Detection of Low Blood Hemoglobin Levels on Pulmonary CT Angiography: A Feasibility Study Combining Dual-Energy CT and Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Dual-Energy CT Protocol
2.3. Image Analysis
2.4. Statistical Analyses and Machine Learning Modeling
3. Results
3.1. Characteristics of the Sample
3.2. Image Analysis
3.3. Machine Learning Modeling
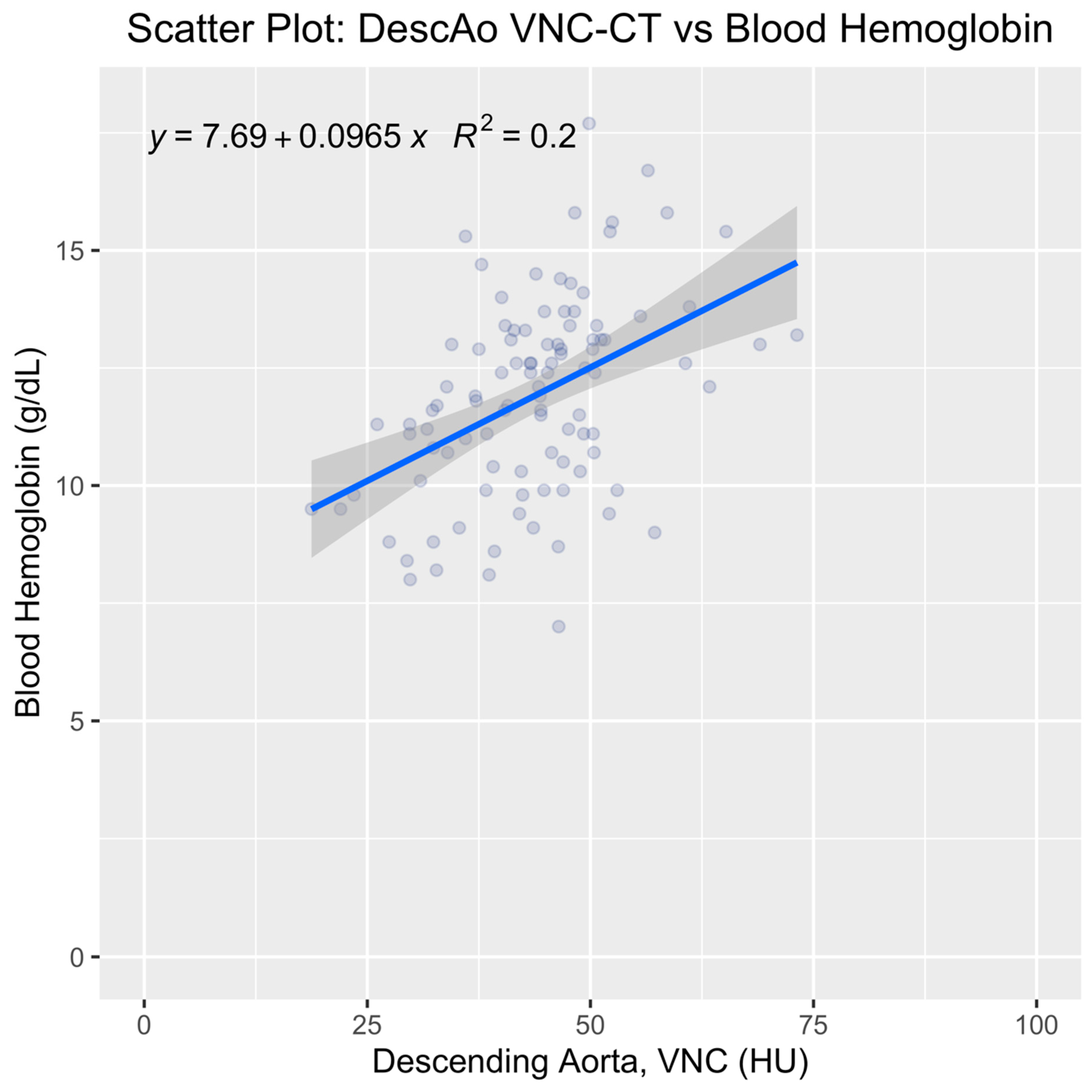
3.4. Univariate Analysis of the Top-Ranked Variable
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanc, B. Nutritional anemias. Report of a WHO Scientific Group. WHO Tech. Rep. Ser. 1968, 405, 1–40. [Google Scholar]
- McLean, E.; Cogswell, M.; Egli, I.; Woidyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Shander, A.; Knight, K.; Thurer, R.; Adamson, J.; Spence, R. Prevalence and outcomes of anemia in surgery: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 58S–69S. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.; Wade, S.; Balducci, L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 11S–26S. [Google Scholar] [CrossRef]
- Wilson, A.; Reyes, E.; Ofman, J. Prevalence and outcomes of anemia in inflammatory bowel disease: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 44S–49S. [Google Scholar] [CrossRef]
- Belperio, P.S.; Rhew, D.C. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 27S–43S. [Google Scholar] [CrossRef]
- Beghé, C.; Wilson, A.; Ershler, W.B. Prevalence and outcomes of anemia in geriatrics: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 3S–10S. [Google Scholar] [CrossRef]
- Beverina, I.; Brando, B. Prevalence of anemia and therapeutic behavior in the emergency department at a tertiary care Hospital: Are patient blood management principles applied? Transfus. Apher. Sci. 2019, 58, 688–692. [Google Scholar] [CrossRef]
- Walsh, T.S.; Saleh, E.-E.-D. Anaemia during critical illness. BJA Br. J. Anaesth. 2006, 97, 278–291. [Google Scholar] [CrossRef]
- Zhou, Q.-Q.; Yu, Y.-S.; Chen, Y.-C.; Ding, B.-B.; Fang, S.-Y.; Yang, X.; Zhang, B.; Zhang, H. Optimal threshold for the diagnosis of anemia severity on unenhanced thoracic CT: A preliminary study. Eur. J. Radiol. 2018, 108, 236–241. [Google Scholar] [CrossRef]
