, S.; Novak, K.; Furtner, J.; Rössler, K.; et al. 5-ALA fluorescence for intraoperative visualization of spinal ependymal tumors and identification of unexpected residual tumor tissue: Experience in 31 patients. J. Neurosurg. Spine 2021, 34, 374–382. [Google Scholar] [CrossRef]
- Falco, J.; Höhne, J.; Broggi, M.; Rubiu, E.; Restelli, F.; Vetrano, I.G.; Schiariti, M.; Mazzapicchi, E.; Bonomo, G.; Ferroli, P.; et al. Fluorescein-guided surgery for the resection of pilocytic astrocytomas: A multicentric retrospective study. Front. Oncol. 2022, 12, 943085. [Google Scholar] [CrossRef]
- Falco, J.; Broggi, M.; Vetrano, I.G.; Rubiu, E.; Schiariti, M.; Restelli, F.; Mazzapicchi, E.; Bonomo, G.; La Corte, E.; Ferroli, P.; et al. Fluorescein sodium in the surgical treatment of pleomorphic xanthoastrocytomas: Results from a retrospective study. Front. Oncol. 2022, 12, 1009769. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, M.; Durmaz, O.; Ozer, I.; Kırık, A.; Yasar, S.; Kural, C.; Temiz, Ç.; Tehli, Ö.; Ezgu, M.C.; Daneyemez, M.; et al. Fluorescein Sodium-Guided Neuroendoscopic Resection of Deep-Seated Malignant Brain Tumors: Preliminary Results of 18 Patients. Oper. Neurosurg. 2020, 20, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Olguner, S.K.; Arslan, A.; Açık, V.; Istemen, I.; Can, M.; Gezercan, Y.; Ökten, A.I. Sodium Fluorescein for Spinal Intradural Tumors. Front. Oncol. 2021, 10, 618579. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.H.; Serva, S.; Chatain, G.P.; Witt, J.-P.; Finn, M. Application of sodium fluorescein for spinal cord lesions: Intraoperative localization for tissue biopsy and surgical resection. Neurosurg. Rev. 2021, 45, 1563–1569. [Google Scholar] [CrossRef]
- Alcazar, P.; Avedillo, A.; Vazquez, S.; Lopez, L.B.; Fustero, D.; Moles, J.; Gonzalez, L.; Orduna, J. The usefulness of intraoperative sodium fluorescein in the surgical treatment of relapsed high-grade brain tumors in pediatric patients. Child’s Nerv. Syst. 2023, 39, 1501–1507. [Google Scholar] [CrossRef]
- Xue, Z.; Kong, L.; Hao, S.; Wang, Y.; Jia, G.; Wu, Z.; Jia, W.; Zhang, J.; Zhang, L. Combined Application of Sodium Fluorescein and Neuronavigation Techniques in the Resection of Brain Gliomas. Front. Neurol. 2021, 12, 747072. [Google Scholar] [CrossRef]
- Falco, J.; Rubiu, E.; Broggi, M.; Farinotti, M.; Vetrano, I.G.; Schiariti, M.; Anghileri, E.; Eoli, M.; Pollo, B.; Moscatelli, M.; et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. J. Clin. Med. 2023, 12, 178. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, J.; Tang, R.; Ruan, J.; Mao, D.; Yang, H. Sodium Fluorescein-Guided Surgery for Resection of Brain Metastases from Lung Cancer: A Consecutive Case Series Study and Literature Review. Cancers 2023, 15, 882. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zhang, X.-H.; Lin, F.-H.; Li, C.; Jin, J.-T.; Zhou, Z.-H.; Zhu, S.-H.; Cheng, Z.-Q.; Zhong, S.; He, Z.-Q.; et al. The application of fluorescein sodium for the resection of medulloblastoma. J. Neuro-Oncol. 2022, 158, 463–470. [Google Scholar] [CrossRef]
- Ott, C.; Proescholdt, M.; Friedrich, M.; Hoehne, J.; Rosengarth, K.; Schmidt, N.-O.; Schebesch, K.-M. The use of the sodium fluorescein and YELLOW 560 nm filter for the resection of pediatric posterior fossa lesions. Child’s Nerv. Syst. 2022, 39, 1495–1500. [Google Scholar] [CrossRef]
- Sun, Z.; Jing, L.; Fan, Y.; Zhang, H.; Chen, L.; Wang, G.; Sharma, H.S.; Wang, J. Fluorescein-guided surgery for spinal gliomas: Analysis of 220 consecutive cases. Int. Rev. Neurobiol. 2020, 151, 139–154. [Google Scholar] [CrossRef]
- Cardali, S.M.; Ricciardo, G.; Garufi, G.; Raffa, G.; Messineo, F.; Scalia, G.; Conti, A.; Germanò, A. Fluorescein-guided surgery for intradural spinal tumors: A single-center experience. Brain Spine 2022, 2, 100908. [Google Scholar] [CrossRef] [PubMed]
