Chitosan-Folic Acid-Coated Quercetin-Loaded PLGA Nanoparticles for Hepatic Carcinoma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
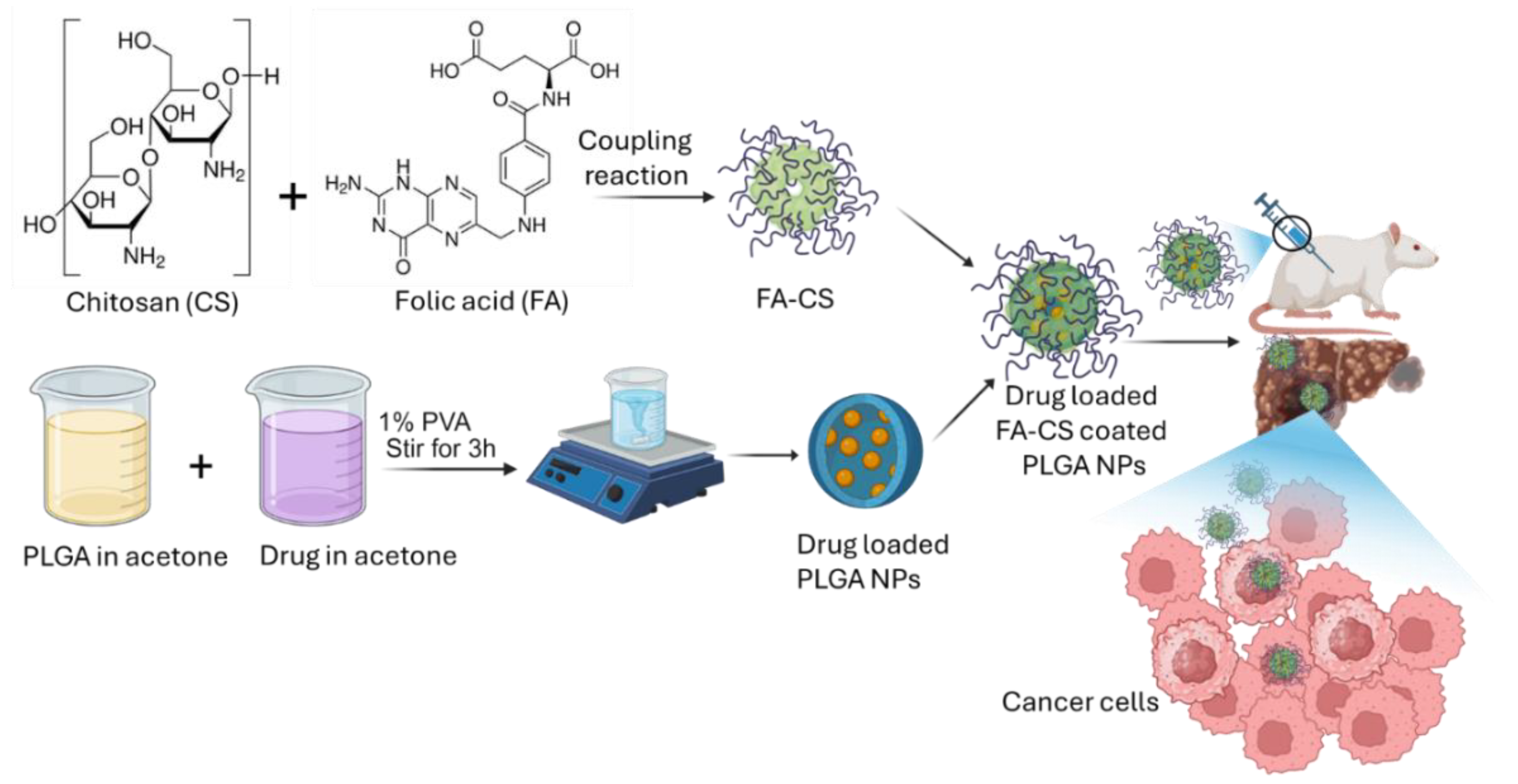
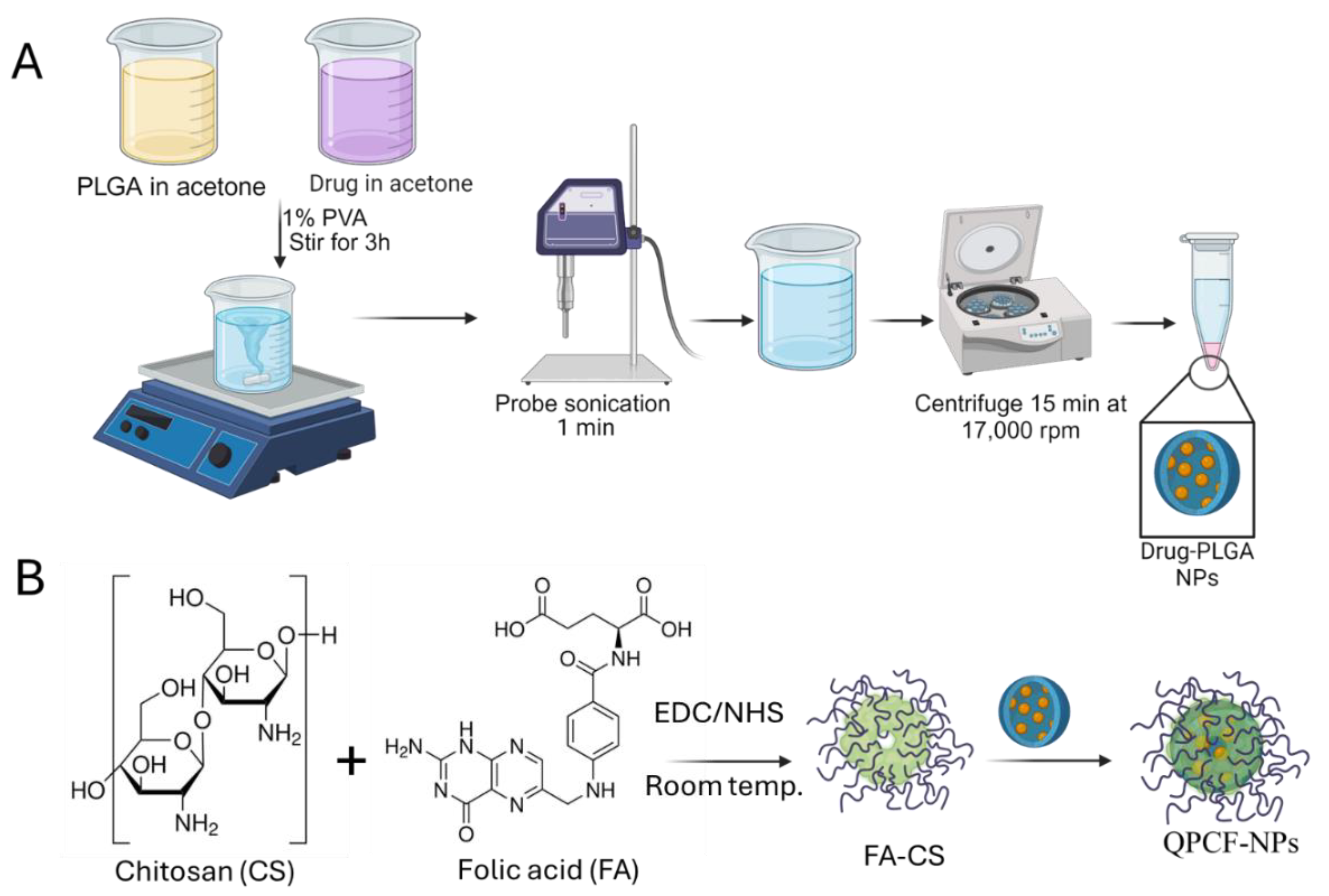
2.2. Preparation of Quercetin-Loaded Drug-Loaded PLGA NPs and Coating with Chitosan-FA
2.3. Characterization of Prepared QPCF-NPs NPs
2.3.1. Particle Size and Zeta Potential
2.3.2. FTIR and XRD Analysis of QPCF-NPs
2.3.3. SEM and HR-TEM Analysis of QPCF-NPs
2.3.4. HPLC Analysis
2.3.5. Drug Loading and Entrapment Efficiency of QPCF-NPs
2.3.6. Drug Release Profile of QPCF-NPs
2.4. In Vitro Cytotoxicity Assay of QPCF-NPs Using MTT Assay
2.5. Animal Study
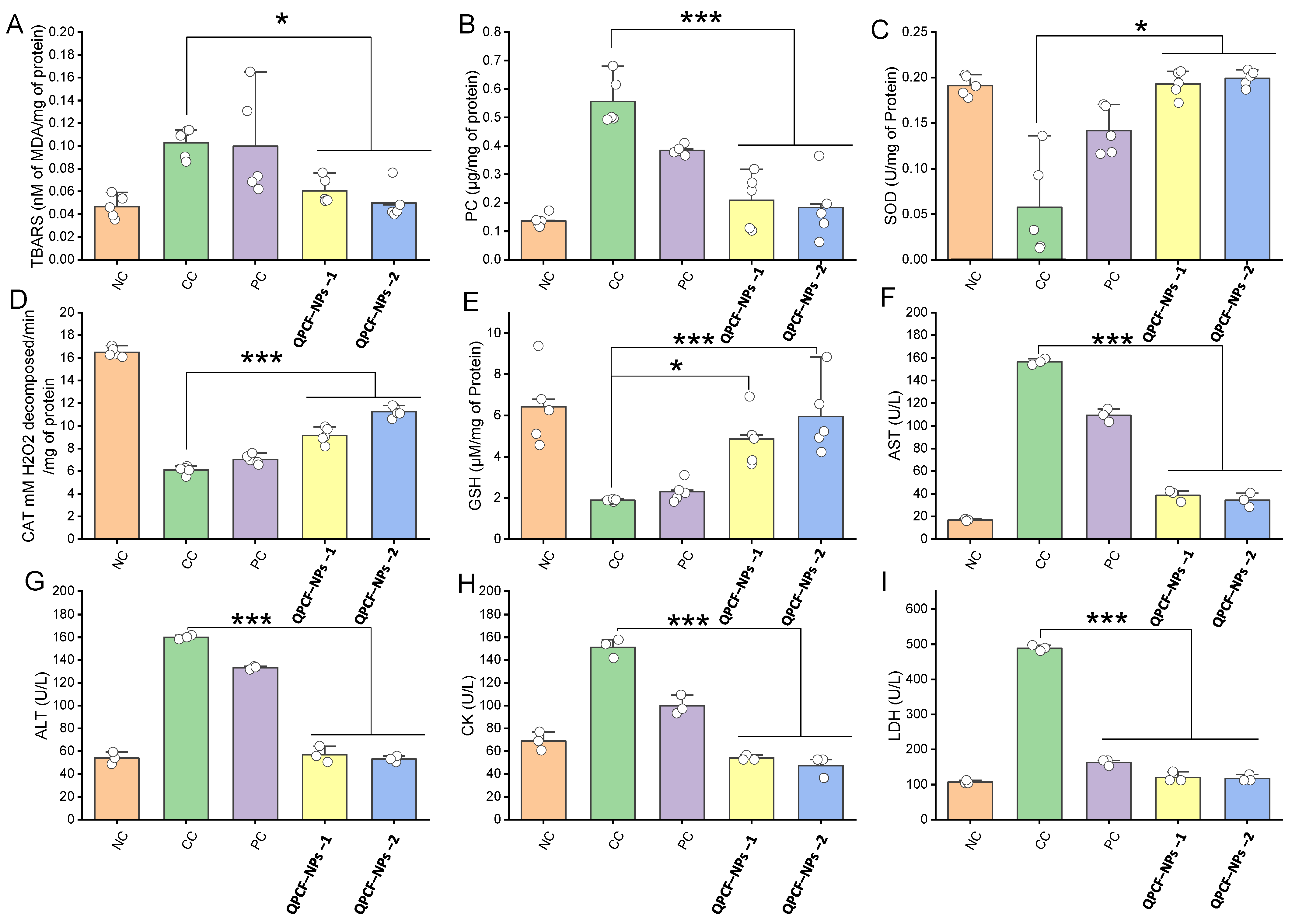
2.6. Biochemical Analysis
2.7. Oxidative Stress Parameters in Hepatic Tissue
2.7.1. Tissue GSH
2.7.2. Tissue PC
2.7.3. Tissue SOD
2.7.4. Tissue CAT
2.7.5. Tissue MDA/TBARS
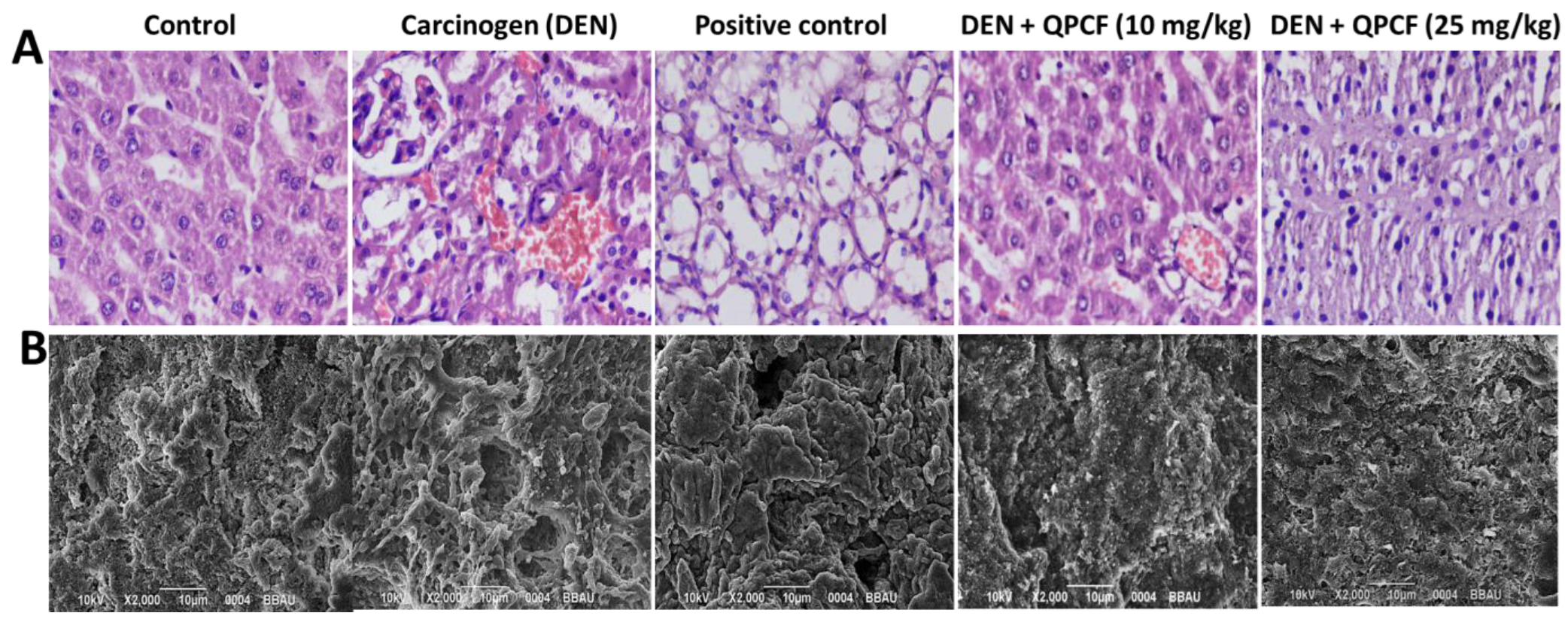
2.8. Histopathological Examination of Hepatic Cancer Tissue After Treatment with QPCF-NPs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of QPCF-NPs
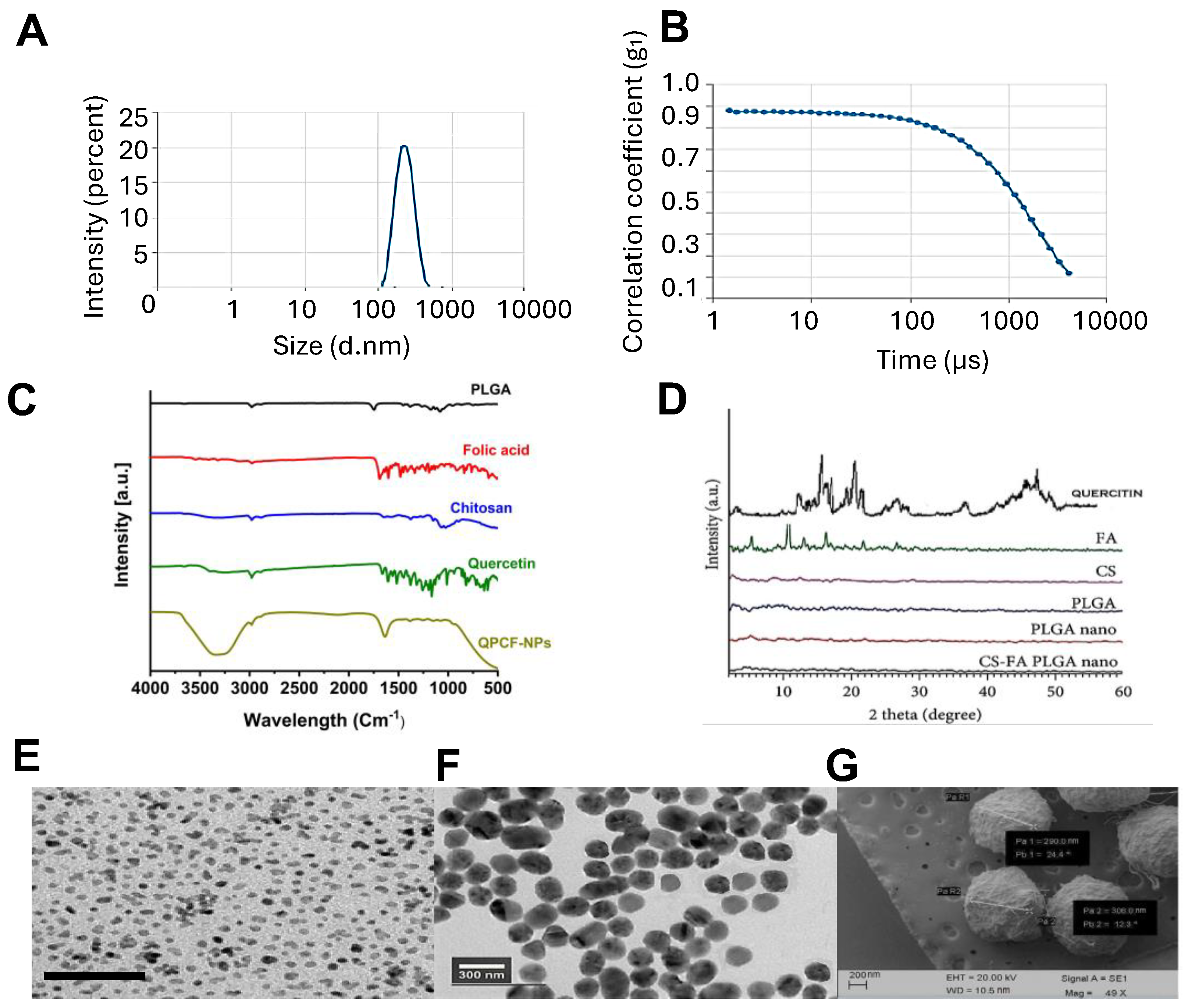
3.1.1. Particle Size and Zeta Potential of QPCF-NPs
3.1.2. Drug Loading Efficiency
3.1.3. FTIR and XRD
3.1.4. SEM and TEM of QPCF-NPs
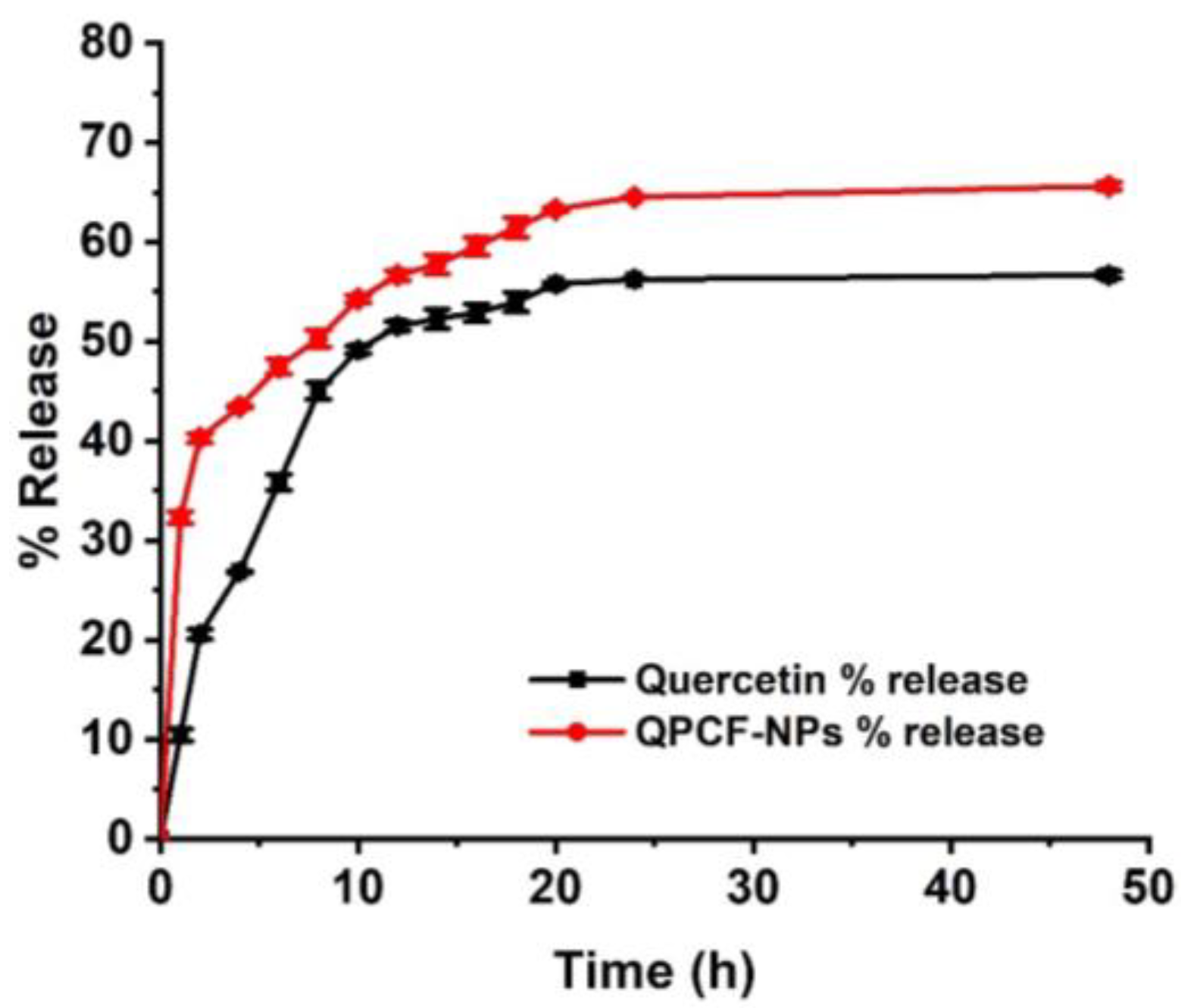
3.2. Drug Release
3.3. Cytotoxicity and Cell Apoptosis Assay
Cell Viability Assay
3.4. Determination of Antioxidative Effects of QPCF-NPs
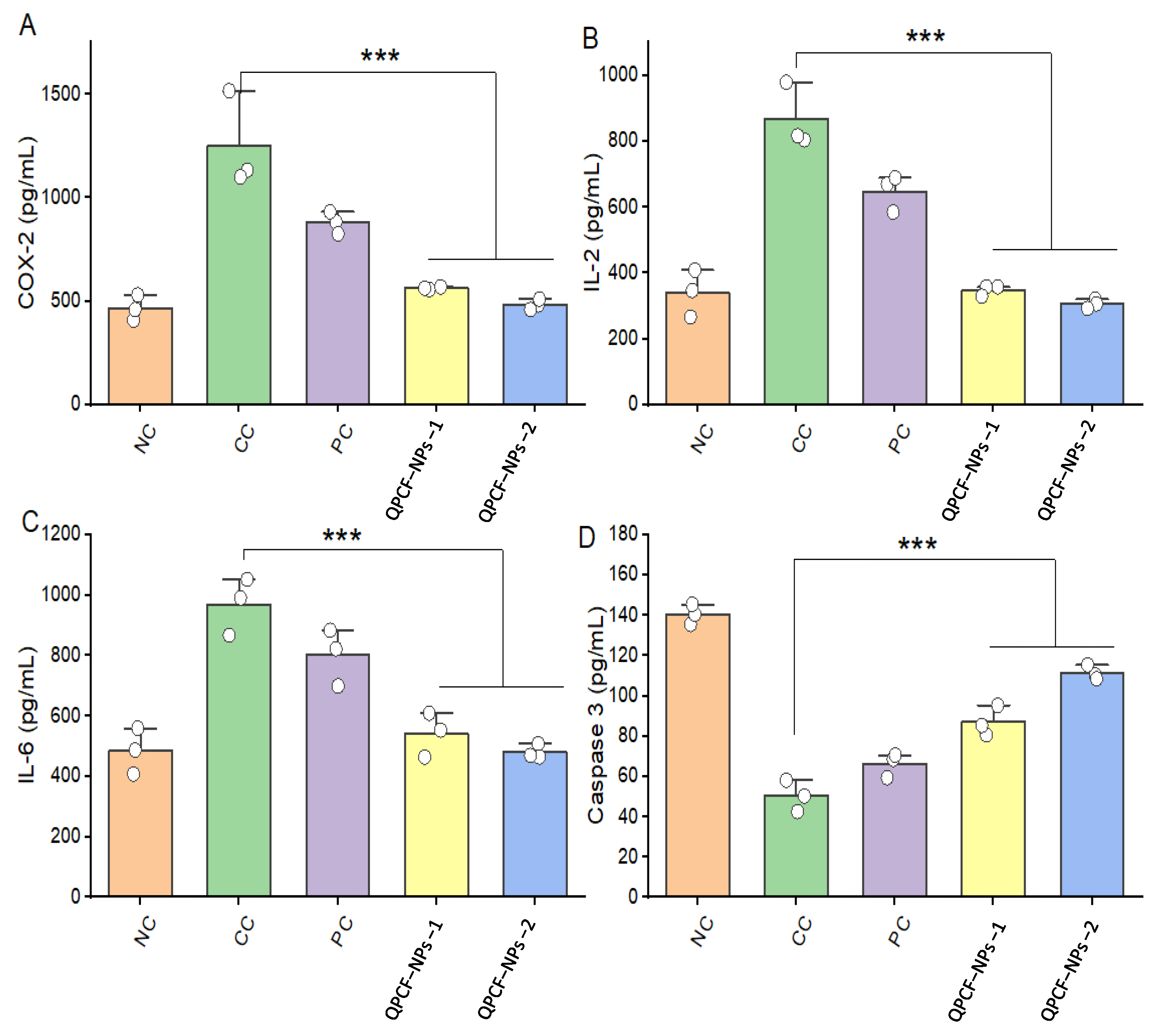
3.5. ELISA of COX-2, IL-2, IL-6, and Caspase-3
3.6. Histopathology and SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| FA | Folic acid |
| PLGA | Polylactic-co-glycolic acid |
| RP | Reversed-phase |