- Riva, E.; Colombo, R.; Moreo, G.; Mandelli, S.; Franchi, C.; Pasina, L.; Tettamanti, M.; Lucca, U.; Mannucci, P.M.; Nobili, A. Prognostic value of degree and types of anaemia on clinical outcomes for hospitalised older patients. Arch. Gerontol. Geriatr. 2017, 69, 21–30. [Google Scholar] [CrossRef]
- Tymińska, A.; Kapłon-Cieślicka, A.; Ozierański, K.; Peller, M.; Balsam, P.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; Jankowska, E.A.; Drożdż, J.; et al. Anemia at Hospital Admission and Its Relation to Outcomes in Patients With Heart Failure (from the Polish Cohort of 2 European Society of Cardiology Heart Failure Registries). Am. J. Cardiol. 2017, 119, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, D.; DiNardo, J.A.; Goobie, S.M. Relationship Between Preoperative Anemia and In-Hospital Mortality in Children Undergoing Noncardiac Surgery. Anesth. Analg. 2016, 123, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Doppman, J.L.; Rienmuller, R.; Lissner, J. The visualized interventricular septum on cardiac computed tomography: A clue to the presence of severe anemia. J. Comput. Assist. Tomogr. 1981, 5, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, J.; Rzymski, K.; Czarnecki, R. Severe anaemia: Its CT findings in the cardiovascular system. Eur. J. Radiol. 1983, 3, 108–111. [Google Scholar]
- Corcoran, H.L.; Cook, D.E.; Proto, A.V. Diagnosis of anemia on computed tomography scans of the thorax. J. Comput. Tomogr. 1988, 12, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Gillespie, S.; Kelly, B.E. Can computed tomography identify patients with anaemia? Ulst. Med. J. 2001, 70, 116–118. [Google Scholar]
- Foster, M.; Nolan, R.L.; Lam, M. Prediction of anemia on unenhanced computed tomography of the thorax. Can. Assoc. Radiol. J. 2003, 54, 26–30. [Google Scholar]
- Title, R.S.; Harper, K.; Nelson, E.; Evans, T.; Tello, R. Observer Performance in Assessing Anemia on Thoracic CT. Am. J. Roentgenol. 2005, 185, 1240–1244. [Google Scholar] [CrossRef]
- Kamel, E.M.; Rizzo, E.; Duchosal, M.A.; Duran, R.; Goncalves-Matoso, V.; Schnyder, P.; Qanadli, S.D. Radiological profile of anemia on unenhanced MDCT of the thorax. Eur. Radiol. 2008, 18, 1863–1868. [Google Scholar] [CrossRef]
