In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation
Abstract
1. Introduction
2. Materials and Methods
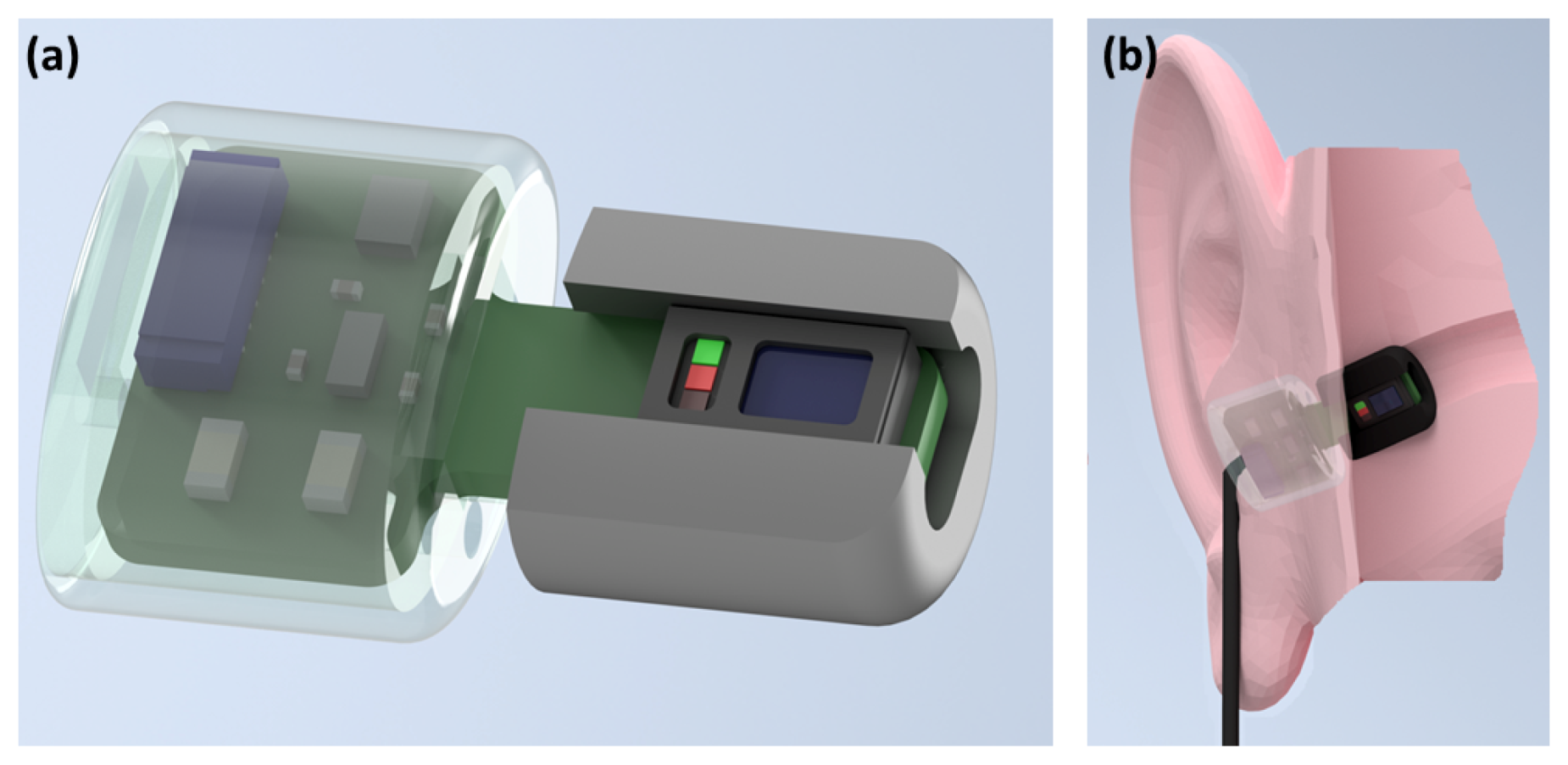
2.1. Hardware
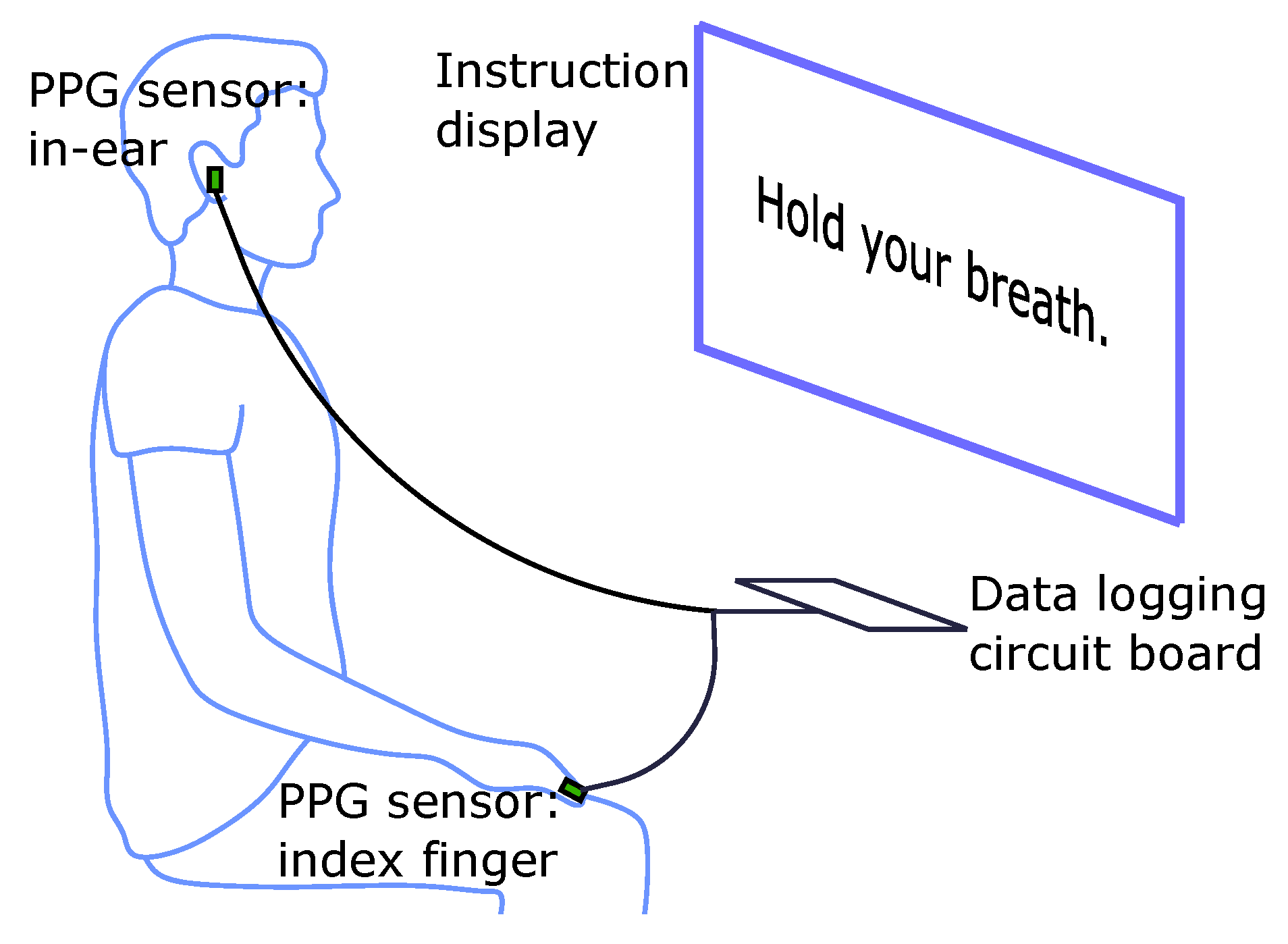
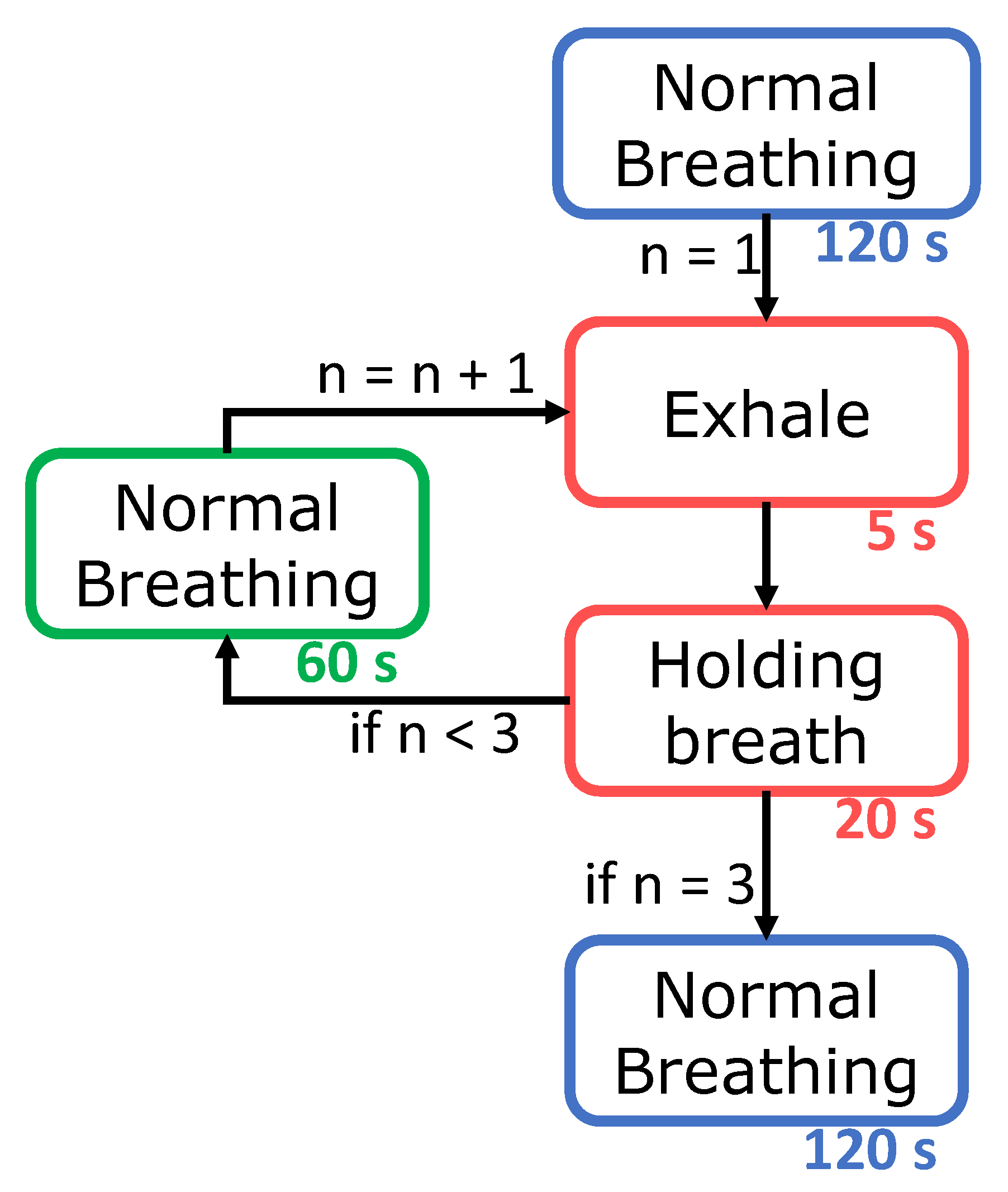
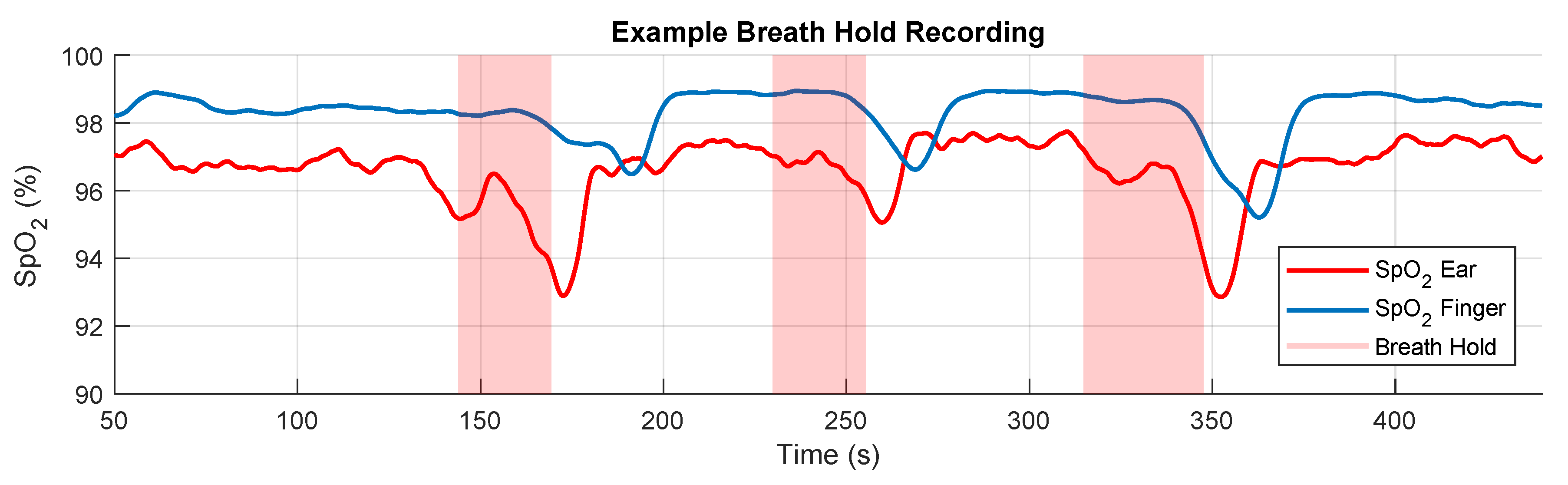
2.2. Experimental Protocol
2.3. Extraction of the SpO2 Signal
2.4. Data Analysis
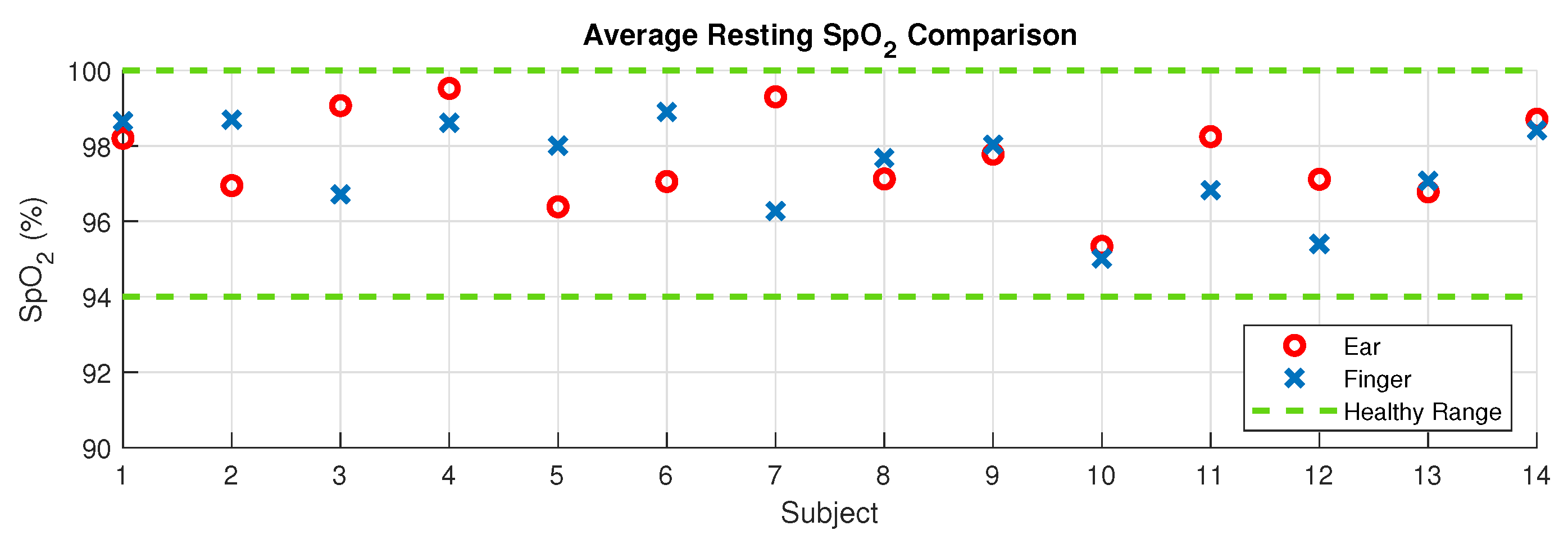
2.4.1. Resting SpO2 Comparison
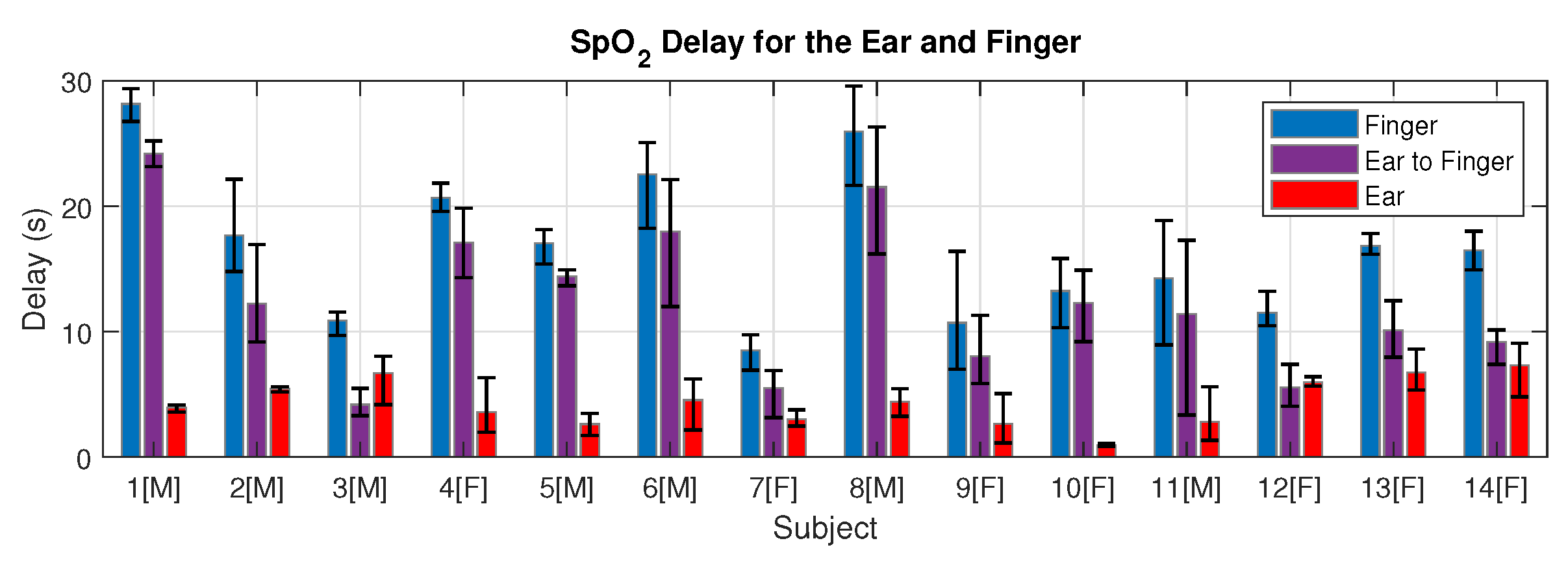
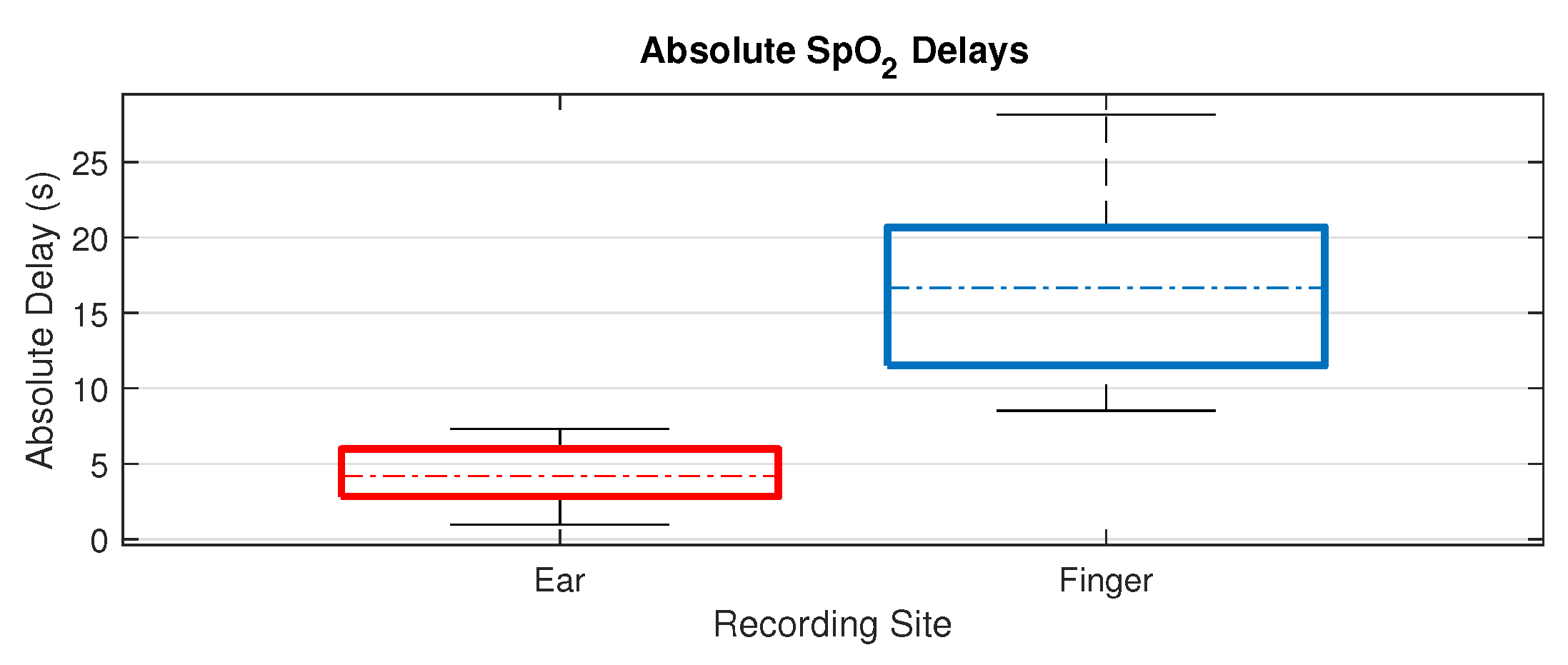
2.4.2. SpO2 Delay
3. Results
3.1. Resting Oxygen Comparison
3.2. Blood Oxygen Delay
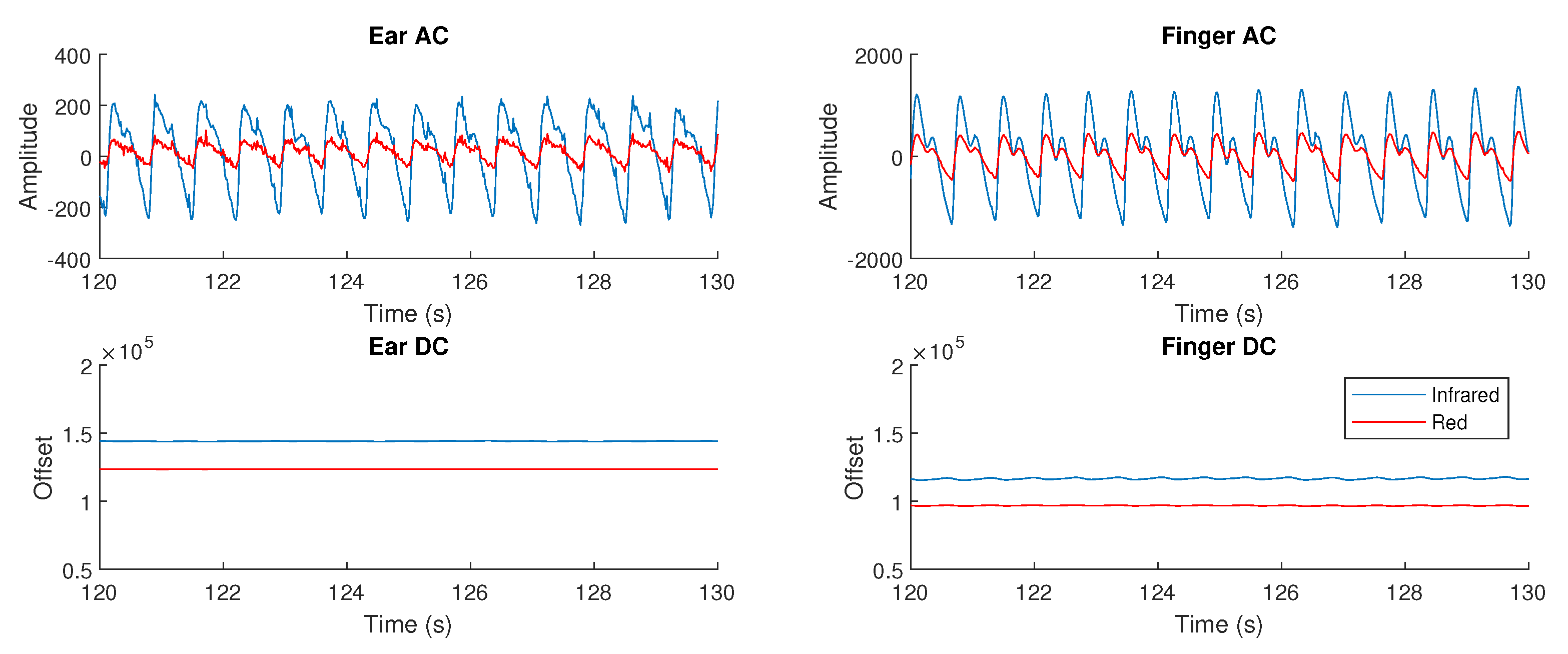
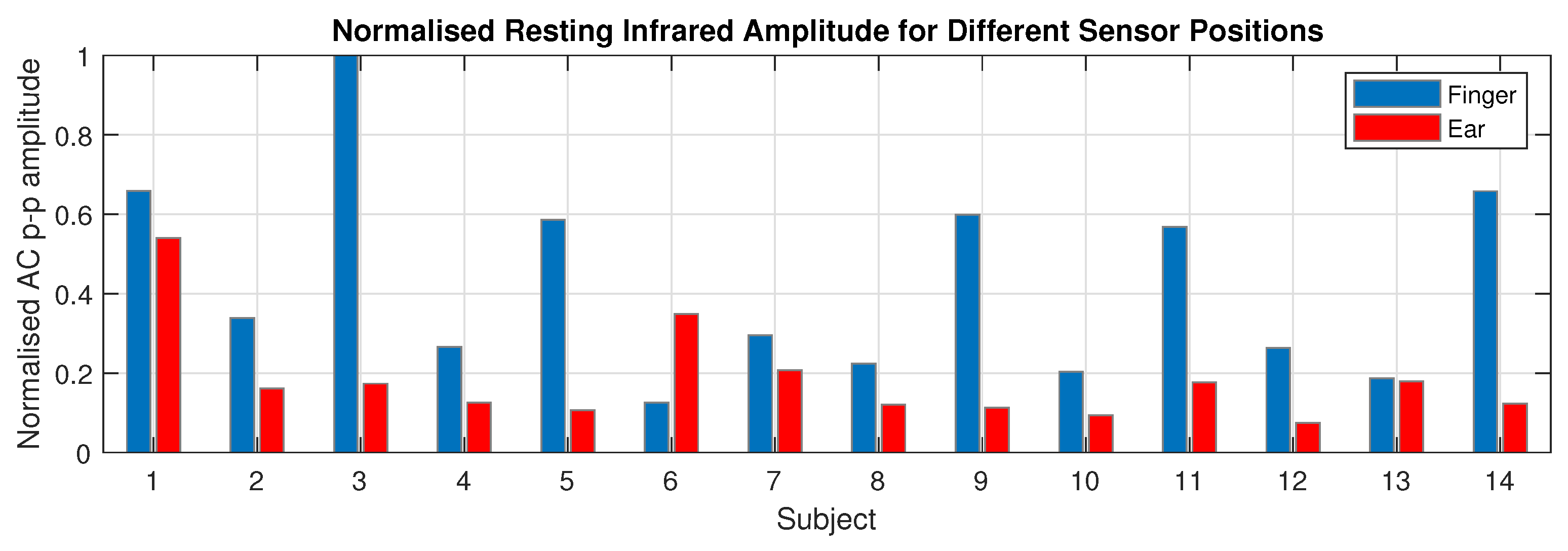
3.3. Photoplethysmogram Amplitude
4. Discussion
4.1. Resting SpO2
4.2. SpO2 Delay
4.3. Photoplethysmogram Amplitude