| EE | Entrapment efficiency |
| QPCF-NPs | Quercetin PLGA-coated folic acid nanoparticles |
| FTIR | Fourier-Transform infrared |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| MDM2 | Mouse double minute-2 homolog |
| PARP | Poly (adenosine diphosphate-ribose) polymerase |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| ERK | Extracellular signal-regulated kinase |
| CDK-2 | Cyclin-dependent kinase 2 |
| PLK-1 | Polo-like kinase-1 |
| TCA | Trichloroacetic acid |
| DEN | Diethyl nitrosamine |
| AST | Aspartate Amino Transferase |
| ALT | Alanine Amino Transferase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| PC | Protein carbonyl |
References
- Abdelhamed, W.; El-Kassas, M. Hepatocellular carcinoma recurrence: Predictors and management. Liver Res. 2023, 7, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.; MacLean, D.R.; Riley, L.M.; d’Espaignet, E.T.; Mathers, C.D.; Stevens, G.A.; Bettcher, D. Monitoring and surveillance of chronic non-communicable diseases: Progress and capacity in high-burden countries. Lancet 2011, 376, 1861–1868. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Ann. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Asghar, U.; Meyer, T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J. Hepatol. 2012, 56, 686–695. [Google Scholar] [CrossRef]
- He, S.; Dong, G.; Wu, S.; Fang, K.; Miao, Z.; Wang, W.; Sheng, C. Small Molecules Simultaneously Inhibiting p53-Murine Double Minute 2 (MDM2) Interaction and Histone Deacetylases (HDACs): Discovery of Novel Multitargeting Antitumor Agents. J. Med. Chem. 2018, 61, 7245–7260. [Google Scholar] [CrossRef]