- de Laurentis, C.; Beuriat, P.A.; Bteich, F.; Mottolese, C.; Szathmari, A.; Vinchon, M.; Di Rocco, F. Pediatric Low-Grade Glioma Surgery with Sodium Fluorescein: Efficient Localization for Removal and Association with Intraoperative Pathological Sampling. Diagnostics 2022, 12, 2927. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus 2021, 50, E19. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.M.; Jeltema, H.-R.; Kruijff, S.; Groen, R.J.M. The application of fluorescence techniques in meningioma surgery—A review. Neurosurg. Rev. 2019, 42, 799–809. [Google Scholar] [CrossRef]
- Mirza, A.B.; Lavrador, J.P.; Christodoulides, I.; Boardman, T.M.; Vastani, A.; Al Banna, Q.; Ahmed, R.; Norman, I.C.F.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic Acid-Guided Resection in Grade III Tumors—A Comparative Cohort Study. Oper. Neurosurg. 2022, 22, 215–223. [Google Scholar] [CrossRef]
- Mirza, A.B.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma—A comparative cohort study of 343 patients. Neuro-Oncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein Angiography Complication Survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef]
- Ibrahim, O.; Hafez, M.A.; Haleem, H.A.; El Maghraby, H. Recent Advances in the Treatment of Gliomas: The Multimodal Care Therapy. Open Access Maced. J. Med. Sci. 2021, 9, 503–508. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic acid for enhanced surgical visualization of high-grade gliomas: A prospective, multicenter study. J. Neurosurg. 2022, 136, 1525–1534. [Google Scholar] [CrossRef]
- Kiesel, B.; Wadiura, L.I.; Mischkulnig, M.; Makolli, J.; Sperl, V.; Borkovec, M.; Freund, J.; Lang, A.; Millesi, M.; Berghoff, A.S.; et al. Efficacy, Outcome, and Safety of Elderly Patients with Glioblastoma in the 5-ALA Era: Single Center Experience of More Than 10 Years. Cancers 2021, 13, 6119. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Widhalm, G.; Goldberg, M.F.; Chelushkin, D.; Spallone, A.; Chernyshov, K.A.; Ryzhova, M.; Pavlova, G.; Revischin, A.; Shishkina, L.; et al. The Role of 5-ALA in Low-Grade Gliomas and the Influence of Antiepileptic Drugs on Intraoperative Fluorescence. Front. Oncol. 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Güresir, Á.; Hamed, M.; Vatter, H.; Herrlinger, U.; Güresir, E. Impact of Levetiracetam Treatment on 5-Aminolevulinic Acid Fluorescence Expression in IDH1 Wild-Type Glioblastoma. Cancers 2022, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.; Steele, C.J.; Rovin, R.A.; Belton, R.J.; Winn, R.J. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5-ALA-mediated PpIX production and cellular retention in glioblastoma cells. J. Neuro-Oncol. 2016, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hefti, M.; Albert, I.; Luginbuehl, V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J. Neuro-Oncol. 2012, 108, 443–450. [Google Scholar] [CrossRef]
- Wadiura, L.I.; Mischkulnig, M.; Hosmann, A.; Borkovec, M.; Kiesel, B.; Rötzer, T.; Mercea, P.A.; Furtner, J.; Hervey-Jumper, S.; Rössler, K.; et al. Influence of Corticosteroids and Antiepileptic Drugs on Visible 5-Aminolevulinic Acid Fluorescence in a Series of Initially Suspected Low-Grade Gliomas Including World Health Organization Grade II, III, and IV Gliomas. World Neurosurg. 2020, 137, e437–e446. [Google Scholar] [CrossRef]
- Schebesch, K.-M.; Höhne, J.; Rosengarth, K.; Noeva, E.; Schmidt, N.O.; Proescholdt, M. Fluorescein-guided resection of newly diagnosed high-grade glioma: Impact on extent of resection and outcome. Brain Spine 2022, 2, 101690. [Google Scholar] [CrossRef]
- Kofoed, M.S.; Pedersen, C.B.; Schulz, M.K.; Kristensen, B.W.; Hansen, R.W.; Markovic, L.; Halle, B.; Poulsen, F.R. Fluorescein-guided resection of cerebral metastases is associated with greater tumor resection. Acta Neurochir. 2022, 164, 451–457. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Demetz, M.; Krigers, A.; Pinggera, D.; Spinello, A.; Thomé, C.; Freyschlag, C.F. Mind the gap—The use of sodium fluoresceine for resection of brain metastases to improve the resection rate. Acta Neurochir. 2023, 165, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Crasto, S.G.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci. 