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jubran, A. Pulse oximetry. Crit. Care 1999, 3, R11. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.B.; Prytherch, D.R.; Watson, D.; Forde, V.; Windsor, A.; Schmidt, P.E.; Featherstone, P.I.; Higgins, B.; Meredith, P. SpO2 values in acute medical admissions breathing air-Implications for the British Thoracic Society guideline for emergency oxygen use in adult patients? Resuscitation 2012, 83, 1201–1205. [Google Scholar] [CrossRef]
- World Health Organization. Pulse Oximetry Training Manual; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 2020, 200370. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Khemani, R.G.; Thomas, N.J.; Venkatachalam, V.; Scimeme, J.P.; Berutti, T.; Schneider, J.B.; Ross, P.A.; Willson, D.F.; Hall, M.W.; Newth, C.J.L. Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Critical Care Med. 2012, 40, 1309–1316. [Google Scholar] [CrossRef]
- Oksenberg, A.; Arons, E.; Nasser, K.; Vander, T.; Radwan, H. REM-related Obstructive Sleep Apnea: The Effect of Body Position. J. Clin. Sleep Med. 2010, 6, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices (Auckl. NZ) 2014, 7, 231. [Google Scholar] [CrossRef]
- Nilsson, L.; Goscinski, T.; Kalman, S.; Lindberg, L.G.; Johansson, A. Combined photoplethysmographic monitoring of respiration rate and pulse: A comparison between different measurement sites in spontaneously breathing subjects. Acta Anaesthesiol. Scand. 2007, 51, 1250–1257. [Google Scholar] [CrossRef]
- Bradke, B.; Everman, B. Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR). Biosensors 2020, 10, 34. [Google Scholar] [CrossRef]
- Dresher, R. Wearable Forehead Pulse Oximetry: Minimization of Motion and Pressure Artifacts. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2006. [Google Scholar]