- Cui, J.; Gong, Z.; Shen, H.M. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1836, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Panel, S.S.; Aishwarya, R.; Vivek, D.; Kakarla, R.R.; Raghuraj, S.C.; Veera, S. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar]
- Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Kappl, R.; Hüttermann, J.; Hyatt, J.A.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.-M. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: Influence on ATPase activity and membrane fluidity. Mol. Pharm. 2007, 4, 465–474. [Google Scholar] [CrossRef]
- Edo, G.I.; Yousif, E.; Al-Mashhadani, M.H. Chitosan: An overview of biological activities, derivatives, properties, and current advancements in biomedical applications. Carbohydr. Res. 2024, 542, 109199. [Google Scholar] [CrossRef]
- Sahdev, A.K.; Raorane, C.J.; Shastri, D.; Raj, V.; Singh, A.; Kim, S.C. Update on modified chitosan frameworks and their applications for food, wastewater, toxic heavy metals, dyes treatment and cancer drug delivery. J. Environ. Chem. Eng. 2022, 10, 108656. [Google Scholar] [CrossRef]
- Tatode, A.; Agrawal, P.R.; Taksande, J.; Qutub, M.; Premchandani, T.; Umekar, M.; Danao, K. Role of folate receptor and CD44 in targeting of docetaxel and paclitaxel fabricated conjugates for efficient cancer therapy. J. Med. Surg. Public Health 2025, 5, 100163. [Google Scholar] [CrossRef]
- Rosch, J.G.; DuRoss, A.N.; Landry, M.R.; Sun, C. Development of a pemetrexed/folic acid nanoformulation: Synthesis, characterization, and efficacy in a murine colorectal cancer model. ACS Omega 2020, 5, 15424–15432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ma, H.; Shao, M.; Fan, Q.; Lv, H.; Peng, J.; Hao, T.; Li, D.; Zhao, C.; Zong, X. Synthesis of folate chitosan nanoparticles loaded with ligustrazine to target folate receptor positive cancer cells. Mol. Med. Rep. 2017, 16, 1101–1108. