Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
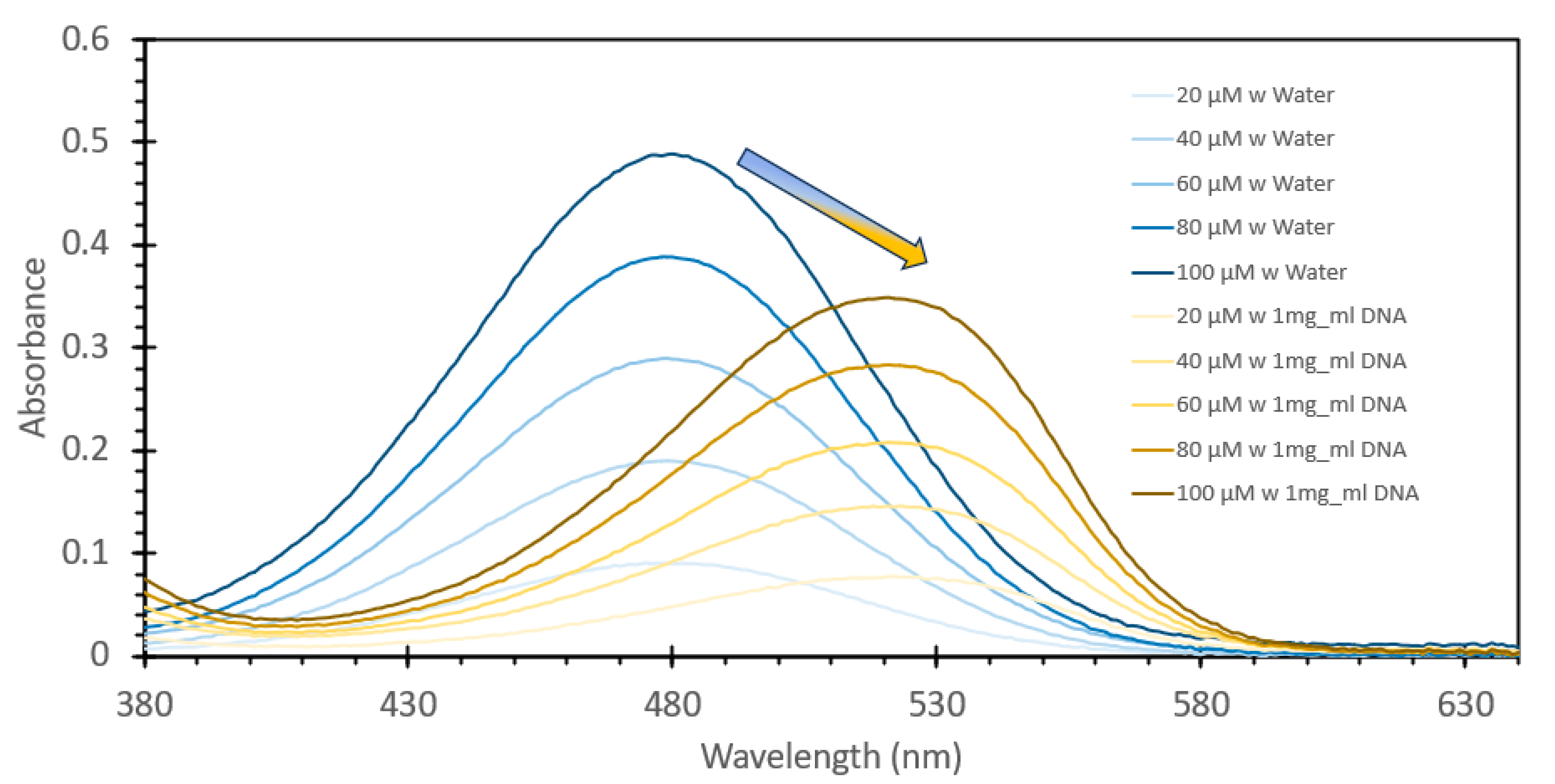
3.1. Detection of Aqueous DNA Using Ethidium Bromide
3.2. Detection of Aqueous DNA Using SYBR Green 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Geddes, C.D.; Aslan, K. Metal-Enhanced Fluorescence: Progress Towards a Unified Plasmon-Fluorophore Description; John Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Eustis, S.; El-Sayed, M. Aspect Ratio Dependence of the Enhanced Fluorescence Intensity of Gold Nanorods: Experimental and Simulation Study. J. Phys. Chem. B 2005, 109, 16350–16356. [Google Scholar] [CrossRef]
- Pelton, M.; Bryant, G.W. Introduction to Metal-Nanoparticle Plasmonics; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Li, J.; Krasavin, A.V.; Webster, L.; Segovia, P.; Zayats, A.V.; Richards, D. Spectral variation of Fluorescence Lifetime Near Single Metal Nanoparticles. Sci. Rep. 2016, 6, 21349. [Google Scholar] [CrossRef]
- Weitz, D.A.; Gersten, S.; Garoff, C.D.; Hanson, T.J.; Gramila, J.I. Fluorescent Lifetimes of Molecules on Silver-Island Films. Opt. Lett. 1982, 7, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dyer, M.J.; Weimer, W.A. Preparation and Characterization of Surface Plasmon Resonance Tunable Gold and Silver Films. J. Appl. Phys. 2002, 92, 5264–5271. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Wei, W.; Zhang, X.; Ren, X. Plasmonic Circular Resonators for Refractive Index Sensors and Filters. Nanoscale Res. Lett. 2015, 10, 211. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, D.M. Principles of Instrumental Analysis; Holt, Rinehart and Winston: New York, NY, USA, 2010. [Google Scholar]
- Minamikawa, T.; Sakaguchi, R.; Harada, Y.; Tanioka, H.; Inoue, S.; Hase, H.; Mori, Y.; Takamatsu, T.; Yamasaki, Y.; Morimoto, Y.; et al. Long-range enhancement for fluorescence and Raman spectroscopy using AG nanoislands protected with column-structured silica overlayer. Light Sci. Appl. 2024, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Kawata, S.; Inouye, Y.; Verma, P. Plamsonic for Near Field Nano Imaging and Super Lensing. Nat. Photonics 2009, 3, 388–394. [Google Scholar] [CrossRef]
- Sultangaziyev, A.; Bukasov, R. Review: Applications of surface-enhanced fluorescence (SEF) spectroscopy in bio-detection and Biosensing. Sens. Bio-Sens. Res. 2020, 30, 100382. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, J.; Geddes, C.D. Plasmonic Electricity: Fluorophore-Induced Plasmonic Current. J. Phys. Chem. C 2019, 123, 27770–27777. [Google Scholar] [CrossRef]
- Moskowitz, J.; Sindi, R.; Geddes, C.D. Plasmonic Electricity II: The Effect of Particle Size, Solvent Permittivity, Applied Voltage, and Temperature on Fluorophore-Induced Plasmonic Current. J. Phys. Chem. C 2020, 124, 5780–5788. [Google Scholar] [CrossRef]
- Pierce, D.R.; Bobbin, M.; Geddes, C.D. Fluorophore-induced plasmonic current generation from aluminum nanoparticle films. J. Phys. Chem. C 2023, 127, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.R.; Saha, L.; Geddes, C.D. Fluorophore-induced plasmonic current generation from copper nanoparticle films. ACS Omega 2024, 9, 25181–25188. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.R.; Geddes, C.D. Fluorophore-Induced Plasmonic Current based Detection of Aqueous Fe3+ ions using a Turn-On Fluorescence Probe and Copper Nanoparticle Substrates. J. Phys. Chem. C 2024, 128, 15537–15541. [Google Scholar] [CrossRef]