- Goverdovsky, V.; Von Rosenberg, W.; Nakamura, T.; Looney, D.; Sharp, D.J.; Papavassiliou, C.; Morrell, M.J.; Mandic, D.P. Hearables: Multimodal physiological in-ear sensing. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Looney, D.; Kidmose, P.; Park, C.; Ungstrup, M.; Rank, M.; Rosenkranz, K.; Mandic, D. The in-the-ear recording concept: User-centered and wearable brain monitoring. IEEE Pulse 2012, 3, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Alqurashi, Y.D.; Morrell, M.J.; Mandic, D.P. Hearables: Automatic Overnight Sleep Monitoring with Standardized In-Ear EEG Sensor. IEEE Trans. Biomed. Eng. 2020, 67, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Budidha, K.; Kyriacou, P.A. Investigation of photoplethysmography and arterial blood oxygen saturation from the ear-canal and the finger under conditions of artificially induced hypothermia. In Proceedings of the IEEE Annual International Conference of the Engineering in Medicine and Biology Society, EMBS, Milan, Italy, 25–29 August 2015; pp. 7954–7957. [Google Scholar]
- Budidha, K.; Kyriacou, P.A. In vivo investigation of ear canal pulse oximetry during hypothermia. J. Clin. Monit. Comput. 2018, 32, 97–107. [Google Scholar] [CrossRef]
- Hamber, E.A.; Bailey, P.L.; James, S.W.; Wells, D.T.; Lu, J.K.; Pace, N.L. Delays in the detection of hypoxemia due to site of pulse oximetry probe placement. J. Clin. Anesth. 1999, 11, 113–118. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG from viscoelastic generic earpieces: Robust and unobtrusive 24/7 monitoring. IEEE Sens. J. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Rusch, T.L.; Sankar, R.; Scharf, J.E. Signal processing methods for pulse oximetry. Comput. Biol. Med. 1996, 26, 143–159. [Google Scholar] [CrossRef]