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, S.K. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system. J. Microbiol. Biotechnol. 2002, 12, 503–507. [Google Scholar]
- Yao, Q.; Nooeaid, P.; Roether, J.A.; Dong, Y.; Zhang, Q.; Boccaccini, A.R. Bioglass®-based scaffolds incorporating polycaprolactone and chitosan coatings for controlled vancomycin delivery. Ceram. Int. 2013, 39, 7517–7522. [Google Scholar] [CrossRef]
- Deepika Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Kaşıkcı, M.B.; Bağdatlıoğlu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. J. 2016, 4 (Suppl. S2), 146–151. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Rath, P.; Chauhan, A.; Ranjan, A.; Choudhary, R.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Rani, I.; Tuli, H.S. Apoptotic mechanisms of quercetin in liver cancer: Recent trends and advancements. Pharmaceutics 2023, 15, 712. [Google Scholar] [CrossRef] [PubMed]
- Nava-Arzaluz, M.G.; Piñón-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223. [Google Scholar]
- Chan, P.; Kurisawa, M.; Chung, J.E.; Yang, Y.Y. Synthesis and characterization of chitosan-g-poly (ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials 2007, 28, 540–549. [Google Scholar]
- Srigopalram, S.; Ilavenil, S.; Jayraaj, I.A. Apoptosis associated inhibition of DEN-induced hepatocellular carcinogenesis by ellagic acid in experimental rats. Biomed. Prev. Nutr. 2012, 2, 1–8. [Google Scholar]