- Prabhakar, A.M.; Misono, A.S.; Hemingway, J.; Hughes, D.R.; Duszak, R. Medicare Utilization of CT Angiography from 2001 through 2014: Continued Growth by Radiologists. J. Vasc. Interv. Radiol. 2016, 27, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Martí, D.; Díaz, G.; César, J.; García-Avello, Á.; Sueiro, A.; Yusen, R.D.; Jiménez, D. Association of anaemia and mortality in patients with acute pulmonary embolism. Thromb. Haemost. 2009, 102, 153–158. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G.; Jacobsen, M.C.; Schellingerhout, D.; Wood, C.A.; Tamm, E.P.; Godoy, M.C.; Sun, J.; et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Syeda-Mahmood, T. Role of Big Data and Machine Learning in Diagnostic Decision Support in Radiology. J. Am. Coll. Radiol. 2018, 15 Pt B, 569–576. [Google Scholar] [CrossRef]
- Johnson, T.R.C.; Krauß, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Graffy, P.M.; Zea, R.; Lee, S.J.; Liu, J.; Sandfort, V.; Summers, R.M. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: A retrospective cohort study. Lancet Digit. Health 2020, 2, e192–e200. [Google Scholar] [CrossRef]
- Spence, R.K. Medical and economic impact of anemia in hospitalized patients. Am. J. Health Pharm. 2007, 64 (Suppl. S11), S3–S10. [Google Scholar] [CrossRef]
- Kim, N.Y.; Im, D.J.; Youn, J.-C.; Hong, Y.J.; Choi, B.W.; Kang, S.-M.; Lee, H.-J. Synthetic Extracellular Volume Fraction Derived Using Virtual Unenhanced Attenuation of Blood on Contrast-Enhanced Cardiac Dual-Energy CT in Nonischemic Cardiomyopathy. AJR Am. J. Roentgenol. 2022, 218, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.; Price, D.; Boyd, D.; Fishman, R.; Newton, T.H. Quantitative Aspects of Computed Tomography of the Blood and Cerebrospinal Fluid. Radiology 1977, 123, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Nishihara, S.; Nishitani, H. Accuracy of computed tomography attenuation measurements for diagnosing anemia. Jpn. J. Radiol. 2010, 28, 53–57. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Gul, M.; Chaudhry, A.; Sheikh, M.; Dunkin, J. Quantitative Evaluation of Noncontrast Computed Tomography of the Head for Assessment of Anemia. J. Comput. Assist. Tomogr. 2015, 39, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Groth, M.; Bley, T.A.; Henes, F.O.; Treszl, A.; Adam, G.; Bannas, P. Assessment of anemia during CT pulmonary angiography. Eur. J. Radiol. 2012, 81, 4196–4202. [Google Scholar] [CrossRef]
