Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution
Abstract
:1. Introduction
2. Research Design and Methods
2.1. CGM Data to Inform Insulin Dosing
2.2. Physical Activity Data to Inform Insulin Dosing
2.3. Multi-Source Smart Insulin Dosing
2.4. Simulation Studies
- Study 1: CGM-Informed Bolus Calculator
- Study 2: Step Count-Informed Bolus Calculator
2.5. Glycemic Risk Indices: LBGI and HBGI
3. Results
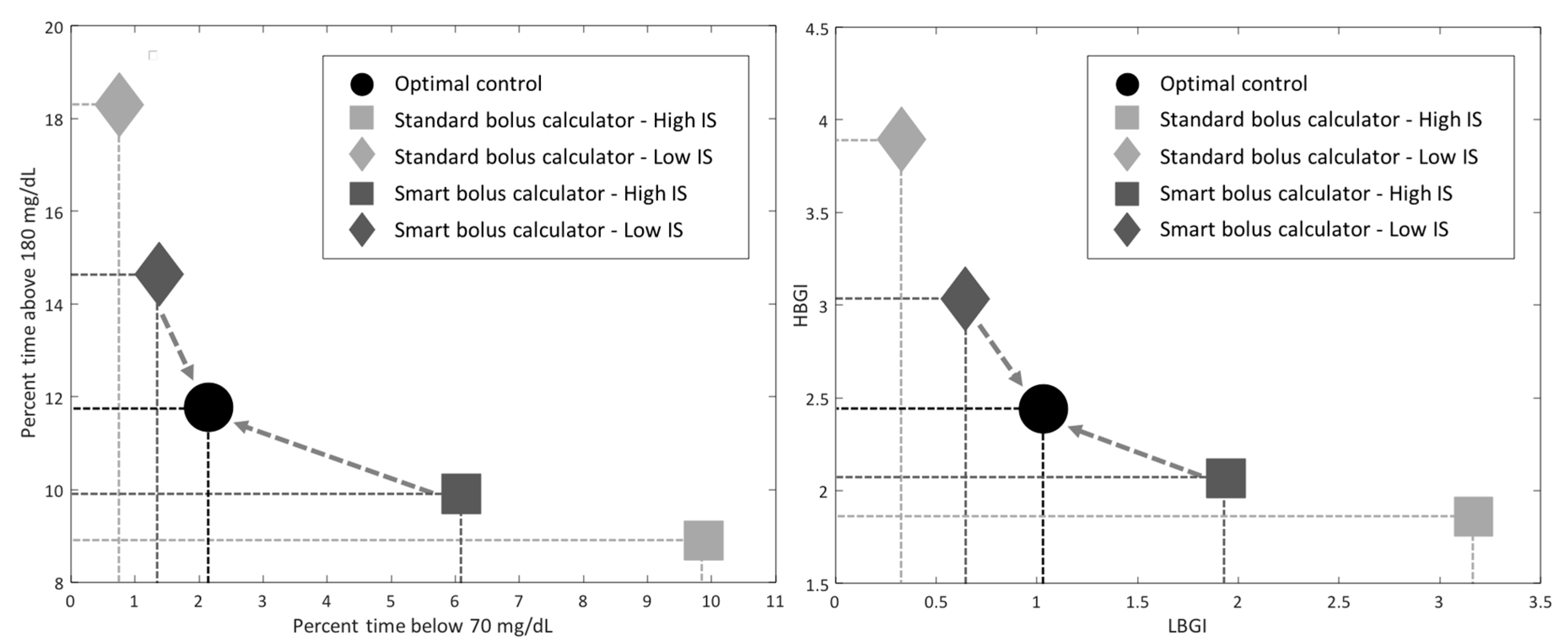
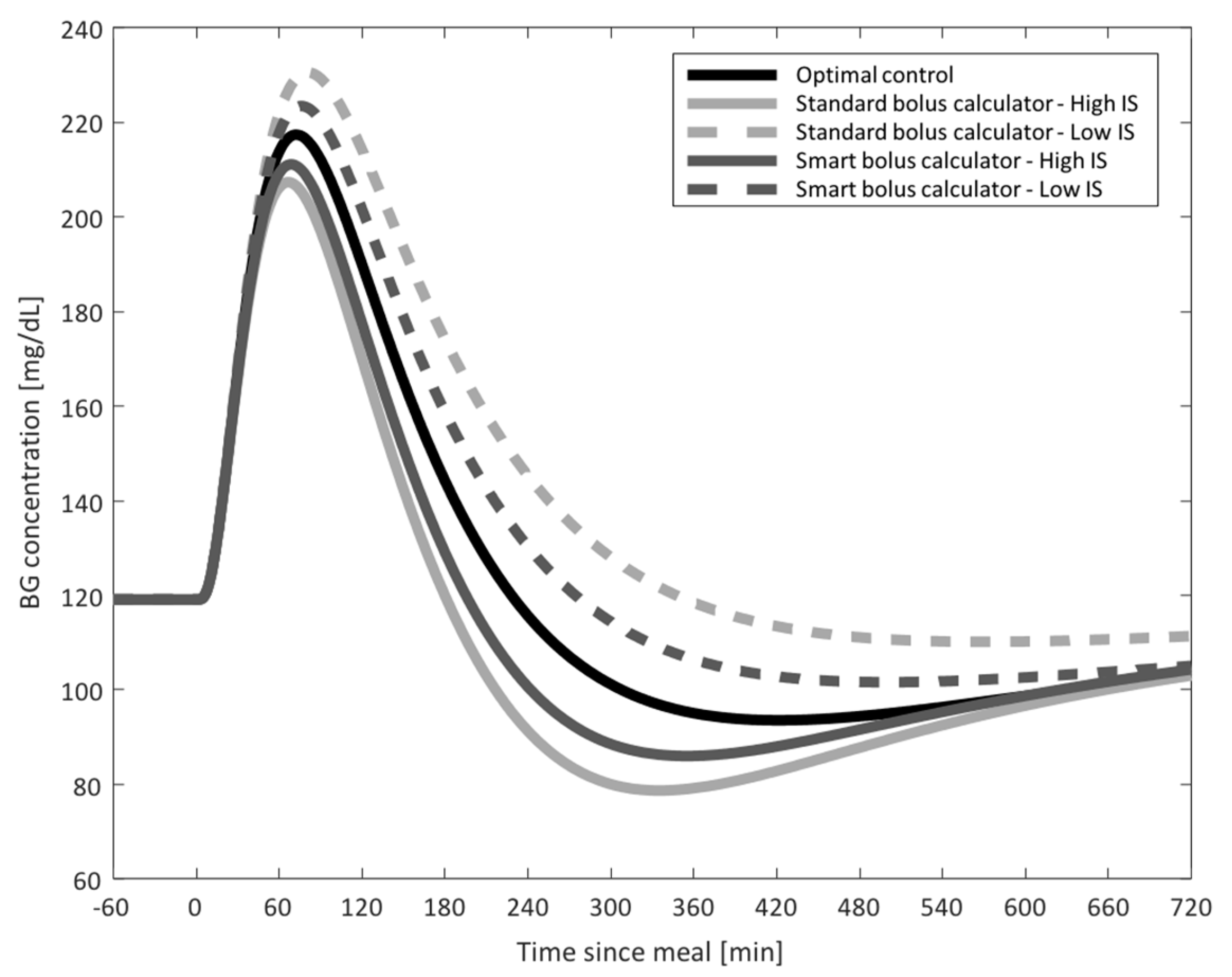
- Study 1: CGM-Informed Bolus Calculator
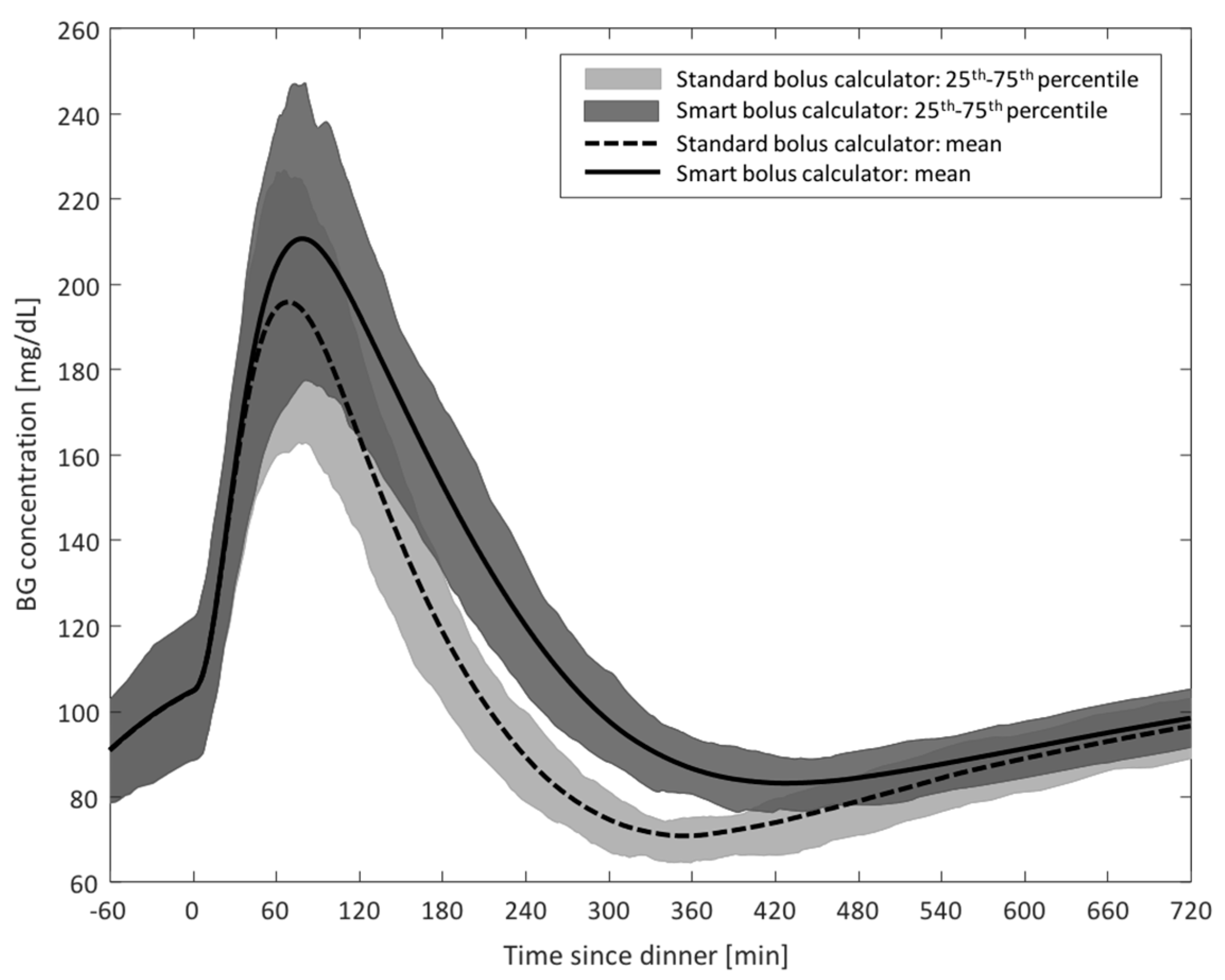
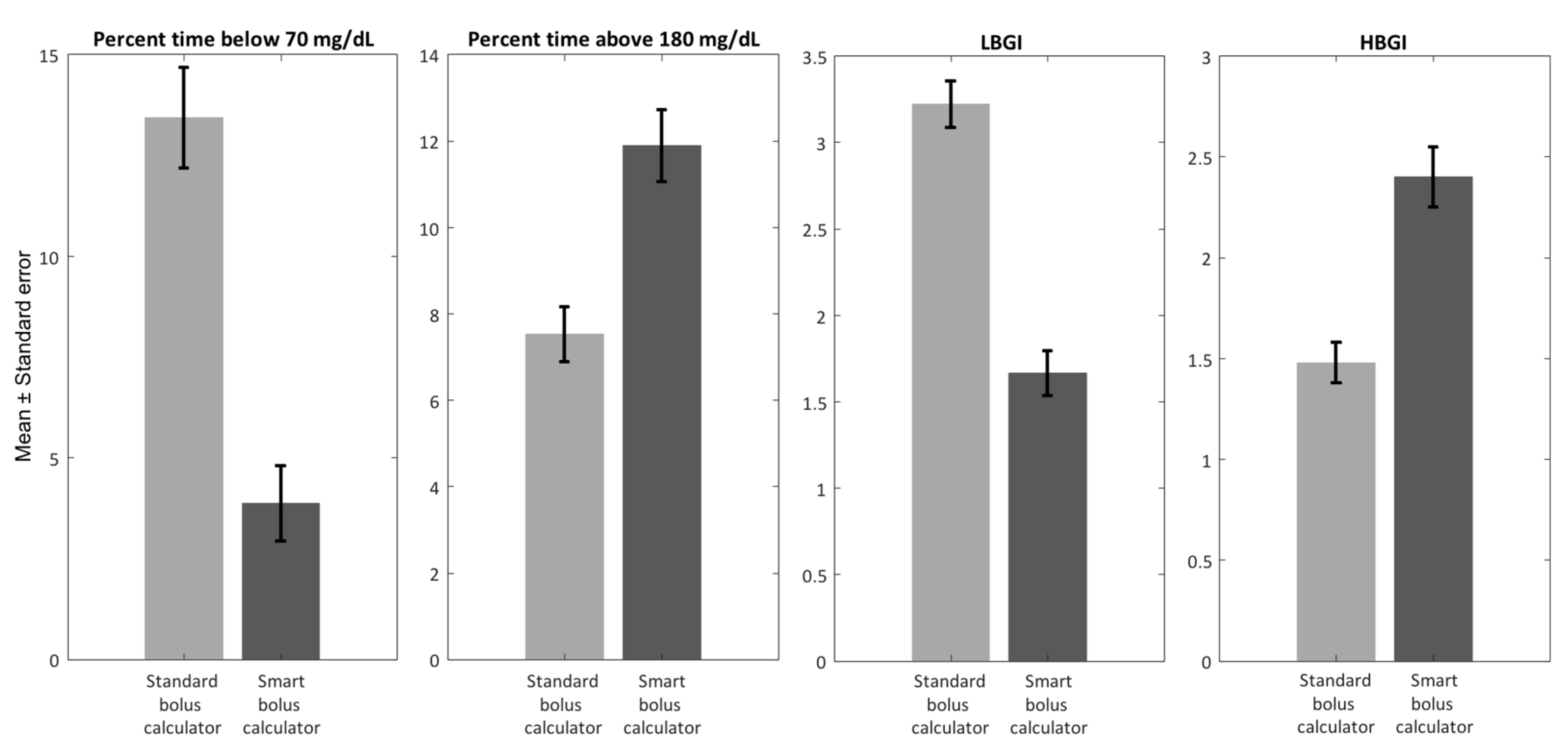
- Study 2: Step Count-Informed Bolus Calculator
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cobelli, C.; Dalla Man, C.; Sparacino, G.; Magni, L.; De Nicolao, G.; Kovatchev, B.P. Diabetes: Models, Signals, and Control. IEEE Rev. Biomed. Eng. 2009, 2, 54–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, E.A.; Weinzimer, S.A.; Steffen, A.T.; Ahern, J.A.; Vincent, M.; Tamborlane, W.V. A Randomized, Prospective Trial Comparing the Efficacy of Continuous Subcutaneous Insulin Infusion with Multiple Daily Injections Using Insulin Glargine. Diabetes Care 2004, 27, 1554–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonoff, D.C.; Prahalad, P. Performance of Cleared Blood Glucose Meters. J. Diabetes Sci. Technol. 