- Fausto, N.; Campbell, J.S. Mouse models of hepatocellular carcinoma. In Seminars in Liver Disease; © Thieme Medical Publishers: New York, NY, USA, 2010; Volume 30, pp. 87–98. [Google Scholar]
- Parashar, P.; Rana, P.; Dwivedi, M.; Saraf, S.A. Dextrose modified bilosomes for peroral delivery: Improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome Res. 2019, 29, 251–263. [Google Scholar]
- Abdelmoneem, M.A.; Elnaggar, M.A.; Hammady, R.S.; Kamel, S.M.; Helmy, M.W.; Abdulkader, M.A.; Zaky, A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-targeted lactoferrin shell-oily core nanocapsules for synergistic targeted/herbal therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 26731–26744. [Google Scholar]
- Park, D.H.; Shin, J.W.; Park, S.K.; Seo, J.N.; Li, L.; Jang, J.J.; Lee, M.J. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol. Lett. 2009, 191, 321–326. [Google Scholar]
- Kushwaha, P.S.; Raj, V.; Singh, A.K.; Keshari, A.K.; Saraf, S.A.; Mandal, S.C.; Yadav, R.K.; Saha, S. Antidiabetic effects of isolated sterols from Ficus racemosa leaves. RSC Adv. 2015, 5, 35230–35237. [Google Scholar] [CrossRef]
- Saha, S.; New, L.S.; Ho, H.K.; Chui, W.K.; Chan, E.C. Direct toxicity effects of sulfo-conjugated troglitazone on human hepatocytes. Toxicol. Lett. 2010, 195, 135–141. [Google Scholar] [PubMed]
- Saha, S.; Chan, D.S.; Lee, C.Y.; Wong, W.; New, L.S.; Chui, W.K.; Yap, C.W.; Chan, E.C.; Ho, H.K. Pyrrolidinediones reduce the toxicity of thiazolidinediones and modify their anti-diabetic and anti-cancer properties. Eur. J. Pharmacol. 2012, 697, 13–23. [Google Scholar] [PubMed]