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Batalov, A.I.; Goryaynov, S.A.; Zakharova, N.E.; Solozhentseva, K.D.; Kosyrkova, A.V.; Potapov, A.A.; Pronin, I.N. Prediction of Intraoperative Fluorescence of Brain Gliomas: Correlation between Tumor Blood Flow and the Fluorescence. J. Clin. Med. 2021, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.B.; Ramina, R.; Neto, M.C.; Machado, G.; Cavalcanti, M.S.; Da Silva, J.F.C. Extending the indications of 5-Aminolevulinic acid for Fluorescence-Guided surgery for different central nervous system tumors: A series of 255 cases in Latin America. Arq. Bras. Neurocir. 2022, 41, e35–e42. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Buklina, S.B.; Khapov, I.V.; Batalov, A.I.; Potapov, A.A.; Pronin, I.N.; Belyaev, A.U.; Aristov, A.A.; Zhukov, V.U.; Pavlova, G.V.; et al. 5-ALA-guided tumor resection during awake speech mapping in gliomas located in eloquent speech areas: Single-center experience. Front. Oncol. 2022, 12, 940951. [Google Scholar] [CrossRef]
- Lavrador, J.P.; Reisz, Z.; Sibtain, N.; Rajwani, K.; Baig Mirza, A.; Vergani, F.; Gullan, R.; Bhangoo, R.; Ashkan, K.; Bleil, C.; et al. H3 G34-mutant high-grade gliomas: Integrated clinical, imaging and pathological characterisation of a single-centre case series. Acta Neurochir. 2023, 165, 1615–1633. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Martinelli, A.; Del Maestro, M.; Savioli, G.; Simoncelli, A.; Lafe, E.; Preda, L.; Galzio, R. Supratentorial high-grade gliomas: Maximal safe anatomical resection guided by augmented reality high-definition fiber tractography and fluorescein. Neurosurg. Focus 2021, 51, E5. [Google Scholar] [CrossRef]
- Maragkos, G.A.; Schüpper, A.J.; Lakomkin, N.; Sideras, P.; Price, G.; Baron, R.; Hamilton, T.; Haider, S.; Lee, I.Y.; Hadjipanayis, C.G.; et al. Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front. Neurol. 2021, 12, 644804. [Google Scholar] [CrossRef]
- Milos, P.; Haj-Hosseini, N.; Hillman, J.; Wårdell, K. 5-ALA fluorescence in randomly selected pediatric brain tumors assessed by spectroscopy and surgical microscope. Acta Neurochir. 2023, 165, 71–81. [Google Scholar] [CrossRef]
- Muscas, G.; Orlandini, S.; Bonaudo, C.; Dardo, M.; Esposito, A.; Campagnaro, L.; Carrai, R.; Fainardi, E.; Della Puppa, A. Functional outcomes, extent of resection, and bright/vague fluorescence interface in resection of glioblastomas involving the motor pathways assisted by 5-ALA. Acta Neurochir. 2022, 164, 3267–3274. [Google Scholar] [CrossRef]
- Müther, M.; Jaber, M.; Johnson, T.D.; Orringer, D.A.; Stummer, W. A Data-Driven Approach to Predicting 5-Aminolevulinic Acid-Induced Fluorescence and World Health Organization Grade in Newly Diagnosed Diffuse Gliomas. Neurosurgery 2022, 90, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Strickland, B.A.; Wedemeyer, M.; Ruzevick, J.; Micko, A.; Shahrestani, S.; Daneshmand, S.; Shiroishi, M.S.; Hwang, D.H.; Attenello, F.; Chen, T.; et al. 5-Aminolevulinic acid-enhanced fluorescence-guided treatment of high-grade glioma using angled endoscopic blue light visualization: Technical case series with preliminary follow-up. J. Neurosurg. 2022, 137, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.F.; Rosoklija, G.; Sheldon, B.L.; Bondoc, M.; Bandlamuri, S.; Adamo, M.A. Comparison of sodium fluorescein and intraoperative ultrasonography in brain tumor resection. J. Clin. Neurosci. 2022, 106, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Takeda, J.; Nonaka, M.; Li, Y.; Isozaki, H.; Kamei, T.; Hashiba, T.; Asai, A. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. Surg. Neurol. Int. 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Dayimu, A.; Matys, T.; Ashkan, K.; Price, S.; Jenkinson, M.D.; Doughton, G.; Mather, C.; Young, G.; Qian, W.; et al. Refining the Intraoperative Identification of Suspected High-Grade Glioma Using a Surgical Fluorescence Biomarker: GALA BIDD Study Report. J. Pers. Med. 2023, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jaman, E.; Habib, A.; Ozpinar, A.; Andrews, E.; Amankulor, N.M.; Zinn, P.O. A Novel 5-Aminolevulinic Acid-Enabled Surgical Loupe System-A Consecutive Brain Tumor Series of 11 Cases. Oper. Neurosurg. 2022, 22, 298–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).