2015, 9, 895–910. [Google Scholar] [CrossRef] [Green Version]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous Glucose Monitoring: A Review of the Technology and Clinical Use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- King, F.; Ahn, D.; Hsiao, V.; Porco, T.; Klonoff, D.C. A Review of Blood Glucose Monitor Accuracy. Diabetes Technol. Ther. 2018, 20, 843–856. [Google Scholar] [CrossRef]
- Breton, M.D.; Patek, S.D.; Lv, D.; Schertz, E.; Robic, J.; Pinnata, J.; Kollar, L.; Barnett, C.; Wakeman, C.; Oliveri, M.; et al. Continuous Glucose Monitoring and Insulin Informed Advisory System with Automated Titration and Dosing of Insulin Reduces Glucose Variability in Type 1 Diabetes Mellitus. Diabetes Technol. Ther. 2018, 20, 531–540. [Google Scholar] [CrossRef]
- Nimri, R.; Ochs, A.R.; Pinsker, J.E.; Phillip, M.; Dassau, E. Decision Support Systems and Closed Loop. Diabetes Technol. Ther. 2019, 21, S42–S56. [Google Scholar] [CrossRef]
- Lind, M.; Svensson, A.M.; Kosiborod, M.; Gudbjörnsdottir, S.; Pivodic, A.; Wedel, H.; Dahlqvist, S.; Clements, M.; Rosengren, A. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Eng. J. Med. 2014, 371, 1972–1982. [Google Scholar] [CrossRef]
- Nishimura, R.; LaPorte, R.E.; Dorman, J.S.; Tajima, N.; Becker, D.; Orchard, T.J. Mortality Trends in Type 1 Diabetes. Diabetes Care 2001, 24, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Bergman, R.N.; Ider, Y.Z.; Bowden, C.R.; Cobelli, C. Quantitative Estimation of Insulin Sensitivity. Am. J. Physiol. 1979, 236, E667–E677. [Google Scholar] [CrossRef] [PubMed]