- Khinchi, P.; Saha, S.; Saraf, S.A.; Kaithwas, G. Combination therapy of gamma-aminobutyric acid derivative promotes proton pump inhibitor-based healing of reflux esophagitis in animal model. Pharmacol. Rep. 2014, 66, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, R.L.; Maity, S.; Kumar, P.; Saraf, S.A.; Kaithwas, G.; Saha, S. Evaluation of mechanism of hepatotoxicity of leflunomide using albino wistar rats. Afr. J. Pharm. Pharmacol. 2013, 7, 1625–1631. [Google Scholar]
- Wang, F.; Wang, Y.; Ma, Q.; Cao, Y.; Yu, B. Development and characterization of folic acid-conjugated chitosan nanoparticles for targeted and controlled delivery of gemcitabinein lung cancer therapeutics. Artif Cells Nanomed. Biotechnol. 2017, 45, 1530–1538. [Google Scholar]
- Sanna, V.; Roggio, A.M.; Posadino, A.M.; Cossu, A.; Marceddu, S.; Mariani, A.; Alzari, V.; Uzzau, S.; Pintus, G.; Sechi, M. Novel docetaxel-loaded nanoparticles based on poly(lactide-co-caprolactone) and poly(lactide-co-glycolide-co-caprolactone) for prostate cancer treatment: Formulation, characterization, and cytotoxicity studies. Nanoscale Res. Lett. 2011, 6, 260. [Google Scholar]
- Vino, A.B.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848. Asian Pac. J. Trop. Biomed. 2012, 2, S334–S341. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical propertiesand antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar]
- Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Mano, C.M.; Bechara, J.E.H.; Godínez, L.A.; Mendoza, S. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Baudrimont, I.; Ahouandjivo, R.; Creppy, E.E. Prevention of lipid peroxidation induced by ochratoxin A in Vero cells in culture by several agents. Chem.-Biol. Interact. 1997, 104, 29–40. [Google Scholar]