- Stolzmann, P.; Frauenfelder, T.; Pfammatter, T.; Peter, N.; Scheffel, H.; Lachat, M.; Schmidt, B.; Marincek, B.; Alkadhi, H.; Schertler, T. Endoleaks after endovascular abdominal aortic aneurysm repair: Detection with dual-energy dual-source CT. Radiology 2008, 249, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, H.; Godoy, M.C.B.; Vlahos, I.; Graser, A.; Babb, J.; Leidecker, C.; Macari, M. Abdominal Aorta: Evaluation with Dual-Source Dual-Energy Multidetector CT after Endovascular Repair of Aneurysms—Initial Observations. Radiology 2008, 249, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Nyman, U.; Gottsäter, A.; Wassélius, J. Reliability of virtual non-contrast computed tomography angiography: Comparing it with the real deal. Acta Radiol. Open 2018, 7, 2058460118790115. [Google Scholar] [CrossRef]
- Toepker, M.; Moritz, T.; Krauss, B.; Weber, M.; Euller, G.; Mang, T.; Wolf, F.; Herold, C.J.; Ringl, H. Virtual non-contrast in second-generation, dual-energy computed tomography: Reliability of attenuation values. Eur. J. Radiol. 2012, 81, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.A.; Huber, A.; Senel, F.; Bette, S.; Braun, F.; Risch, F.; Woźnicki, P.; Becker, J.; Popp, D.; Haerting, M.; et al. Anemia Detection by Hemoglobin Quantification on Contrast-enhanced Photon-counting CT Data Sets. Radiology 2022, 305, 650–652. [Google Scholar] [CrossRef] [PubMed]
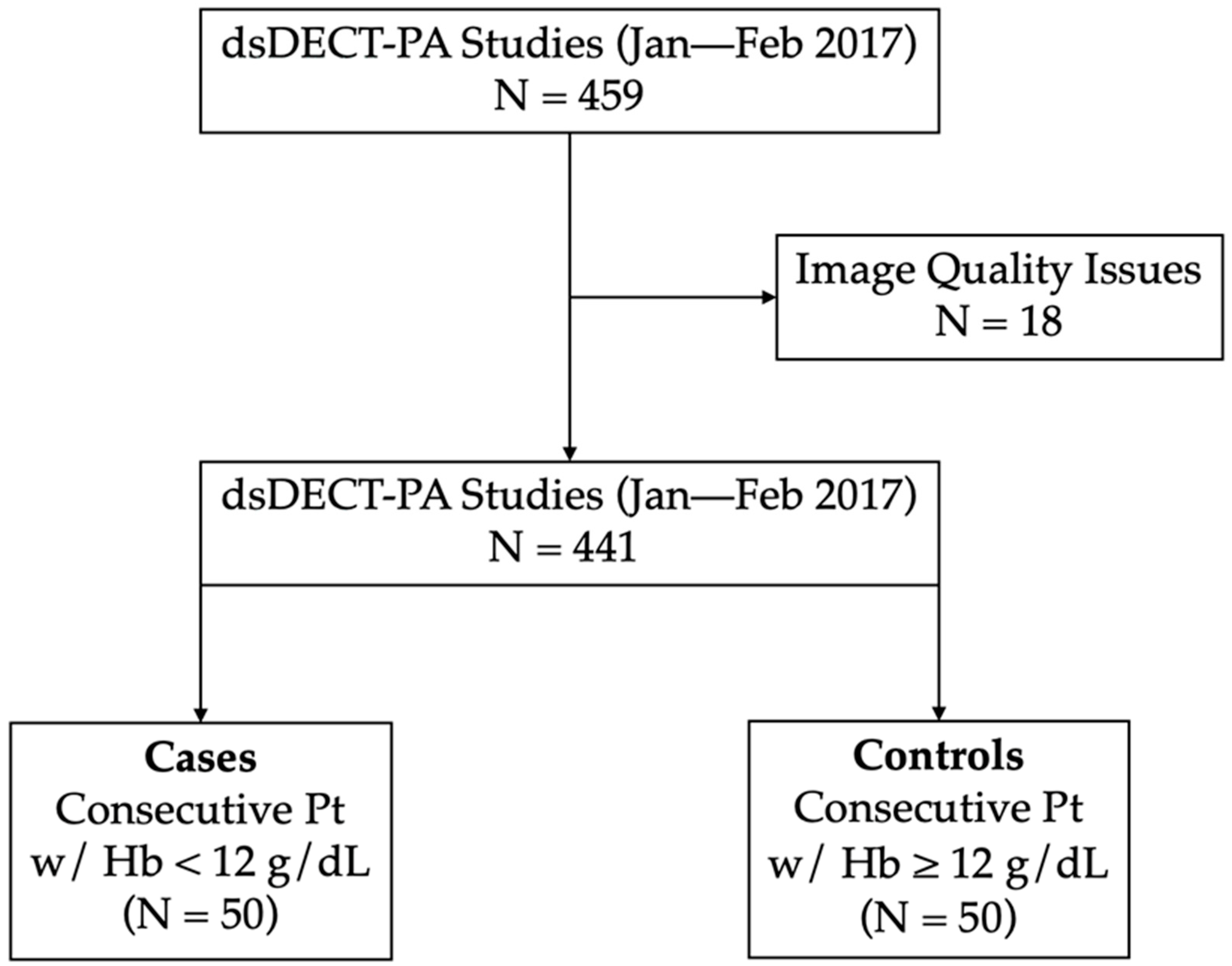




| Variables | Cases | Controls | p-Value |
|---|---|---|---|
| Age (years) | 50.3 ± 15.4 | 52.3 ± 14.3 | 0.51 |
| Male-to-female ratio | 22/28 | 20/30 | 0.84 |
| BMI (kg/m2) | 29.9 ± 9.9 | 30.1 ± 9.5 | 0.88 |
| RBC (million cells/mcL) | 3.6 ± 0.5 | 4.6 ± 0.5 | <0.001 |
| Hematocrit % | 31.3 ± 3.7 | 40.6 ± 3.2 | <0.001 |
| Hb (g/dL) | 10.2 ± 1.2 | 13.6 ± 1.3 | <0.001 |
| CTDI (mGy) | 8.1 ± 4.5 | 9.6 ± 4.8 | 0.12 |
| Contrast volume | 85.9 ± 22.1 | 84.1 ± 13.8 | 0.63 |
| Variables | Training | Test | p-Value |
|---|---|---|---|
| Age (years) | 50.1 ± 13.9 | 55.2 ± 16.9 | 0.13 |
| Male-to-female ratio | 33/43 | 9/15 | 0.64 |
| BMI (kg/m2) | 29.4 ± 9.5 | 31.8 ± 10.0 | 0.29 |
| RBC (million cells/mcL) | 4.10 ± 0.7 | 4.16 ± 0.7 | 0.72 |
| Hematocrit % | 35.9 ± 5.7 | 36.1 ± 6.3 | 0.92 |
| Hb (g/dL) | 11.9 ± 2.1 | 11.9 ± 2.2 | 0.98 |
| CTDI (mGy) | 8.5 ± 4.2 | 10.0 ± 5.8 | 0.24 |
| Contrast volume | 84.3 ± 20.1 | 87.2 ± 11.4 | 0.37 |
| ROI Location | Average Bias | 97.5th Limit | 2.5th Limit |
|---|---|---|---|
| RA | −0.64 | 36.86 | −38.13 |