- Dalla Man, C.; Caumo, A.; Cobelli, C. The Oral Glucose Minimal Model: Estimation of Insulin Sensitivity from a Meal Test. IEEE Trans. Biomed. Eng. 2002, 49, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Cobelli, C.; Dalla Man, C.; Toffolo, G.; Basu, R.; Vella, A.; Rizza, R. The Oral Minimal Model Method. Diabetes 2014, 63, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisser, H.; Jovanovic, L.; Doyle, F.J., 3rd; Ospina, P.; Owens, C. Run-To-Run Control of Meal-Related Insulin Dosing. Diabetes Technol. Ther. 2005, 7, 48–57. [Google Scholar] [CrossRef]
- Owens, C.; Zisser, H.; Jovanovic, L.; Srinivasan, B.; Bonvin, D.; Doyle, F.J., 3rd. Run-To-Run Control of Blood Glucose Concentrations for People with Type 1 Diabetes Mellitus. IEEE Trans. Biomed. Eng. 2006, 53, 996–1005. [Google Scholar] [CrossRef]
- Herrero, P.; Pesl, P.; Reddy, M.; Oliver, N.; Georgiou, P.; Toumazou, C. Advanced Insulin Bolus Advisor Based on Run-To-Run Control and Case-Based Reasoning. IEEE J Biomed. Health Inf. 2015, 19, 1087–1096. [Google Scholar]
- Palerm, C.C.; Zisser, H.; Bevier, W.C.; Jovanovic, L.; Doyle, F.J., 3rd. Prandial Insulin Dosing Using Run-To-Run Control: Application of Clinical Data and Medical Expertise to Define a Suitable Performance Metric. Diabetes Care 2007, 30, 1131–1136. [Google Scholar] [CrossRef] [Green Version]
- Palerm, C.C.; Zisser, H.; Jovanovic, L.; Doyle, F.J., 3rd. A Run-To-Run Control Strategy to Adjust Basal Insulin Infusion Rates in Type 1 Diabetes. J. Process. Control. 