- MaximIntegrated. Recommended Configurations and Operating Profiles for MAX30101/MAX30102 EV Kits; Technical Report; MaximIntegrated: San Jose, CA, USA, 2018. [Google Scholar]
- Basaranoglu, G.; Bakan, M.; Umutoglu, T.; Zengin, S.U.; Idin, K.; Salihoglu, Z. Comparison of SpO2 values from different fingers of the hands. SpringerPlus 2015, 4, 561. [Google Scholar] [CrossRef]
- Baquero, H.; Alviz, R.; Castillo, A.; Neira, F.; Sola, A. Avoiding hyperoxemia during neonatal resuscitation: Time to response of different SpO2 monitors. Acta Paediatr. 2011, 100, 515–518. [Google Scholar] [CrossRef]
- Morozoff, E.P.; Smyth, J.A. Evaluation of three automatic oxygen therapy control algorithms on ventilated low birth weight neonates. In Proceedings of the 31st IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Minneapolis, MI, USA, 3–6 September 2009; pp. 3079–3082. [Google Scholar]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.M.; Vaughn, B.V. AASM | Scoring Manual Version 2.4 The AASM Manual for the Scoring of Sleep and Associated Events; American Academy of Sleep Medicine: Darien, IL, USA, 2017. [Google Scholar]
- Mannheimer, P.D. The Light–Tissue Interaction of Pulse Oximetry. Anesth. Analg. 2007, 105, S10–S17. [Google Scholar] [CrossRef]
- Alvord, L.S.; Farmer, B.L. Anatomy and Orientation of the Human External Ear. J.-Am. Acad. Audiol. 1997, 8, 383–390. [Google Scholar] [PubMed]
- Kelly, R.; Hayward, C.; Avolio, A.; O’Rourke, M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989, 80, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
| Relative | Finger | Ear Canal | |
|---|---|---|---|
| Female | 9.70 ± 4.07 | 14.00 ± 4.25 | 4.33 ± 2.37 |
| Male | 15.13 ± 6.76 | 19.49 ± 6.28 | 4.36 ± 1.42 |
| Total | 12.40 ± 6.06 | 16.75 ± 5.88 | 4.35 ± 1.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors 2020, 20, 4879. https://doi.org/10.3390/s20174879
Davies HJ, Williams I, Peters NS, Mandic DP. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors. 2020; 20(17):4879. https://doi.org/10.3390/s20174879
Chicago/Turabian StyleDavies, Harry J., Ian Williams, Nicholas S. Peters, and Danilo P. Mandic. 2020. "In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation" Sensors 20, no. 17: 4879. https://doi.org/10.3390/s20174879
APA StyleDavies, H. J., Williams, I., Peters, N. S., & Mandic, D. P. (2020). In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors, 20(17), 4879. https://doi.org/10.3390/s20174879