| RV | −0.17 | 23.84 | −24.18 |
| LA | −0.45 | 18.50 | −19.40 |
| LV | 0.48 | 17.51 | −16.56 |
| AscAo | −2.21 | 17.56 | −21.98 |
| DescAo | −0.68 | 10.96 | −12.32 |
| PA | −0.43 | 17.39 | −18.24 |
| AoArch | 0.23 | 16.22 | −15.77 |
| Location | Component | Cases (HU) | Controls (HU) | p-Value |
|---|---|---|---|---|
| RA | VNC | 48.14 ± 12.97 | 53.01 ± 12.73 | 0.06 |
| CM | 133.49 ± 271.17 | 247.73 ± 97.44 | 0.14 | |
| RV | VNC | 46.97 ± 11.85 | 56.12 ± 12.08 | <0.001 |
| CM | 250.34 ± 105.32 | 245.54 ± 85.54 | 0.80 | |
| LA | VNC | 43.02 ± 9.84 | 51.62 ± 10.30 | <0.001 |
| CM | 180.67 ± 50.21 | 182.65 ± 60.75 | 0.86 | |
| LV | VNC | 42.75 ± 8.91 | 52.42 ± 11.33 | <0.001 |
| CM | 165.61 ± 50.54 | 163.37 ± 55.27 | 0.83 | |
| Asc Ao | VNC | 41.09 ± 11.52 | 46.89 ± 9.63 | <0.01 |
| CM | 169.61 ± 54.83 | 158.25 ± 63.74 | 0.34 | |
| Desc Ao | VNC | 39.09 ± 8.75 | 48.24 ± 8.44 | <0.001 |
| CM | 154.05 ± 64.81 | 133.32 ± 61.66 | 0.10 | |
| PA | VNC | 50.04 ± 14.13 | 57.63 ± 13.48 | <0.01 |
| CM | 239.87± 96.01 | 238.06 ± 84.39 | 0.92 | |
| Ao Arch | VNC | 40.94 ± 9.26 | 45.35 ± 7.18 | <0.01 |
| CM | 162.19 ± 63.91 | 147.36 ± 69.73 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kay, F.U.; Lumby, C.; Tanabe, Y.; Abbara, S.; Rajiah, P. Detection of Low Blood Hemoglobin Levels on Pulmonary CT Angiography: A Feasibility Study Combining Dual-Energy CT and Machine Learning. Tomography 2023, 9, 1538-1550. https://doi.org/10.3390/tomography9040123
Kay FU, Lumby C, Tanabe Y, Abbara S, Rajiah P. Detection of Low Blood Hemoglobin Levels on Pulmonary CT Angiography: A Feasibility Study Combining Dual-Energy CT and Machine Learning. Tomography. 2023; 9(4):1538-1550. https://doi.org/10.3390/tomography9040123
Chicago/Turabian StyleKay, Fernando U., Cynthia Lumby, Yuki Tanabe, Suhny Abbara, and Prabhakar Rajiah. 2023. "Detection of Low Blood Hemoglobin Levels on Pulmonary CT Angiography: A Feasibility Study Combining Dual-Energy CT and Machine Learning" Tomography 9, no. 4: 1538-1550. https://doi.org/10.3390/tomography9040123
APA StyleKay, F. U., Lumby, C., Tanabe, Y., Abbara, S., & Rajiah, P. (2023). Detection of Low Blood Hemoglobin Levels on Pulmonary CT Angiography: A Feasibility Study Combining Dual-Energy CT and Machine Learning. Tomography, 9(4), 1538-1550. https://doi.org/10.3390/tomography9040123