2008, 18, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Patek, S.D.; Lv, D.; Campos-Nanez, E.; Breton, M.D. Retrospective Optimization of Daily Insulin Therapy Parameters: Control Subject to a Regenerative Disturbance Process. IFAC-PapersOnLine 2016, 49, 773–778. [Google Scholar] [CrossRef]
- Toffanin, C.; Sandri, A.; Messori, M.; Cobelli, C.; Magni, L. Automatic Adaptation of Basal Therapy for Type 1 Diabetic Patients: A Run-To-Run Approach. IFAC Proc. Vol. 2014, 47, 2070–2075. [Google Scholar] [CrossRef]
- Tuo, J.; Sun, H.; Shen, D.; Wang, H.; Wang, Y. Optimization of Insulin Pump Therapy Based on High Order Run-To-Run Control Scheme. Comput. Methods Programs Biomed. 2015, 120, 123–134. [Google Scholar] [CrossRef]
- Scott, A.R.; Macdonald, I.A.; Bowman, C.A.; Jeffcoate, W.J. Effect of Phase of Menstrual Cycle on Insulin Sensitivity, Peripheral Blood Flow and Cardiovascular Responses to Hyperinsulinaemia in Young Women with Type 1 Diabetes. Diabet. Med. 1990, 7, 57–62. [Google Scholar]
- Moberg, E.; Kollind, M.; Lins, P.E.; Adamson, U. Day-To-Day Variation of Insulin Sensitivity in Patients with Type 1 Diabetes: Role of Gender and Menstrual Cycle. Diabet. Med. 1995, 12, 224–228. [Google Scholar] [CrossRef]
- Trout, K.K.; Rickels, M.R.; Schutta, M.H.; Petrova, M.; Freeman, E.W.; Tkacs, N.C.; Teff, K.L. Menstrual Cycle Effects on Insulin Sensitivity in Women with Type 1 Diabetes: A Pilot Study. Diabetes Technol. Ther. 2007, 9, 176–182. [Google Scholar] [CrossRef]