- Galindo-Murillo, R.; Cheatham, T.E. Ethidium bromide interactions with DNA: An exploration of a classic DNA–ligand complex with unbiased molecular dynamics simulations. Nucleic Acids Res. 2021, 49, 3735–3747. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Misra, A.; Banerjee, S.; Dam, B. Adaptation of ethidium bromide fluorescence assay to monitor activity of efflux pumps in bacterial pure cultures or mixed population from environmental samples. J. King Saud Univ.-Sci. 2020, 32, 939–945. [Google Scholar] [CrossRef]
- Bonasera, V.; Alberti, S.; Sacchetti, A. Protocol for high-sensitivity/long linear-range spectrofluorimetric DNA quantification using ethidium bromide. BioTechniques 2007, 43, 173–176. [Google Scholar] [CrossRef]
- Singer, V.L.; Haugland, R.P. Ethidium Bromide. In Fluorescent and Luminescent Probes for Biological Activity; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Batt, C.A.; Tortorello, M.L. Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Tien, W.-P.; Lim, G.; Yeo, G.; Chiang, S.N.; Chong, C.-S.; Ng, L.-C.; Hapuarachchi, H.C. SYBR green-based one step quantitative real-time polymerase chain reaction assay for the detection of zika virus in field-caught mosquitoes. Parasites Vectors 2017, 10, 427. [Google Scholar] [CrossRef]
- Rahmasari, R.; Raekiansyah, M.; Aliyah, S.H.; Yodi, P.; Baihaqy, F.; Irhamsyah, M.; Sari, K.C.; Suryadi, H.; Moi, M.L.; Sauriasari, R. Development and validation of cost-effective SYBR green-based RT-qPCR and its evaluation in a sample pooling strategy for detecting SARS-COV-2 infection in the Indonesian setting. Sci. Rep. 2024, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, F.; Toomes, C.; Bransfield, K.; Leong, F.T.; Douglas, S.H.; Field, S.L.; Bell, S.M.; Combaret, V.; Puisieux, A.; Mighell, A.J.; et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the taqman assay for a relative quantification of gene rearrangements, Gene Amplifications and micro gene deletions. BMC Biotechnol. 2003, 3, 18. [Google Scholar] [CrossRef] [PubMed]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierce, D.R.; Nichols, Z.; Cunningham, C.; Villaver, S.A.; Bajwah, A.; Oluwarotimi, S.; Halaa, H.; Geddes, C.D. Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach. Sensors 2024, 24, 7985. https://doi.org/10.3390/s24247985
Pierce DR, Nichols Z, Cunningham C, Villaver SA, Bajwah A, Oluwarotimi S, Halaa H, Geddes CD. Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach. Sensors. 2024; 24(24):7985. https://doi.org/10.3390/s24247985
Chicago/Turabian StylePierce, Daniel R., Zach Nichols, Clifton Cunningham, Sean Avryl Villaver, Abdullah Bajwah, Samuel Oluwarotimi, Herbert Halaa, and Chris D. Geddes. 2024. "Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach" Sensors 24, no. 24: 7985. https://doi.org/10.3390/s24247985
APA StylePierce, D. R., Nichols, Z., Cunningham, C., Villaver, S. A., Bajwah, A., Oluwarotimi, S., Halaa, H., & Geddes, C. D. (2024). Detection and Quantification of DNA by Fluorophore-Induced Plasmonic Current: A Novel Sensing Approach. Sensors, 24(24), 7985. https://doi.org/10.3390/s24247985