- Lin, C.; Zimmer, S.G.; Lu, Z.; Holland, R.E., Jr.; Dong, Q.; Chambers, T.M. The involvement of a stress-activated pathway in equine influenza virus-mediated apoptosis. Virology 2001, 287, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Tripathi, A.K.; Parveen, A.; Parveen, S.; Banerjee, M. PLGA-quercetin nano-formulation inhibits cancer progression via mitochondrial dependent caspase-3, 7 and independent FoxO1 activation with concomitant PI3K/AKT suppression. Pharmaceutics 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Study on the Anti-cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- McIntyre, N.; Rosalki, S. Biochemical investigations in the management of liver disease. In Hepatobiliary Diseases; Springer: Berlin/Heidelberg, Germany, 1992; pp. 39–71. [Google Scholar] [CrossRef]
- Markowska, J.; Kasprzak-Drozd, K.; Niziński, P.; Dragan, M.; Kondracka, A.; Gondek, E.; Oniszczuk, T.; Oniszczuk, A. Quercetin: A Promising Candidate for the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Molecules 2024, 29, 5245. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Gourley, M. Diagnostic testing and interpretation of tests for autoimmunity. J. Allergy Clin. Immunol. 2010, 125, S238–S247. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Combination treatment of murine colon cancer with doxorubicin and redox nanoparticles. Mol. Pharm. 2016, 13, 449–455. [Google Scholar] [CrossRef]
- Rawat, A.; Chaturvedi, S.; Singh, A.K.; Guleria, A.; Dubey, D.; Keshari, A.K.; Raj, V.; Rai, A.; Prakash, A.; Kumar, U.; et al. Metabolomics approach discriminates toxicity index of pyrazinamide and its metabolic products, pyrazinoic acid and 5-hydroxy pyrazinoic acid. Hum. Exp. Toxicol. 2018, 37, 373–389. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, B.; Meyer, D.J. Gluthathione transferases: A possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1989, 214, 33–40. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]