- Brown, S.A.; Jiang, B.; McElwee-Malloy, M.; Wakeman, C.; Breton, M.D. Fluctuations of Hyperglycemia and Insulin Sensitivity Are Linked to Menstrual Cycle Phases in Women With T1D. J. Diabetes Sci. Technol. 2015, 9, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, X.; Zhou, W.; Messina, J.L. Acute Psychological Stress Results in the Rapid Development of Insulin Resistance. J. Endocrinol. 2013, 217, 175–184. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S.K.; Ferreira, L.D.; Ratnam, N.; Davey, R.J.; Youngs, L.M.; Davis, E.A.; Fournier, P.A.; Jones, T.W. Glucose Requirements to Maintain Euglycemia after Moderate-Intensity Afternoon Exercise in Adolescents with Type 1 Diabetes Are Increased in a Biphasic Manner. J. Clin. Endocrinol. Metab. 2007, 92, 963–968. [Google Scholar] [CrossRef]
- Maran, A.; Pavan, P.; Bonsembiante, B.; Brugin, E.; Ermolao, A.; Avogaro, A.; Zaccaria, M. Continuous Glucose Monitoring Reveals Delayed Nocturnal Hypoglycemia After Intermittent High-Intensity Exercise in Nontrained Patients with Type 1 Diabetes. Diabetes Technol. Ther. 2010, 12, 763–768. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Jones, T.W.; Fournier, P.A. Intermittent High-Intensity Exercise Does Not Increase the Risk of Early Postexercise Hypoglycemia in Individuals with Type 1 Diabetes. Diabetes Care 2005, 28, 416–418. [Google Scholar] [CrossRef] [Green Version]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Effects of Performing Resistance Exercise Before Versus After Aerobic Exercise on Glycemia in Type 1 Diabetes. Diabetes Care 2012, 35, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise Management in Type 1 Diabetes: A Consensus Statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Dalla Man, C.; Breton, M.D.; Cobelli, C. Physical Activity into the Meal Glucose-Insulin Model of Type 1 Diabetes: In Silico Studies. J. Diabetes Sci. Technol. 2009, 3, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Catorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.P.; Locke, C.D. Breaking Up Prolonged Sitting with Light-Intensity Walking Improves Postprandial Glycemia, But Breaking Up Sitting with Standing Does Not. J. Sci. Med. Sport. 2015, 18, 294–298. [Google Scholar] [CrossRef]
- Peddie, M.C.; Bone, J.L.; Rehrer, N.J.; Skeaff, C.M.; Gray, A.R.; Perry, T.L. Breaking Prolonged Sitting Reduces Postprandial Glycemia in Healthy, Normal-Weight Adults: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2013, 98, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Manohar, C.; Levine, J.A.; Nandy, D.K.; Saad, A.; Dalla Man, C.; McCrady-Spitzer, S.K.; Basu, R.; Cobelli, C.; Carter, R.E.; Basu, A.; et al. The Effect of Walking on Postprandial Glycemic Excursion in Patients with Type 1 Diabetes and Healthy People. Diabetes Care 2012, 35, 2493–2499. [Google Scholar] [CrossRef] [Green Version]
- Visentin, R.; Campos-Nanez, E.; Schiavon, M.; Lv, D.; Vettoretti, M.; Breton, M.; Kovatchev, B.P.; Dalla Man, C.; Cobelli, C. The UVA/Padova Type 1 Diabetes Simulator Goes from Single Meal to Single Day. J. Diabetes Sci. Technol. 2018, 12, 273–281. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Cox, D.J.; Gonder-Frederick, L.; Young-Hyman, D.; Schlundt, D.; Clarke, W. Assessment of Risk for Severe Hypoglycemia Among Adults with IDDM. Validation of the Low Blood Glucose Index. Diabetes Care 1998, 21, 1870–1875. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Straume, M.; Cox, D.J.; Farhy, L.S. Risk Analysis of Blood Glucose Data: A Quantitative Approach to Optimizing the Control of Insulin Dependent Diabetes. J. Theor. Med. 2000, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fabris, C.; Patek, S.D.; Breton, M.D. Are Risk Indices Derived from CGM Interchangeable with SMBG-Based Indices? J. Diabetes Sci. Technol. 2015, 10, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Padulo, J.; Chamari, K.; Ardigò, L.P. Walking and Running on Treadmill: The Standard Criteria for Kinematics Studies. Muscles Ligaments Tendons J. 2014, 4, 159–162. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, C.; Ozaslan, B.; Breton, M.D. Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution. Sensors 2019, 19, 5386. https://doi.org/10.3390/s19245386
Fabris C, Ozaslan B, Breton MD. Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution. Sensors. 2019; 19(24):5386. https://doi.org/10.3390/s19245386
Chicago/Turabian StyleFabris, Chiara, Basak Ozaslan, and Marc D. Breton. 2019. "Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution" Sensors 19, no. 24: 5386. https://doi.org/10.3390/s19245386