| NPS | Probe Sonication Times (s) | Drug:Polymer Ratio | Average Diameter (nm) | Average PdI (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| PLGA-NPS | 60 s | 0:5 | 192 ± 14 | 0.2001 ± 0.171 | −21 ± 15 |
| QPCF-NPs-1 | 60 s | 1:5 | 290 ± 0.055 | 0.039 ± 0.016 | 36 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Sahdev, A.; Raorane, C.J.; Ali, M.A.; Mashay Al-Anazi, K.; Manoharan, R.K.; Raj, V.; Singh, A. Chitosan-Folic Acid-Coated Quercetin-Loaded PLGA Nanoparticles for Hepatic Carcinoma Treatment. Polymers 2025, 17, 955. https://doi.org/10.3390/polym17070955
Kumar Sahdev A, Raorane CJ, Ali MA, Mashay Al-Anazi K, Manoharan RK, Raj V, Singh A. Chitosan-Folic Acid-Coated Quercetin-Loaded PLGA Nanoparticles for Hepatic Carcinoma Treatment. Polymers. 2025; 17(7):955. https://doi.org/10.3390/polym17070955
Chicago/Turabian StyleKumar Sahdev, Anil, Chaitany Jayprakash Raorane, Mohammad Ajmal Ali, Khalid Mashay Al-Anazi, Ranjith Kumar Manoharan, Vinit Raj, and Anita Singh. 2025. "Chitosan-Folic Acid-Coated Quercetin-Loaded PLGA Nanoparticles for Hepatic Carcinoma Treatment" Polymers 17, no. 7: 955. https://doi.org/10.3390/polym17070955
APA StyleKumar Sahdev, A., Raorane, C. J., Ali, M. A., Mashay Al-Anazi, K., Manoharan, R. K., Raj, V., & Singh, A. (2025). Chitosan-Folic Acid-Coated Quercetin-Loaded PLGA Nanoparticles for Hepatic Carcinoma Treatment. Polymers, 17(7), 955. https://doi.org/10.3390/polym17070955










